Abstract
In recent years, therapeutic peptides have garnered great interest in the pharmaceutical industry for the treatment of diabetes. Lactic acid bacteria (LAB) are an appealing vehicle for safe and convenient oral delivery of bioactive peptide and protein drugs. Exendin-4 (Exd4) is a glucagon-like protein-1 (GLP-1) receptor agonist that is considered an excellent therapeutic peptide drug for type 2 diabetes due to its longer-lasting bioactivity, resulting from resistance to dipeptidyl peptidase 4. We explored Lactococcus lactis with the nisin-controlled gene expression (NICE) system as an oral delivery system for recombinant (r) Exd4 peptide in situ. Heterologous expression and secretion of rExd4 by L. lactis NZ9000/pNZ8048-rExd4 were successful and efficient under the NICE system. In vitro treatment with rExd4 significantly enhanced insulin secretion of INS-1 cells and activated the PI3-K/AKT signal pathway with protein levels of AKT and p-AKT increasing 1.6- to 1.8-fold compared to negative controls, similar to the positive GLP-1 controls. INS-1 cells treated with rExd4 also showed enhanced proliferation and inhibited apoptosis, corresponding with the effects of the standard Exd4 and GLP-1 treatments. Our data suggest that the rExd4 secreted by L. lactis is a bioactive insulinotropic peptide and functional GLP-1 receptor agonist that enhances glucose-dependent insulin secretion and activates the PI3-K/AKT signal pathway; furthermore, it may be involved in improving proliferation and inhibiting apoptosis of INS-1 cells in in vitro treatments. Therefore, L. lactis producing rExd4 may potentially serve as a novel strategy for oral treatment of diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes is a prevalent chronic metabolic disorder. According to the International Diabetes Federation (www.diabetesatlas.org), it affected about 415 million people worldwide in 2015 and is expected to affect 642 million by 2040. Type 2 diabetes accounts for 90–95% of the diabetic population (Engelgau et al. 2000). In the last decades, the peptidic incretin glucagon-like peptide-1 (GLP-1), which can stimulate glucose-dependent insulin secretion, promote beta-cell proliferation, and inhibit beta-cell apoptosis, has been considered a promising therapy for the management of type 2 diabetes (Holst 2007; Wang et al. 2004). However, the native GLP-1 is unsuitable for therapeutic use because of its extremely short half-life in vivo (~1–2 min), due to its rapid degradation by the ubiquitous dipeptidyl peptidase 4 (DPP-4) (Mentlein 2005). GLP-1 receptor agonists (GLP-1RAs) are structurally homologous to GLP-1 and can bind to GLP-1 receptors; they therefore share the same biological functions as GLP-1, but they are naturally resistant to DPP-4 degradation, giving them a longer half-life. They have thus garnered attention as potential therapeutic drugs for use in humans. Exenatide was the first GLP-1RA approved by the US Food and Drug Administration in 2005. It is the synthetic form of exendin-4 (Exd4), a 39 amino-acid peptide with 53% homology to human GLP-1 found in the salivary secretions of the Gila monster Heloderma suspectum (Eng et al. 1992). Exenatide has a longer half-life (2.4 h) and is injected either twice daily as an immediate-release formulation (Byetta, 2014) or once weekly as an extended-release suspension (Bydureon, 2014). However, its administration via subcutaneous injection is inconvenient, largely affecting patient compliance. Therefore, development of a new, more convenient and efficient delivery route and formulation of exenatide for type 2 diabetes treatment are warranted.
Oral administration is the most attractive option for drug delivery due to convenience of administration, patient acceptance, and long-term compliance (Rekha and Sharma 2013). However, this strategy is not feasible for peptide or protein drugs, due to poor biochemical stability (degradation and denaturation) in the harsh gastrointestinal environment (Morishita and Peppas 2006) and low epithelial permeability (Bouttefeux et al. 2016). Several strategies for oral peptide delivery have been extensively explored to improve peptide stability and its absorption through the intestinal epithelium (Chen et al. 2015), for example, using enzyme inhibitors to reduce peptide degradation, enhancing intestinal absorption by opening tight junctions and physicochemical modification of the peptides (Bouttefeux et al. 2016).
Lactococcus lactis, part of the human body’s normal flora, is a generally regarded as safe (GRAS) lactic acid bacterium (LAB) that has been proposed for use as probiotics (Balca’zar et al., 2007). This model LAB species is widely used for fermented products in the food industry and can be found in various traditional fermented foods. Compared to lactobacilli, it is the best studied LAB and many useful genetic tools have been developed for use in L. lactis (Peterbauer et al. 2011). In the past two decades, L. lactis has been demonstrated as a promising candidate for the oral delivery of heterologous proteins with therapeutic or prophylactic effects (Wyszyńska et al. 2015; Cano-Garrido et al. 2015; Wang et al. 2016). Recombinant L. lactis-mediated delivery is more attractive than other oral-delivery systems such as nanoparticles, liposomes, and microspheres involving different strategies (Chen et al. 2015; Bouttefeux et al. 2016). L. lactis can survive transit through the human gastrointestinal tract (Klijn et al. 1995), which facilitates mucosal targeting of therapeutic molecules, particularly for chronic diseases, and has a specific cell-physiological role in the opening of tight junctions of the intestinal epithelium and enhancing the absorption of peptides (Kaushal et al. 2006). A study on the use of transformed L. lactis to secrete interleukin (IL)-10 to treat Crohn’s disease has recently passed phase I clinical trials, demonstrating that L. lactis is indeed an effective oral-delivery platform for heterologous protein delivery. Some articles exploring the potential of transformed L. lactis for the treatment of diabetes have also been published, including its use to deliver insulin analog (Ng and Sarkar 2011; Mao et al. 2017), GLP-1 (Agarwal et al. 2014), and heat shock protein (HSP) 60 (Ma et al. 2014), as well as the combined expression of glutamic acid decarboxylase (GAD65) and IL-10 (Robert et al. 2014).
In this study, genetic engineering of an L. lactis strain harboring a nisin-controlled expression (NICE) system for expression and secretion of bioactive recombinant (r) Exd4 was explored as a potential live bacterial vector for oral delivery of Exd4. The primary bioactivities of the L. lactis-produced rExd4 were assessed for the insulinotropic ability of GLP-1RAs and relative cell-pathway regulatory effects with the in vitro pancreatic beta-cell line INS-1 as a model.
Materials and methods
Bacterial strains, plasmids, and growth conditions
The bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli strains were incubated in Luria-Bertani (LB) medium at 37 °C with shaking at 220 rpm. L. lactis NZ9000 was propagated statically in M17 (Difco) medium containing 0.5% (w/v) glucose (GM17) at 30 °C. To select for and to grow the recombinant L. lactis strains, chloramphenicol (Cm) was added at 10 μg mL−1.
DNA manipulation and transformation
rTaq DNA polymerase, restriction endonuclease, and T4 DNA ligase were purchased from Takara (Dalian, China). The complementary DNA (cDNA) sequence of exendin-4 was obtained from GenBank (accession number DI206426.1) and was codon-optimized for L. lactis using the Codon Adaptation Tool (JCAT). An oligonucleotide carrying a fusion of signal peptide Usp45 and LEISSTCDA propeptide sequences (Zhang et al. 2016), codon-optimized exendin-4, and Sac I and Hin dIII cDNA restriction sites between Usp45 and exendin-4 were synthesized by GENEWIZ Biological Technology Co. Ltd. (Suzhou, China) and then incorporated into pNZ8048, the NICE vector (plasmid courtesy of Dr. Oscar P. Kuipers, University of Groningen), to yield pNZ8048-rExd4. The plasmids pNZ8048-rExd4 and empty pNZ8048 were transformed into competent L. lactis NZ9000 by electroporation.
Inducible expression and secretion of rExd4 by L. lactis NZ9000
For inducible expression, recombinant L. lactis NZ9000/pNZ8048-rExd4 and blank control (NZ9000/pNZ8048) transformants were grown overnight in GM17 medium containing 10 μg mL−1 Cm. A 2% (v/v) inoculum of the L. lactis strains was transferred to fresh GM17 broth and propagated statically at 30 °C to mid-log phase with OD600 ~0.4–0.6 and induced with 10 ng mL−1 nisin (50 IU mL−1, Sigma) for another 3 h. For long-term expression experiments, the recombinant NZ9000/pNZ8048-rExd4 was first induced with 10 ng mL−1 nisin at OD600 ~0.4–0.6 for 1 h and then centrifuged. The cell pellet was washed three times with PBS, resuspended in the same volume of GM17 medium, and incubated for another 2 h.
To prepare rExd4 (NZ9000/pNZ8048-rExd4)-conditioned medium for INS-1 cell experiments, RPMI 1640 medium was used for rExd4 secretion (RPMI 1640-rExd4 medium). Overnight cultures of L. lactis NZ9000/pNZ8048-rExd4 and NZ9000/pNZ8048 cells were transferred to fresh GM17 broth at 2% inoculum, incubated statically at 30 °C to mid-log phase with OD600 ~0.4–0.6, and induced with 10 ng mL−1 nisin for 1 h. Cells were centrifuged, washed three times with PBS, resuspended in the same volume of RPMI 1640 medium, and incubated for another 2 h. The culture was centrifuged at 8000×g for 10 min at 4 °C. The supernatants were collected, adjusted to pH 7.4 with sodium hydroxide, and sterilized by filtration through a 0.22-μm membrane as pulse nisin-induced L. lactis cell-conditioned RPMI 1640 media.
Western blot analysis and ELISA quantification of rExd4
Soluble cytoplasmic and secreted protein fractions were prepared separately. The induced recombinant L. lactis was centrifuged at 6000×g for 10 min at 4 °C. The cell pellets were washed three times with PBS, then resuspended in PBS (pH 7.4) and ultrasonically disrupted. The supernatant samples were subjected to precipitation with trichloroacetic acid (10% w/v) for 2 h at 4 °C followed by centrifugation at 12,000×g for 10 min at 4 °C. The pellet was washed four times with ice-cold acetone and dissolved in PBS. Both cytoplasm and supernatant samples were mixed with 4× SDS loading buffer (12% w/v SDS, 6% v/v β-mercaptoethanol, 30% w/v glycerol, 0.05% w/v Coomassie blue G-250, 150 mM Tris–HCl pH 7.0) for analysis by tricine SDS-PAGE as described previously (Schägger 2006) to separate the target protein. Proteins were then transferred to a PVDF membrane and detected by polyclonal rat anti-Exd4 antibody. The concentration of the secreted rExd4 was determined by ELISA kit (Xinyu Biotech Co. Ltd.).
INS-1 cell culture
Rat insulinoma INS-1 cells (passage 20–30) were cultured in RPMI 1640 medium supplemented with 10% (v/v) fetal bovine serum, 10 mM HEPES, 50 μM β-mercaptoethanol, 1 mM sodium pyruvate, 100 U mL−1 penicillin, and 100 μg mL−1 streptomycin. Cells were maintained at 37 °C in a humidified atmosphere with 5% CO2. The culture medium was replaced every other day.
Insulin-secretion assay
INS-1 cells were seeded at a density of 105 cells per well in 24-well plates and grown until 100% confluence. Cells were then quickly rinsed in 1 mL HEPES balanced salt solution (HBSS) (Hohmeier et al. 2000) with 3 mM glucose followed by a 2-h preincubation with the same buffer. The buffer was discarded, and L. lactis cell-conditioned RPMI 1640 media (pulse nisin-induced NZ9000/pNZ8048-rExd4 and NZ9000/pNZ8048 cells as above), diluted 1:1 (v/v) with HBSS (600 μL per well), were added, respectively. Standard Exd4 (100 nmol L−1) (Sigma Aldrich, St. Louis, MO) and GLP-1 (100 nmol L−1) (Sigma Aldrich, St. Louis, MO) in RPMI 1640 medium were used as positive controls. The mixture was incubated for 2 h, and then supernatant was collected and centrifuged for 10 min at 4000×g at 4 °C. The insulin concentration was determined by ELISA kit (Millipore, St. Charles, MO, USA).
Cell-proliferation assay
INS-1 cells were seeded at a density of 2 × 104 cells per well in 96-well plates. After 36 h incubation, the medium was discarded and replaced with L. lactis cell-conditioned RPMI 1640 media (as above) diluted 1:1 (v/v) with fresh RPMI 1640 medium containing 10% fetal bovine serum (100 μL per well). Standard Exd4 and GLP-1 in RPMI 1640 medium (100 nmol L−1) were used as positive treatment controls. Blank control cells were cultured in pure medium with 10% fetal bovine serum. The mixture was incubated for 24 h. Then, the number of viable cells was determined by cell counting kit-8 (CCK-8) assay (Biyuntian Biotech Co. Ltd., Shanghai, China) based on the formation of formazan with sensitive changes in OD450 values.
Cell-apoptosis assay
INS-1 cells were seeded at a density of 105 cells per well in a 24-well plate and grown to 90% confluence. The cells were incubated with different L. lactis-conditioned RPMI 1640 media diluted 1:1 (v/v) with fresh RPMI 1640 containing 10% fetal bovine serum (600 μL per well) for 24 h as above. Respective positive treatment controls consisted of 100 nmol L−1 Exd4 or 100 nmol L−1 GLP-1 in RPMI 1640 medium. Cells were treated with staurosporine (250 nmol L−1) (Macklin Biochemical Co. Ltd., Shanghai, China) for 1 h and then with Annexin V-PI (propidium iodide) cell apoptosis kit (Biyuntian Biotech). Blank control cells were cultured as above. Cell apoptosis was measured by flow cytometry using a FACScan (Becton Dickinson).
Detection of AKT and phosphor-AKT (p-AKT) expression
INS-1 cells were grown in 6-well plates to 80% confluence and treated with different conditioned RPMI 1640 media diluted 1:1 (v/v) with RPMI 1640 containing 10% fetal bovine serum for 1 h as above. Positive (100 nmol L−1 GLP-1) and blank controls were as above. Cells were then lysed in 200 μL cell lysis buffer (Biyuntian Biotech) supplemented with 1 nM PMSF for 30 min on ice and transferred to 1.5-mL Eppendorf tubes for ultrasonic disruption (9 s, two times). Cell debris was removed by centrifugation at 12,000×g for 10 min at 4 °C, and protein content was measured by BCA Protein Assay Kit (Biyuntian Biotech). Equal amounts of protein were separated by 12% SDS-PAGE for western blot analysis with anti-AKT, anti-phosphor-AKT, and anti-actin (used as an internal control) monoclonal antibodies (Cell Signaling Technology).
Statistical analysis
All experiments were performed in triplicate, and data are presented as mean ± SD. Statistical analyses were carried out by one-way ANOVA, and significant differences (P < 0.05) between means were identified by Duncan’s test procedures using SPSS 20.
Results
Cloning and expression of rExd4 in L. lactis NZ9000
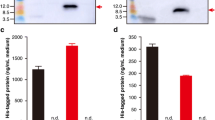
The whole exendin-4 gene was obtained from GenBank (DI206426.1). The DNA oligonucleotide carrying a fused sequence of Usp45 signal peptide and LEISSTCDA propeptide, and the codon-optimized exendin-4 sequence was synthesized and cloned into the nisin-inducible vector pNZ8048 (Fig. 1a). The resulting recombinant plasmid pNZ8048-rExd4 and empty plasmid pNZ8048 were respectively transformed into L. lactis NZ9000. The ability of strain L. lactis NZ9000/pNZ8048-rExd4 to produce and secrete rExd4 was examined by western blot and ELISA. The western blot assay revealed the expected 4.2-kD protein product in both the cell extract and supernatant fractions of the recombinant strain L. lactis NZ9000/pNZ8048-rExd4 (Fig. 1b). The results suggested that rExd4 is successfully expressed by L. lactis NZ9000/pNZ8048-rExd4 with the NICE system. ELISA showed an increase in rExd4 production to a maximum detectable concentration of 249 nmol L−1 by the bacteria at a concentration of 6 × 108 cfu mL−1 after 2.5-h nisin induction (Fig. 1c). The secretion of rExd4 plateaued at this concentration for 4–6 h and then decreased in the presence of nisin.
Expression of recombinant exendin-4 (rExd4) by L. lactis NZ9000. a Schematic DNA sequence of NICE system expression cassette for rExd4 on pNZ8048. PnisA NICE nisA promoter, SP Usp45 sequence of the Usp45 signal peptide, LEISSTCDA sequence of the synthetic propeptide for secretion. The cDNA sequence of codon-optimized exendin-4 is also shown. b Western blot analysis of rExd4 expressed by plasmid-transformed L. lactis NZ9000 strains. c ELISA of secreted rExd4 concentration in the culture broth with different durations of nisin induction
De novo production of rExd4 by L. lactis after 1-h pulse induction with nisin
Because we wanted to construct a recombinant L. lactis vector that secretes rExd4 in order to deliver rExd4 in vivo in the future, we evaluated this strain’s ability to secrete rExd4 “de novo” after nisin induction, following a previously reported method (Bermúdez-Humarán et al. 2003). Two separate batches of the recombinant strain (A and B) were first induced with nisin for 1 h, then cells in culture A were washed and suspended in fresh nisin-free medium while culture B contained nisin throughout the assay. As shown in Fig. 2, culture A produced much more rExd4 than culture B in both the cell extract and supernatant fractions of the transformed strain. Since the extracellular nisin was removed from culture A at 1 h of induction, the rExd4 secreted during the subsequent 2 h corresponded to “de novo” protein, possibly resulting from the operon which was “switched on” by nisin during the first hour of induction. The pulse nisin induction of culture A produced much more rExd4 than the continuous nisin induction of culture B, which may be attributed to decreased toxicity of nisin to the cells in culture A, since neither the NZ9000 strain nor the pNZ8048 strain has nisin-immunity genes (de Ruyter et al. 1996; Kuipers et al., 1998). The secretion efficiency (the ratio of rExd4 secreted in the supernatant vs. cellular rExd4) in cultures A and B was around 37 and 7.3%, respectively. Based on the results of Fig. 2, we prepared the pulse nisin-treated strain to condition cell culture media with rExd4 for further in vitro cell treatments and assays.
De novo secretion of recombinant exendin-4 (rExd4) in the absence of nisin. Synthesis and secretion of rExd4 by the L. lactis NZ9000/pNZ8048-rExd4 strain with nisin (10 ng mL−1) induction. Culture A, pulse induction for 1 h then nisin is washed away and incubation for 2 h for “de novo” rExd4 expression and secretion. Culture B, persistent nisin induction and incubation for 3 h
Secreted rExd4 promotes insulin secretion by INS-1 cells
To evaluate whether rExd4 retains its biological activity, we first tested its ability to stimulate insulin secretion from INS-1 rat pancreatic beta cells. As observed in Fig. 3, L. lactis NZ9000/pNZ8048-rExd4-conditioned medium significantly stimulated insulin secretion to 69.96 ± 0.74 ng mL−1. This was 10% higher than the blank control (with only RPMI 1640, 63.6 ± 0.45 ng mL−1) (P < 0.05); 8% higher than the negative control (L. lactis NZ9000 with empty plasmid pNZ8048-conditioned medium, 64.52 ± 0.78 ng mL−1) (P < 0.05); and equivalent to secretion in the positive controls, stimulated with 100 nM Exd4 and 100 nM GLP-1 (67.12 ± 0.69 and 71.24 ± 1.26 ng mL−1, respectively). Thus, it could be concluded that the L. lactis-secreted rEdx4 is functionally involved in stimulation of insulin secretion from INS-1 cells, similar to GLP-1 and the GLP-1RA Exd4.
Secreted rEdx4 enhances INS-1 cell proliferation and decreases staurosporine-induced cell apoptosis
Previous studies have shown that Exd4 can also increase beta cell proliferation and decrease their apoptosis (Thum et al. 2002; Xu et al., 2006). Here, we sought to verify whether rExd4 secreted by L. lactis is a functional GLP-1RA, showing the same features of proliferation and apoptosis of INS-1 cells. As shown in Fig. 4, in the treatment with rExd4-conditioned medium, the proliferation rate of INS-1 cells increased significantly, to 104.1% that of the blank control (RPMI 1640 medium) and the negative control (NZ9000/pNZ8048-conditioned medium) (P < 0.01) and equivalent to that of the positive controls, 100 nmol L−1 standard Exd4 and 100 nmol L−1 GLP-1 (108.9 and 108.1% of the blank control, respectively, Fig. 4a). These results indicated that the rExd4 secreted by L. lactis NZ9000 can moderately improve INS-1 cell proliferation.
L. lactis-secreted recombinant exendin-4 (rExd4) promotes proliferation and inhibits staurosporine-induced apoptosis of INS-1 cells. a rExd4 promotes INS-1 cell proliferation. Data represent means ± SD of three independent experiments. **P < 0.01; ***P < 0.001 vs. control cells with pure cell media. b rExd4 inhibits staurosporine-induced INS-1 cell apoptosis. Data represent means ± SD of three independent experiments. **P < 0.01 vs. control cells with no stauroporine; # P < 0.05 vs. cells with only staurosporine
Staurosporine, a general kinase inhibitor, induces apoptosis in a broad spectrum of cells (Chae et al. 2000; Belmokhtar et al. 2001; Thuret et al. 2003). After treatment with 250 nmol L−1 staurosporine for 1 h, the number of INS-1 cells in the earliest stages of apoptosis increased significantly to 116.4% of the control with no staurosporine (P < 0.01), while pretreatment with rExd4-conditioned media reduced the apoptosis rate to 96.4% (P < 0.05) (Fig. 4b) of the control. However, pretreatment with pNZ8048-conditioned medium did not reduce the apoptosis rate of INS-1 cells, with no difference compared to the staurosporine-apoptosis model group (Fig. 4b). The rExd4 inhibited INS-1 cell apoptosis to the same extent as 100 nmol L−1 standard Exd4 and GLP-1. These results showed that the rExd4 secreted by L. lactis NZ9000 decreases staurosporine-induced beta cell apoptosis.
Secreted rEdx4 upregulates AKT and phosphor-AKT expression of INS-1 cells
One of the main functions of GLP-1 is to activate the PI3-K/AKT signaling pathway, enhancing beta-cell proliferation and decreasing beta-cell apoptosis (Buteau et al. 1999; Wang and Brubaker 2002; Wang et al. 2004; Gigoux and Fourmy 2013). AKT (also called PKB) is a serine–threonine kinase which is phosphorylated by phosphatidylinositol-3-kinase (PI3-K) upon stimulation (Kandel and Hay, 1999; Hajduch et al. 2001). To examine whether L. lactis-secreted rExd4 is a functional GLP-1RA with respect to its effect on the GLP-1R (PI3-K)/AKT signaling pathway, AKT and p-AKT protein expression in INS-1 cells was measured by western blot (Fig. 5). The intensities of both AKT and p-AKT protein bands in the cells treated with L. lactis-secreted rExd4 were strongly increased, to the same level as those in the positive GLP-1 treatments, compared to the control groups (Fig. 5a) (blank control and L. lactis NZ9000/pNZ8048 negative control). As shown in Fig. 5b, the relative expression levels of AKT and p-AKT in the rExd4 treatment groups were 1.6- and 1.8-fold those of the blank and negative controls, respectively (P < 0.01). These results suggested that phosphorylation of AKT in INS-1 cells is enhanced by the rExd4 treatment, exerting the functional effects of GLP-1RA on INS-1 cell proliferation and apoptosis.
L. lactis-secreted recombinant exendin-4 (rExd4) increases AKT and p-AKT protein expression. a Western blot assays. b Relative AKT and p-AKT protein abundances show increased AKT pathway activation and capacity. GLP-1 group: positive treatment; blank control group: pure cultures; negative control group: L. lactis NZ9000/pNZ8048-conditioned medium treatment; rExd4 experimental group: L. lactis NZ9000/pNZ8048-rExd4-conditioned medium treatment. Data represent means ± SD of three independent experiments, **P < 0.01
Discussion
It has been widely confirmed that food-grade L. lactis is safe as an oral vector for mucosal delivery of therapeutic and prophylactic drugs (Wyszyńska et al. 2015; Cano-Garrido et al. 2015; Wang et al. 2016; Zhang et al. 2016). Bioactive drugs delivered by L. lactis include insulin analogs SCI-57, SCI-59, and GLP-1 for diabetes; HPV-16 E7 for cancer; and superoxide dismutase, IL-10, IL-27, and catalase for inflammatory bowel disease (Cano-Garrido et al. 2015; Mao et al. 2017). In this study, we further verified L. lactis as a promising oral-delivery vector for secretion of the bioactive rExd4 peptide, as a functional GLP-1RA to combat diabetes.
We successfully constructed the engineered strain L. lactis NZ9000/pNZ8048-rExd4 under control of the NICE system. To guarantee considerable secretion of Exd4 by the recombinant strain, the Usp45 signal peptide and LEISSTCDA leading peptide were concatenated with the Exd4 sequence. Insertion of this leading peptide potentially facilitates processing of the precursor and facilitates its translocation, thus enhancing the secretion efficiency of heterologous proteins (Le Loir et al. 1998). As shown in Fig. 1b, c, mature rExd4 was successfully expressed and secreted by L. lactis NZ9000/pNZ8048-rExd4, with a maximum detectable concentration of 249 nmol L−1 after 2.5 h by 6 × 108 cfu mL−1 bacterial cells in the presence of 10 ng mL−1 nisin. Figure 2 shows more efficient secretion of rExd4 under de novo synthesis conditions with pulse nisin induction. Our results were consistent with a previous report in which preinduction of L. lactis with a 1-h nisin pulse resulted in the secretion of recombinant Nuc protein for 10 h. The study showed that the Nuc expression level was approximately doubled at 2 h (~ 60 mg L−1) and then maintained to 25 mg L−1 for even 10 h after the nisin pulse. They further suggested that the remaining protein expression is largely due to the continuation of transcription of Nuc, since the transcript synthesis blocker rifampicin has a dramatic influence on the production of Nuc (Bermúdez-Humarán et al. 2003). In addition, the level of recombinant Nuc was higher in the sample in which nisin was washed away than in that in which nisin was retained, which is in accordance with the rExd4 expression in this study. This effect might be due to lack of toxicity of the added nisin, since the wild-type strain NZ9000 lacks intrinsic nisin immunity genes (Bermúdez-Humarán et al. 2003; de Ruyter et al. 1996). Genetically expressing Exd4 from a nisin-producing strain might improve the heterologous Exd4 expression under nisin induction. These results demonstrated that the recombinant strain has the ability to express and secrete rExd4 after nisin has been washed away, which would be very meaningful for future application of the Exd4-secreting strain as an oral-administration vector in vivo.
Exd4 has been shown to exert its therapeutic effect by promoting glucose-dependent insulin secretion and increasing beta-cell mass by enhancing beta cells’ proliferation and decreasing their apoptosis in vitro and in vivo (Edwards et al. 2001; Wang and Brubaker, 2002; Xu et al., 2006). We therefore tested whether our rExd4 peptide had such bioactivity. Our results confirmed that the rExd4 secreted by the engineered L. lactis NZ9000/pNZ8048-rExd4 is biologically active and can significantly stimulate insulin secretion from beta cells and enhance beta-cell proliferation and inhibit apoptosis. Furthermore, previous studies have reported that Exd4 exerts these latter proliferative and inhibitory actions via activation of AKT (Wang et al. 2004). Thus, we also explored the effects of the secreted rExd4 on AKT protein expression. The L. lactis-secreted rExd4 significantly increased the targeted cellular p-AKT/AKT ratio, further confirming that rExd4 is a physiologically functional GLP-1RA.
Peptide drugs such as insulin with high molecular weight and low lipophilicity are usually poorly permeable across the intestinal wall (Gundogdu and Yurdasiper, 2014). Therefore, penetrating the gastrointestinal barrier remains a big challenge for oral-peptide delivery. L. lactis has the ability to survive passage through the human gastrointestinal tract (Klijn et al. 1995) and temporarily colonize the epithelial cells, leading to sustainable secretion of the recombinant proteins and a high substrate concentration effect that accelerates the transport. Previous studies have reported that the transport rate of β-lactamase is doubled and GLP-1 increases 8-fold when delivered by L. lactis (Shao and Kaushal, 2004; Agarwal et al. 2014). In this study, rExd4 delivery by L. lactis NZ9000/pNZ8048-rExd4 was 5.5-fold the rate of in situ delivery of the control free Exd4 solution using the Caco-2 cell monolayer model (Supplemental Fig. S1). In this respect, it is also a good strategy to use the engineered L. lactis NZ9000/pNZ8048-rExd4 toward improved intestinal permeability for Exd4.
In summary, the anti-diabetic drug Exd4 was successfully expressed and secreted by L. lactis. The secreted rExd4 retained the biological activity of a GLP-1RA, significantly stimulating insulin secretion, promoting proliferation, and inhibiting apoptosis of beta cells via activation of the AKT signaling pathway. Our study suggests the feasibility of expression and secretion of bioactive rExd4 by the food-grade microorganism L. lactis under the NICE system. This strategy opens up the possibility of using L. lactis as a system to orally deliver Exd4 for diabetes treatment, and further improvements and an in vivo assay are warranted.
References
Agarwal P, Khatri P, Billack B, Low WK, Shao J (2014) Oral delivery of glucagon like peptide-1 by a recombinant Lactococcus lactis. Pharm Res 31:3404–3414. doi:10.1007/s11095-014-1430-3
Balca’zar JL, De Blas I, Ruiz-Zarzuela I, Vendrell D, Calvo AC, Ma’rquez I, Girone’s O, Muzquiz JL (2007) Changes in intestinal microbiota and humoral immune response following probiotic administration in brown trout (Salmo trutta). Brit J Nutr 97:522–527. doi:10.1017/S0007114507432986
Belmokhtar CA, Hillion J, Ségal-Bendirdjian E (2001) Staurosporine induces apoptosis through both caspase-dependent and caspase-independent mechanisms. Oncogene 20:3354–3362
Bermúdez-Humarán LG, Langella P, Commissaire J, Gilbert S, Le Loir Y, L’Haridon R, Corthier G (2003) Controlled intra- or extracellular production of staphylococcal nuclease and ovine omega interferon in Lactococcus lactis. FEMS Microbiol Lett 224(2):307–313. doi:10.1016/S0378-1097(03)00475-0
Bouttefeux O, Beloqui A, Préat V (2016) Delivery of peptides via the oral route: diabetes treatment by peptide-loaded nanoparticles. Curr Pharm Design 22:1161–1176
Buteau J, Roduit R, Susini S, Prentki M (1999) Glucagonlike peptide-1 promotes DNA synthesis, activates phosphatidylinositol 3-kinase and increases transcription factor pancreatic and duodenal homeobox gene 1 (PDX-1) DNA binding activity in β (INS-1)-cells. Diabetologia 42:856–864. doi:10.1007/s001250051238
Byetta (exenatide) injection: US prescribing information (2014) Wilmington, DE: AstraZeneca LP
Bydureon (exenatide extended-release for injectable suspension): US prescribing information (2014) Wilmington, DE: AstraZeneca LP
Cano-Garrido O, Seras-Franzoso J, Garcia-Fruitós E (2015) Lactic acid bacteria: reviewing the potential of a promising delivery live vector for biomedical purposes. Microb Cell Factories 14:137. doi:10.1186/s12934-015-0313-6
Chae HJ, Kang JS, Byun JO, Han KS, Kim DU, Oh SM, Kim HM, Chae SW, Kim HR (2000) Molecular mechanism of staurosporine-induced apoptosis in osteoblasts. Pharmacol Res 42:373–381. doi:10.1006/phrs.2000.0700
Chen C, Zhu X, Dou Y, Xu J, Zhang J, Fan T, Du J, Liu K, Deng Y, Zhao L, Huang Y (2015) Exendin-4 loaded nanoparticles with a lipid shell and aqueous core containing micelles for enhanced intestinal absorption. J Biomed Nanotechnol 11:865–876. doi:10.1166/jbn.2015.1971
de Ruyter PG, Kuipers OP, de Vos WM (1996) Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl Environ Microbiol 62:3662–3667
Edwards CM, Stanley SA, Davis R, Brynes AE, Frost GS, Seal LJ, Ghatei MA, Bloom SR (2001) Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab 281:E155–E161
Eng J, Kleinman WA, Singh L, Singh G, Raufman JP (1992) Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem 267:7402–7405
Engelgau MM, Narayan KM, Herman WH (2000) Screening for type 2 diabetes. Diabetes Care 23:1563–1580. doi:10.2337/diacare.23.10.1563
Gigoux V, Fourmy D (2013) Acting on hormone receptors with minimal side effect on cell proliferation: a timely challenge illustrated with GLP-1R and GPER. Front Endocrinol 4:50. doi:10.3389/fendo.2013.00050
Gundogdu E, Yurdasiper A (2014) Drug transport mechanism of oral antidiabetic nanomedicines. Int J Endocrinol Metab 12:e8984. doi:10.5812/ijem.8984
Hajduch E, Litherland GJ, Hundal HS (2001) Protein kinase B (PKB/Akt)—a key regulator of glucose transport? FEBS Lett 492:199–203. doi:10.1016/S0014-5793(01)02242-6
Hohmeier HE, Mulder H, Chen GX, Henkel-Rieger R, Prentki M, Newgard CB (2000) Isolation of INS-1–derived cell lines with robust ATP-sensitive K+ channel-dependent and-independent glucose-stimulated insulin secretion. Diabetes 49:424–430. doi:10.2337/diabetes.49.3.424
Holst JJ (2007) The physiology of glucagon-like peptide 1. Physiol Rev 87:1409–1439. doi:10.1152/physrev.00034.2006
Kandel ES, Hay N (1999) The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp Cell Res 253:210–229. doi:10.1006/excr.1999.4690
Kaushal G, Trombetta L, Ochs RS, Shao J (2006) Delivery of TEM beta-lactamase by gene-transformed Lactococcus lactis subsp. lactis through cervical cell monolayer. Int J Pharm 313(1–2):29–35. doi:10.1016/j.ijpharm.2006.01.013
Klijn N, Weerkamp AH, de Vos WM (1995) Genetic marking of Lactococcus lactis shows its survival in the human gastrointestinal tract. Appl Environ Microbiol 61(7):2771–2774
Kuipers OP, de Ruyter PG, Kleerebezem M, de Vos WM (1998) Quorum sensing-controlled gene expression in lactic acid bacteria. J Biotechnol 64:15–21. doi:10.1016/S0168-1656(98)00100-X
Le Loir Y, Gruss A, Ehrlich SD, Langella P (1998) A nine-residue synthetic propeptide enhances secretion efficiency of heterologous proteins in Lactococcus lactis. J Bacteriol 180(7):1895–1903
Ma Y, Liu J, Hou J, Dong Y, Lu Y, Jin L, Cao R, Li T, Wu J (2014) Oral administration of recombinant Lactococcus lactis expressing HSP65 and tandemly repeated P277 reduces the incidence of type I diabetes in non-obese diabetic mice. PLoS One 9:e105701. doi:10.1371/journal.pone.0105701
Mao RF, Wu DL, Hu SM, Zhou KP, Wang M, Wang YF (2017) Secretory expression and surface display of a new and biologically active single-chain insulin (SCI-59) analog by lactic acid bacteria. Appl Microbiol Biotechnol 101:3259–3271. doi:10.1007/s00253-017-8125-8
Mentlein R (2005) Therapeutic assessment of glucagons-like peptide-1 agonists compared with dipeptidyl peptidase IV inhibitors as potential antidiabetic drugs. Expert Opin Investig Drugs 14:57–64. doi:10.1517/13543784.14.1.57
Morishita M, Peppas NA (2006) Is the oral route possible for peptide and protein drug delivery? Drug Discov Today 11:905–910. doi:10.1016/j.drudis.2006.08.005
Ng DT, Sarkar CA (2011) Nisin-inducible secretion of a biologically active single-chain insulin analog by Lactococcus lactis NZ9000. Biotechnol Bioeng 108:1987–1996. doi:10.1002/bit.23130
Peterbauer C, Maischberger T, Haltrich D (2011) Food-grade gene expression in lactic acid bacteria. Biotechnol J 6:1147–1161. doi:10.1002/biot.201100034
Rekha MA, Sharma CP (2013) Oral delivery of therapeutic protein/peptide for diabetes—future perspectives. Int J Pharm 440:48–62. doi:10.1016/j.ijpharm.2012.03.056
Robert S, Gysemans C, Takiishi T, Korf H, Spagnuolo I, Sebastiani G, Van Huynegem K, Steidler L, Caluwaerts S, Demetter P, Wasserfall CH, Atkinson MA, Dotta F, Rottiers P, Van Belle TL, Mathieu C (2014) Oral delivery of glutamic acid decarboxylase (GAD)-65 and IL10 by Lactococcus lactis reverses diabetes in recent-onset NOD mice. Diabetes 63:2876–2887. doi:10.2337/db13-1236
Schägger H (2006) Tricine-sds-page. Nat Protoc 1:16–22. doi:10.1038/nprot.2006.4
Shao J, Kaushal G (2004) Normal flora: living vehicles for non-invasive protein drug delivery. Int J of Pharm 286:117–124. doi:10.1016/j.ijpharm.2004.08.004
Thum A, Hupe-Sodmann K, Goke R, Voigt K, Goke B, McGregor GP (2002) Endoproteolysis by isolated membrane peptidases reveal metabolic stability of glucagon-like peptide-1 analogs, exendins-3 and -4. Exp Clin Endocrinol Diabetes 110:113–118. doi:10.1055/s-2002-29087
Thuret G, Chiquet C, Herrag S, Dumollard JM, Boudard D, Bednarz J, Campos L, Gain P (2003) Mechanisms of staurosporine induced apoptosis in a human corneal endothelial cell line. Br J Ophthalmol 87:346–352. doi:10.1136/bjo.87.3.346
Wang Q, Brubaker PL (2002) Glucagon-like peptide-1 treatment delays the onset of diabetes in 8 week-old db/db mice. Diabetologia 45:1263–1273. doi:10.1007/s00125-002-0828-3
Wang Q, Li L, Xu E, Wong V, Rhodes C, Brubaker PL (2004) Glucagon-like peptide-1 regulates proliferation and apoptosis via activation of protein kinase B in pancreatic INS-1 beta cells. Diabetologia 47:478–487. doi:10.1007/s00125-004-1327-5
Wang M, Gao ZQ, Zhang YG, Pan L (2016) Lactic acid bacteria as mucosal delivery vehicles: a realistic therapeutic option. Appl Microbiol Biotechnol 100:5691–5701. doi:10.1007/s00253-016-7557-x
Wyszyńska A, Kobierecka P, Bardowski J, Jagusztyn-Krynicka EK (2015) Lactic acid bacteria—20 years exploring their potential as live vectors for mucosal vaccination. Appl Microbiol Biotechnol 99:2967–2977. doi:10.1007/s00253-015-6498-0
Xu G, Kaneto H, Lopez-Avalos MD, Weir GC, Bonner-Weir S (2006) GLP-1/exendin-4 facilitates β-cell neogenesis in rat and human pancreatic ducts. Diabetes Res Clin Pract 73:107–110. doi:10.1016/j.diabres.2005.11.007
Zhang B, Li AD, Zuo FL, Yu R, Zeng Z, Ma HQ, Chen SW (2016) Recombinant Lactococcus lactis NZ9000 secretes a bioactive kisspeptin that inhibits proliferation and migration of human colon carcinoma HT-29 cells. Microb Cell Factories 15:102. doi:10.1186/s12934-016-0506-7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was founded by the Beijing Advanced Innovation Center for Food Nutrition and Human Health.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Supplemental Fig. S1
(PDF 496 kb)
Rights and permissions
About this article
Cite this article
Zeng, Z., Yu, R., Zuo, F. et al. Recombinant Lactococcus lactis expressing bioactive exendin-4 to promote insulin secretion and beta-cell proliferation in vitro. Appl Microbiol Biotechnol 101, 7177–7186 (2017). https://doi.org/10.1007/s00253-017-8410-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8410-6