Abstract
In this study, a novel strain capable of degrading sulfamethoxazole (SMX) was isolated and identified as Acinetobacter sp. The effect of influencing factors, such as initial SMX concentration (5–240 mg/L), temperature (15–35 °C), and pH (5–7), on SMX degradation was investigated. The results showed that when the initial SMX concentration was in the range of 5–240, the removal efficiency was 100%. The optimal condition for SMX biodegradation and microbial growth was determined to be 25 °C and pH = 7.0 in terms of the removal efficiencies of SMX and total organic carbon (TOC). Four metabolite compounds were detected during the process of SMX biodegradation, and the degradation pathways were tentatively proposed. In summary, Acinetobacter sp. was highly efficient in mineralizing SMX, which has the potential to be used for degrading SMX in water and wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antibiotics are ubiquitous in the environment as a result of their widespread application. Sulfamethoxazole (SMX) is one of the most commonly used antibiotics, which enters the environment mainly through human and animal excretion. SMX has been frequently detected in wastewater with concentrations ranging from nanograms per liter to micrograms per liter (Karthikeyan and Meyer 2006; Xu et al. 2007; Gibs et al. 2013; Wang and Wang 2016). SMX has been proved to have adverse influence on the ecological system (García-Galán et al. 2011; Wang and Xu 2012). However, it cannot be effectively removed by conventional wastewater treatment processes, such as the activated sludge process (Blair et al. 2015; Kasprzyk-Hordern et al. 2009; Miège et al. 2009); it is necessary to research and develop new technology to remove SMX from water and wastewater (Wang and Chu 2016; Wang and Bai 2017).
Bioaugmentation of activated sludge systems with specialized microorganisms capable of degrading toxic pollutants is a powerful tool to improve the wastewater treatment processes, for example, to improve the performance of the activated sludge and to enhance the removal efficiency of recalcitrant pollutants (Wang et al. 2002a, b). Generally, the biological treatment system can be enhanced by introducing highly efficient strains capable of degrading targeted pollutants. A series of strains have been isolated for degrading SMX, such as Bacillus subtilis, Pseudomonas aeruginosa, and Rhodococcus equi (Larcher and Yargeau 2011); Microbacterium sp. SMXB24 and Brevundimonas sp. SMXB12 (Herzog et al. 2013); Achrombacter sp. BR3 (Bouju et al. 2012); and Pseudomonas psychrophila HA-4 (Jiang et al. 2014). Among these strains, Microbacterium sp. SMXB24 and Brevundimonas sp. SMXB12 can completely degrade 10 mg/L SMX within 10 days with biodegradation rates of 1.25 and 1.0 mg/L/day, respectively. However, no information is available for the mineralization of SMX by these strains.
The objective of this study is to investigate the biodegradation of SMX by a newly isolated strain, Acinetobacter sp. W1; the effect of initial concentration, temperature, and pH on the degradation of SMX was examined, and the biodegradation pathway of SMX was tentatively proposed.
Materials and methods
Chemicals
SMX was purchased from Aladdin Company with purity higher than 98% (China). The desired concentration of SMX was prepared by directly adding SMX to the sterilized culture medium overnight to make it totally dissolved. The composition of liquid medium was given in the next section. The solid medium used to isolate the strain contained the same ingredients as the liquid medium, and agar powder (2.5% wt/vol) and quantified SMX were added. All other chemicals and solvents used in this study were of reagent grades.
Isolation and identification of strain
The strain used in this study was isolated from the activated sludge collected from a wastewater treatment plant in Beijing. The activated sludge was acclimated to SMX for 3 months prior to the isolation of the strain. To isolate the SMX-degrading strain, 10 mL of acclimated activated sludge was centrifuged (7100g for 4 min at 4 °C). The pellet was washed with the sterilized phosphate buffer for three times. Then the pellet was re-suspended in 100 mL medium (as stated below), diluted in tenfold increments serially, and plated on solid medium containing 50 mg/L of SMX. After 2 days of incubation at 25 °C, the colonies exhibiting good growth were transferred to a solid medium containing 200 mg/L of SMX. The transfer was repeated for several times to obtain the pure strain.
The isolated strain was identified to be Acinetobacter sp. W1 based on the analysis of 16S ribosomal DNA (rDNA) (GenBank accession KX622562; CGMCC No. 13236). The detailed procedure has been reported in the previous studies (Rohwer et al. 2001; Wang et al. 2014). Finally, the phylogenetic tree was constructed using software Mega 5.10. The maximum composite likelihood method was used with the bootstrap replications of 1000.
The strain was grown on the basal medium which contained Na2CO3 0.1 g/L, Na2HPO4·12H2O 0.89 g/L, KH2PO4 0.34 g/L, (NH4)2SO4 0.24 g/L, MgSO4·7H2O 0.06 g/L, CaCl2 0.004 g/L, and 1 mL/L of trace elements. The composition of trace elements was as follows:FeCl2·4H2O 1.5 g/L, CoCl2·6H2O 0.19 g/L, MnSO4·7H2O 0.1 g/L, ZnCl2 0.07 g/L, NiCl2·4H2O 0.024 g/L, Na2MoO4·2H2O 0.024 g/L, MnCl2·4H2O 0.006 g/L, CuCl2·2H2O 0.002 g/L.
Preparation for cell suspensions
To conduct the biodegradation experiment with Acinetobacter sp. W1, exponentially growing cells were harvested by centrifugation (5000g for 10 min) and then washed twice with 0.1 M phosphate buffer solution. Thereafter, the cell suspensions were diluted to an optical density of 0.2 at 600 nm (Wang et al. 2014).
Degradation experiments
The initial concentrations of SMX were set as 5, 40, 80, 160, and 240 mg/L. The biodegradation experiments were performed in a 200-mL glass bottle consisting of 120 mL of sterilized culture medium. The temperature and pH were adjusted to the desired values prior to biodegradation experiments. The experiments were initiated by adding cell suspensions into the bottles, which were shaken at 160 rpm on an orbital shaker. Aerobic conditions were maintained throughout the experiments, and the dissolved oxygen concentration was kept at about 5 mg/L. Prior to the measurement, the samples were centrifuged (5000g for 10 min at 4 °C), and the supernatant was collected and kept at 4 °C until analysis. To evaluate the adsorption of SMX by Acinetobacter sp., the culture medium containing the strain was sterilized by an autoclave at 121 °C for 30 min. In addition, the experiments with only SMX in the absence of bacteria were set to evaluate the SMX losses caused by the abiotic process. All samples were carried out in duplicate.
Analytical methods
SMX was measured by high-performance liquid chromatography (HPLC) (Agilent 1200 Series) equipped with a C18 reversed-phase column (5 μm, 4.6 × 150 mm) and a diode array detector (DAD) set to 275 ± 8 nm. The column temperature was kept at 30 °C, and the flow rate was maintained at 1 mL/min. The initial mobile phase consisted of 70% water containing 0.1% formic acid (A) and 30% acetonitrile (B). The level of solvent B increased to 80% within 5 min and maintained for 2 min, and returned to initial settings in 4 min. The retention time for SMX is 5.4 min. The injected volume is 50 μL.
The HPLC equipped with a photodiode array (PDA) detector coupled to a Shimadzu 2010EV mass spectrometer with ESI ion source (LC-MS) was used to detect the metabolite compounds of SMX. The ion mode was positive. The injected volume is 50 μL. The intermediate compounds were tentatively identified according to the m/z, and standard compounds if applicable.
A total organic carbon analyzer (TOC, Multi N/C 2100, Jena, Germany) was used to measure TOC. The injected volume is 25 μL. The bacterial growth was measured by the optical density at 600 nm using a spectrophotometer.
Results
Effect of initial concentration on SMX biodegradation
The strain capable of degrading SMX was isolated from the acclimated activated sludge and identified to be Acinetobacter sp. according to the analysis of 16S rDNA. The phylogenetic tree is shown in Fig. 1. To our best knowledge, no information about the removal of SMX by Acinetobacter sp. has been reported.
Different initial concentrations of SMX (5 to 240 mg/L) were used to investigate the effect of the initial concentration on its biodegradation. The initial pH was adjusted to 7. The temperature was set as 25 °C. Figure 2a shows the biodegradation of SMX. The concentration of SMX decreased with time under all conditions. The time for achieving 100% removal of SMX increased with increase in initial concentration. It took 7 h for Acinetobacter sp. W1 to completely remove SMX when its initial concentration was 240 mg/L, while it just took 1 h to degrade 5 mg/L of SMX. It is noted that the maximum acclimated concentration of SMX for the activated sludge was 160 mg/L. In the experiments, no lag phase was observed when the initial concentration was increased to 240 mg/L. In addition, the value of O.D.600 increased with the degradation of SMX under all conditions (Fig. 2b). Moreover, the maximal value of O.D.600 was found to be 0.46 when the SMX initial concentration was 240 mg/L. Figure 2a, b shows that the value of O.D.600 remained unchanged after SMX was totally degraded.
In addition, TOC concentration was tested during the experiments. Figure 2c shows the change in TOC concentration with time under all the conditions. When the initial concentration of SMX was 5 mg/L, SMX was completely mineralized within 24 h. When the initial concentration of SMX ranged from 40 to 160 mg/L, the decrease in TOC concentration mainly occurred within 24 h. Thereafter, the TOC concentration remained unchanged. At the end, the residual concentrations of TOC were 0.8, 1.2, and 3.0 mg/L for 40, 80, and 160 mg/L, respectively, and the corresponding removal efficiency of TOC reached 95, 97, and 96%. When the initial concentration of SMX was 240 mg/L, the TOC concentration continued decreasing within 48 h, and the final removal efficiency achieved 96%.
Effect of temperature on SMX biodegradation
Temperature has a great effect on the microbial activity. Different temperatures were thus used to investigate the effect of temperature on the biodegradation of SMX by Acinetobacter sp. W1 as shown in Fig. 3. Under all the tested temperatures, the concentration of SMX was completely removed within 48 h. But the biodegradation kinetics of SMX varied obviously with the temperature. When the temperature was 25 °C, Acinetobacter sp. W1 needs 5 h to totally eliminate SMX. When the temperature increased to 35 °C, Acinetobacter sp. W1 degraded 99% of SMX within 12 h. No SMX was detected after 24 h. When the temperature decreased to 15 °C, the concentration of SMX decreased slowly within 9 h. The biodegradation of SMX became very fast after 9 h. At the end, no SMX was found. Based on the biodegradation of SMX at different temperatures, it is concluded that 25 °C might be very close to the optimum temperature for Acinetobacter sp. W1 to degrade the SMX. Nonetheless, Acinetobacter sp. W1 can still completely remove the SMX at 15 and 35 °C, although longer time is needed.
Figure 3b reflects the change of O.D.600 at different temperatures. In line with the trend of biodegradation of SMX, the value of O.D.600 reached the maximum for all the temperatures when the SMX was completely removed. When the temperature was 15 °C, the bacterial growth was slower compared to other temperatures within 9 h, which explains the slow biodegradation of SMX within 9 h. After 9 h, the value of O.D.600 increased significantly with time and eventually reached the maximum.
Figure 3c shows the change in TOC concentration at different temperatures. In accord with the biodegradation of SMX, the maximum removal efficiency of TOC was obtained at 25 °C reaching 96%. The removal efficiency of TOC at 15 and 35 °C were 86 and 91%, respectively.
Effect of pH on SMX biodegradation
The effect of pH on the biodegradation of SMX was studied. Figure 4a shows the variation of SMX concentration with time at different pH values. SMX was completely degraded within 5 h when pH was 7.0. In comparison, SMX was totally removed within 9 h when pH was 9. When pH was 5, slight biodegradation of SMX was observed. At the end, SMX concentration was 144.7 mg/L. It is thus concluded that neutral pH was suitable for the biodegradation of SMX by Acinetobacter sp. W1. The alkaline pH condition slightly delayed the biodegradation of SMX while the acidic pH condition significantly inhibited the biodegradation of SMX.
Figure 4b shows the change in O.D.600 value at different pH. It is easy to see that the value of O.D.600 significantly increased with time and then remained unchanged at pH 7.0 and 9.0. The biomass was different at pH 7.0 and pH 9.0. At pH 7.0, the biomass reached 0.38, while the biomass was 0.31 at pH 9.0. A slight increase in the value of O.D.600 was observed when the pH was 5.0, which is consistent with the biodegradation of SMX at pH 5.0.
Figure 4c depicts the change in TOC concentration with time. At pH 5.0, the TOC concentration slightly decreased at the end of the experiment. The removal efficiency of TOC was about 5%, which is consistent with the low removal efficiency of SMX. When pH was 7.0 and 9.0, the TOC concentration decreased with time. The removal rate of TOC was faster at pH 7.0 than at pH 9.0. At the end, the removal efficiency of TOC was 96 and 95%, respectively.
Biodegradation pathway of SMX
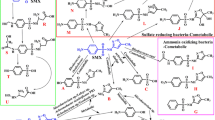
To better understand the biodegradation of SMX, the metabolite compounds during the experiment were tentatively identified. Four metabolites were detected by LC-MS. The figures of mass spectrum and their proposed structures are exhibited in Fig. 5.
Based on the metabolites identified during the experiments, the biodegradation pathway of SMX by Acinetobacter sp. W1 was as proposed in Fig. 6.
Discussion
The value of O.D.600 increased with the initial concentration of SMX, and it remained unchanged after the complete removal of SMX, which indicated that Acinetobacter sp. W1 can use SMX as the carbon source to synthesize the biomass. The decrease in TOC concentration under all conditions demonstrated that biodegradation played a major role for the removal of SMX. Moreover, control experiments showed that adsorption of SMX by Acinetobacter sp. was less than 4%. And the abiotic loss of SMX can be neglected (data not known). This further proved that biodegradation made a major contribution to the removal of SMX. In the previous studies, the biodegradation of SMX was by the pure culture, the maximum concentration of SMX was 127 mg/L, and 44% of SMX was mineralized within 400 h (Bouju et al. 2012). In comparison, 240 mg/L of SMX was used in this study, and the removal efficiency of TOC reached 96% within 48 h, indicating that Acinetobacter sp. W1 had higher capacity for mineralizing SMX.
In contrast to Pseudomonas psychrophila HA-4 mentioned above, Acinetobacter sp. W1 was not inhibited by higher concentration of SMX (240 mg/L), suggesting that Acinetobacter sp. W1 can tolerate higher SMX concentration. In addition, R. equi degraded 15% SMX when its initial concentration was 6 mg/L, which resulted in the increase of bacterial growth by 0.4 (Larcher and Yargeau 2011). While the maximum increment of bacterial growth was only 0.25 in this study, even though the maximum initial concentration of SMX was 240 mg/L. This discrepancy indicated that different degradation mechanisms were involved in the biodegradation of SMX for R. equi and Acinetobacter sp. W1.
The tested temperature affected the growth rate of Acinetobacter sp. W1, but not the maximum biomass. The maximum biomass is dependent on the initial concentration of SMX (Fig. 3a, b). Within the tested temperature, the discrepancy of TOC removal efficiency at different temperatures might be attributed to the effect of temperature on the enzyme involved in the biodegradation of SMX transformation products.
The pH had a significant influence on the removal of SMX by Acinetobacter sp. Acidic condition inhibited obviously the growth of Acinetobacter sp. Alkaline condition slightly inhibited the growth of Acinetobacter sp. (Fig. 4b). But SMX was still completely degraded within the tested periods, suggesting that alkaline condition might not inhibit the enzyme involved in the transformation of SMX, but decreased the biomass of Acinetobacter sp.
Previous study indicated that 3-amino-5-methyl-isoxazole and 4-hydroxy-sulfamethoxazole were the main degradation intermediate products when SMX was used as the sole sources of carbon and nitrogen (Fischer and Majewsky 2014). In addition, the hydrolytic cleavage of the sulfonamide group resulted in the formation of sulfanilic acid and 3-amino-5-methyl-isoxazole (Fischer and Majewsky 2014; Jiang et al. 2014). However, none of these metabolites were detected in this study. Instead of sulfanilic acid, a new metabolite (4-hydroxyl-benzenesulfonic acid) was detected (Fig. 5d). Similarly, instead of 3-amino-5-methyl-isoxazole, a new metabolite (Fig. 5e) was found. In addition, N-hydroxy-acetyl-sulfamethoxazole was detected in the previous study. In this study, a metabolite similar to N-hydroxy-acetyl-sulfamethoxazole (Fig. 5b) which appeared at m/z 317 was observed, which might derive from the hydroxylation and oxygenation of the 5-methyl-isoxazole functional group in the structure of N-hydroxy-acetyl-sulfamethoxazole. Another metabolite appeared at m/z 292 (Fig. 5c). It might be the precursor of N-hydroxy-acetyl-sulfamethoxazole. The accumulation of 3-amino-5-methyl-isoxazole was observed for SMX degradation by Achromobacter denitrificans (Reis et al. 2014). In comparison, no significant accumulation of 3-amino-5-methyl-isoxazole and other metabolites was observed, further demonstrating that different degradation mechanisms were involved in the biodegradation of SMX between Achromobacter denitrificans PR1 and Acinetobacter sp. W1.
To date, the degradation mechanism pertaining to the biodegradation of SMX is still unclear. An NADH-dependent type I hydroxylation was reported to be responsible for the SMX breakdown by Microbacterium sp. strain BR1 (Ricken et al. 2004). Screening the structure of the metabolites, it is easy to see that hydroxylation occurred for most of metabolites, indicating that a NADH-dependent type I hydroxylation might be involved in the biodegradation of SMX by Acinetobacter sp. W1. In addition, monooxygenase or dioxygenase, arylamine N-acetyltransferases, amidases, and N-acetyl-phenylethylamine hydrolase might be also involved in the biodegradation of SMX by Acinetobacter sp. W1 according to the structure of metabolite compounds.
As demonstrated in the previous study (Müller et al. 2013), the metabolites with m/z of 317 and 292 occurred when SMX was provided as the sole source of carbon and nitrogen, which further demonstrated that Acinetobacter sp. W1 can use SMX as the sole source of carbon and nitrogen. In this study, different metabolites were found compared to the metabolites reported in the previous study, which might explain the fast mineralization of SMX.
In summary, Acinetobacter sp. W1 showed high efficiency for removing SMX. Elimination of SMX by Acinetobacter sp. W1 mainly depends on biodegradation. No substrate inhibition phenomenon was observed within the tested initial concentration of SMX (from 5 to 240 mg/L). Both temperature and pH had a significant effect on the biodegradation rate of SMX. Based on the results of TOC concentration during the experiment, Acinetobacter sp. W1 can mineralize more than 95% of SMX within 48 h at the optimum condition. Acinetobacter sp. W1 is thus a potential candidate for removing SMX from water and wastewater.
References
Blair B, Nikolaus A, Hedman C, Klaper R, Grundl T (2015) Evaluating the degradation, sorption, and negative mass balances of pharmaceuticals and personal care products during wastewater treatment. Chemosphere 134:395–401
Bouju H, Ricken B, Beffa T, Corvini PF, Kolvenbach BA (2012) Isolation of bacterial strains capable of sulfamethoxazole mineralization from an acclimated membrane bioreactor. Appl Environ Microbiol 78:5550–5558
Fischer K, Majewsky M (2014) Cometabolic degradation of organic wastewater micropollutants by activated sludge and sludge-inherent microorganisms. Appl Microbiol Biotechnol 98:6583–6597
García-Galán MJ, Díaz-Cruz MS, Barceló D (2011) Occurrence of sulfonamide residues along the Ebro river basin: removal in wastewater treatment plants and environmental impact assessment. Environ Int 37:462–473
Gibs J, Heckathorn HA, Meyer MT, Klapinski FR, Alebus M, Lippincott RL (2013) Occurrence and partitioning of antibiotic compounds found in the water column and bottom sediments from a stream receiving two wastewater treatment plant effluents in Northern New Jersey, 2008. Sci Total Environ 458–460:107–116
Herzog B, Lemmer H, Horn H, Müller E (2013) Characterization of pure cultures isolated from sulfamethoxazole-acclimated activated sludge with respect to taxonomic identification and sulfamethoxazole biodegradation potential. BMC Microbiol 13:276–285
Jiang B, Li A, Cui D, Cai R, Ma F, Wang Y (2014) Biodegradation and metabolic pathway of sulfamethoxazole by Pseudomonas psychrophila HA-4, a newly isolated cold-adapted sulfamethoxazole-degrading bacterium. Appl Microbiol Biotechnol 98:4671–4681
Karthikeyan KG, Meyer MT (2006) Occurrence of antibiotics in wastewater treatment facilities in Wisconsin, USA. Sci Total Environ 361:196–207
Kasprzyk-Hordern B, Dinsdale RM, Guwy AJ (2009) The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res 43:363–380
Larcher S, Yargeau V (2011) Biodegradation of sulfamethoxazole by individual and mixed bacteria. Appl Microbiol Biotechnol 91:211–218
Miège C, Choubert J, Ribeiro L, Eusèbe M, Coquery M (2009) Fate of pharmaceuticals and personal care products in wastewater treatment plants—conception of a database and first results. Environ Pollut 157:1721–1726
Müller E, Schüssler W, Horn H, Lemmer H (2013) Aerobic biodegradation of the sulfonamide antibiotic sulfamethoxazole by activated sludge applied as co-substrate and sole carbon and nitrogen source. Chemosphere 92:969–978
Reis PJ, Reis AC, Ricken B, Kolvenbach BA, Manaia CM, Corvini PF, Nunes OC (2014) Biodegradation of sulfamethoxazole and other sulfonamides by Achromobacter denitrificans PR1. J Hazard Mater 280:741–749
Ricken B, Corvini PFX, Cichocka D, Parisi M, Lenz M, Wyss D, Martínezlavanchy PM, Müller JA, Shahgaldian P, Tulli LG (2004) ipso-Hydroxylation and subsequent fragmentation: a novel microbial strategy to eliminate sulfonamide antibiotics. Appl Environ Microbiol 79:5550–5558
Rohwer F, Breitbart M, Jara J, Azam F, Knowlton N (2001) Diversity of bacteria associated with the Caribbean coral Montastraea franksi. Coral Reefs 20:85–91
Wang JL, Bai ZY (2017) Fe-based catalysts for heterogeneous catalytic ozonation of emerging contaminants in water and wastewater. Chem Eng J 312:79–98
Wang JL, Chu LB (2016) Irradiation treatment of pharmaceutical and personal care products (PPCPs) in water and wastewater: an overview. Radiat Phys Chem 125:56–64
Wang JL, Wang SZ (2016) Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: a review. J Environ Manag 182:620–640
Wang JL, Xu LJ (2012) Advanced oxidation processes for wastewater treatment: formation of hydroxyl radical and application. Crit Rev Environ Sci Technol 42:251–325
Wang JL, Quan XC, Han LP, Qian Y, Werner H (2002a) Microbial degradation of quinoline by immobilized cells of Burkholderia pickettii. Water Res 36:2288–2296
Wang JL, Quan XC, LB W, Qian Y, Werner H (2002b) Bioaugmentation as a tool to enhance the removal of refractory compound in coke plant wastewater. Process Biochem 38:777–781
Wang SZ, Yang Q, Zhang LN, Wang YY (2014) Kinetics of the aerobic co-metabolism of 1, 1-dichloroethylene by Achromobacter sp.: a novel benzene-grown culture. Biotechnol Lett 36:1271–1278
Xu W, Zhang G, Li X, Zou S, Li P, Hu Z, Li J (2007) Occurrence and elimination of antibiotics at four sewage treatment plants in the Pearl River Delta (PRD), South China. Water Res 41:4526–4534
Acknowledgements
This research was supported by the National Natural Science Foundation of China (51338005), the Program for Changjiang Scholars and Innovative Research Team in University (IRT-13026), and the China Postdoctoral Science Foundation (2017M610920).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, S., Wang, J. Biodegradation and metabolic pathway of sulfamethoxazole by a novel strain Acinetobacter sp.. Appl Microbiol Biotechnol 102, 425–432 (2018). https://doi.org/10.1007/s00253-017-8562-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8562-4