Abstract
Varicella is a highly contagious disease caused by primary infection of Varicella zoster virus (VZV). Varicella can be severe or even lethal in susceptible adults, immunocompromised patients and neonates. Determination of the status of immunity to VZV is recommended for these high-risk populations. Furthermore, measurement of population immunity to VZV can help in developing proper varicella vaccination programmes. VZV glycoprotein E (gE) is an antigen that has been demonstrated to be a highly accurate indicator of VZV-specific immunity. In this study, recombinant gE (rgE) was used to establish a double antigen sandwich enzyme-linked immunosorbent assay (ELISA). The established sandwich ELISA showed high specificity and sensitivity in the detection of human sera, and it could detect VZV-specific antibodies at a concentration of 11.25 m IU/mL with a detection linearity interval of 11.25 to 360 m IU/mL (R 2 = 0.9985). The double gE antigen sandwich ELISA showed a sensitivity of 95.08 % and specificity of 100 % compared to the fluorescent-antibody-to-membrane-antigen (FAMA) test, and it showed a sensitivity of 100 % and a specificity of 94.74 % compared to a commercial neutralizing antibody detection kit. Thus, the established double antigen sandwich ELISA can be used as a sensitive and specific quantitative method to evaluate immunity to VZV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Varicella zoster virus (VZV) is a human alpha herpesvirus, which causes varicella and herpes zoster (HZ) upon infection. Primary infection with VZV causes varicella, while reactivation of the latent virus results in HZ (Drolet et al. 2010). Varicella is a highly contagious disease and can be complicated by skin superinfections, pneumonia, encephalomyelitis and myelitis (Bourre et al. 2013; Choo et al. 1995; Sawanyawisuth et al. 2007). In particular, laboratory determination of the status of immunity to VZV has been recommended for immunocompromised patients, pregnant women and health workers after exposure to VZV (Sauerbrei and Wutzler 2006). A live attenuated varicella vaccine has been introduced and has dramatically reduced varicella incidence (Takahashi et al. 1975; Vazquez et al. 2001). However, the effectiveness of the varicella vaccine has been reported to be 80–85 %, and global vaccine coverage remains limited. Thus, varicella remains a public health concern worldwide (Marin et al. 2008; Vazquez et al. 2001).
Antibodies in the serum have been used as a reliable indicator for the evaluation of VZV-specific immunity (Sauerbrei et al. 2004). The varicella vaccine induces a one tenth or lower titre of VZV-specific antibodies than a natural infection (Hammond et al. 2006); highly sensitive methods are needed for the measurement of VZV-specific immunity. Several methods have been developed to measure VZV-specific antibodies (Baba et al. 1984; Caunt and Shaw 1969; Gershon et al. 1994; Grose et al. 1979; Han et al. 2015; Larussa et al. 1987; Maple et al. 2006; Park et al. 2015; Sauerbrei et al. 2004; Wasmuth and Miller 1990; Williams et al. 1974). The fluorescent-antibody-to-membrane-antigen (FAMA) test is the classical method to evaluate immunity to varicella, and extensive clinical validation has shown that the FAMA test facilitates the determination of susceptibility to varicella (Gershon and Gershon 2010; Williams et al. 1974). However, the FAMA test is labour intensive and has low throughput. The enzyme-linked immunosorbent assay (ELISA), based on purified VZV glycoproteins (gpELISA), was developed by Merck Research Laboratories, and it has been used to determine the seroconversion rate in vaccinated groups (Hammond et al. 2006; Maple et al. 2006; Wasmuth and Miller 1990). However, it is difficult to obtain VZV glycoproteins (gps) and control gps, and the effective antigen in VZV gps varies from batches and should be standardized with an in-house panel of sera (Wasmuth and Miller 1990). In addition, this assay is not commercially available, and it is difficult to use this method as a universal method to quantitatively evaluate VZV-specific immunity.
Antibodies specific for VZV membrane proteins are the main detection objects of the FAMA test and gpELISA. Glycoprotein E (gE) is the major and most immunogenic membrane protein of VZV, and it can induce robust humoral and cellular immunity (Baiker et al. 2010; Davison et al. 1986). This study investigated if gE-specific antibodies could be used as an indicator to quantitatively determine VZV-specific antibodies. In this study, recombinant gE (rgE) was expressed using a baculovirus expression system and was subsequently purified. A double gE antigen sandwich ELISA was established and showed high sensitivity and specificity in the detection of human serum. The assay showed good detection linearity in the detection of standard serum and can be used to quantitatively determine VZV-specific antibodies. Furthermore, the established ELISA showed high coincidence with the FAMA test and a commercial neutralizing antibody detection kit in sera evaluation.
Materials and methods
Cell, virus, serum and glycosidase
Spodoptera frugiperda (Sf21) cells (Invitrogen, Carlsbad, CA, USA) were routinely maintained in CCM3 medium (Hyclone, Logan, UT, USA) with 2 % foetal bovine serum (FBS, PAA, Hyclone, Logan, UT, USA). Human acute retinal pigment epithelial cells (ARPE-19, purchased from the ATCC, Manassas, VA, USA) were routinely maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10 % FBS.
The vaccine Oka strain (v-Oka) was maintained in ARPE-19. Baculovirus (Autographa californica nuclear polyhedrosis virus, AcMNPV) was purchased from Clontech (Mountain View, CA, USA).
The collection of 125 human sera was stored by Beijing Wantai Biological Pharmacy Enterprise, and another collection of 240 human sera was obtained from members of the National Institute of Diagnostics and Vaccine Development in Infectious Diseases. Written informed consent was obtained from the donors, and Independent Ethics Committee approval was obtained from the Ethics Committee of the National Institute of Diagnostics and Vaccine Development in Infectious Diseases. A VZV-positive serum, which was calibrated with the international anti-varicella zoster serum W1044, was used as the reference serum. Glycosylases and RNase B were obtained from New England BioLabs (NEB, Ipswich, England).
Expression and purification of rgE
The fragment of gE (1-537aa, GenBank No. AAY57748) with a His tag fused to it was expressed using the baculovirus expression system (Kimura et al. 1997; Liu et al. 2015). rgE was secreted into the culture supernatant, and two obvious bands (gE total) were purified with a Ni-NTA column (QIAGEN, Venlo, The Netherlands). The two bands were further separated by a DEAE Sepharose fast flow column (GE Healthcare, Uppsala, Sweden).
Glycosylation analysis
Purified gE protein and RNase B (control) were digested with peptide-N-glycosidase F (PNGase F), endoglycosidase H (Endo H) or O-glycosidase and neuraminidase, respectively. Treated or untreated proteins were analysed by SDS-PAGE and Coomassie brilliant blue staining.
Indirect ELISA based on VZV gps (gpELISA) and gE (gE ELISA)
gpELISA and gE ELISA adopt a detection mode of indirect ELISA, and VZV gps was purified according to a previous report (Wasmuth and Miller 1990). Purified rgE or VZV gps diluted in coating buffer (0.05 M sodium carbonate, pH 9.6) was coated onto ELISA plates (100 ng/well) and incubated overnight at 4 °C. The plates were saturated for 2 h at 37 °C with saturation buffer (PBS containing 0.25 % casein and 1 % gelatine). After removal of the saturation buffer, diluted serum was added, and the plates were incubated for 1 h at 37 °C. The plates were washed five times with washing buffer (PBS containing 0.05 % Tween 20), and HRP-conjugated goat anti-human IgG diluted in saturation buffer was then added. The plates were then incubated at 37 °C for 30 min. After being washed five times, the plates were incubated with a solution of tetramethylbenzidine (TMB) for 15 min, and the reaction was stopped with 2.1 M H2SO4. The plates were read at 450/620 nm on a microplate reader.
Double gE antigen sandwich ELISA
Purified rgE diluted in coating buffer was coated onto ELISA plates (100 ng/well), which were incubated overnight at 4 °C. The plates were saturated for 2 h at 37 °C with saturation buffer. After removal of the saturation buffer, diluted serum was added, and the plates were incubated for 1 h at 37 °C. The plates were washed five times with washing buffer, and HRP-conjugated rgE was then added. The plates were incubated at 37 °C for 30 min. After being washed five times, the plates were incubated with TMB solution for 15 min, and the reaction was stopped with 2.1 M H2SO4. The plates were read at 450/620 nm on a microplate reader.
FAMA test
The FAMA test was initially validated as an indicator of VZV-specific immunity in 1974 (Williams et al. 1974). The standard version of the FAMA test was performed with modifications as described by many reports (Han et al. 2014; Sauerbrei et al. 2004). In brief, ARPE-19 cells infected by v-Oka were collected when the cytopathic effect was present in approximately 70–80 % of the cells, and they were washed two times with PBS. Cells (8 × 103) were coated onto each well of a 5-mm 12-well slide and fixed in cold acetone for 30 min. The slides were saturated with 10 % goat serum and were air-dried and stored at −80 °C until use. The slides were incubated with twofold serially diluted human sera at 37 °C for 30 min. After three washes with PBS, fluorescein isothiocyanate (FITC)-conjugated goat anti-human IgG premixed with 0.01 % Evans blue was incubated with the cells at 37 °C for 30 min. The slides were washed and evaluated with an IX71 fluorescence microscope (Olympus, Tokyo, Japan).
Detection of neutralizing antibodies with a commercial ELISA kit
The commercial ELISA kit (product code: VZV-0196) was obtained from Beijing Wantai Biological Pharmacy Enterprise (Beijing, China), and it was used to qualitatively determine VZV-specific neutralizing antibodies based on a blocking ELISA. The procedures for serum detection are described in the manual. In brief, 90 μL of sample diluent and 12.5 μL of human serum/positive control/negative control were added to each well of the ELISA plates, which were incubated at 37 °C for 1 h. After washing five times, detection reagent was added, and the plates were incubated at 37 °C for 45 min. After being washed five times, the plates were incubated with TMB solution for 15 min, and the reaction was stopped with 2.1-M H2SO4. The plates were read at 450/620 nm on a microplate reader. A blocking rate ≥50 % indicated the presence of neutralizing antibodies.
Results
Expression, purification and analysis of recombinant gE protein
The fragment of gE (1–537 aa) was expressed using the baculovirus expression system, and rgE was confirmed to be expressed in the medium. The expression levels of rgE were monitored using a previously reported quantitative assay (Liu et al. 2015). rgE was expressed efficiently (>10 mg/L) in different batches. In previous studies, rgE was purified with a Ni-NTA column, and the eluent was analysed by SDS-PAGE to display two clear bands (gE-total). In this study, the two forms of rgE were further separated by a DEAE Sepharose fast flow column. The gE protein with low molecular weight (gE-down) was eluted by 200 mM NaCl, and the protein with large molecular weight (gE-up) was eluted by 300 mM NaCl (Fig. 1a).
Purification and analysis of recombinant VZV gE. a Purification of rgE. rgE was initially purified by a Ni-NTA column, and the elution from Ni-NTA showed two obvious bands (gE-total) on SDS-PAGE. The two bands were separated by a DEAE Sepharose fast flow column. M marker, lane 1 eluent from Ni-NTA, lane 2 eluent from DEAE column by 200 mM NaCl, lane 3 eluent from DEAE column by 300 mM NaCl. b Glycosylation analysis of rgE-up. Purified rgE-up was digested with glycosidases and analysed by SDS-PAGE and Coomassie blue staining. M marker, lane 1 untreated rgE-up, lane 2 rgE-up digested with PNGase F, lane 3 rgE-up digested with Endo H, lane 4 rgE-up digested with O-glycosidase and neuraminidase. c Glycosylation analysis of rgE-down. Purified rgE-down was digested with glycosidases and analysed by SDS-PAGE and Coomassie blue staining. M marker, lane 1 untreated rgE-down, lane 2 rgE-down digested with PNGase F, lane 3 rgE-down digested with Endo H; lane 4: rgE-down digested with O-glycosidase and neuraminidase. d Glycosylation analysis of RNase B. RNase B was digested with glycosidases and analysed by SDS-PAGE and Coomassie blue staining. M marker, lane 1 untreated RNase B, lane 2 RNase B digested with PNGase F, lane 3 RNase B digested with Endo H, lane 4 RNase B digested with O-glycosidase and neuraminidase. e Analysis of gE-up/down by mass spectrometry. Bands of gE-up and gE-down were excised, and their sequences were identified by mass spectrometry. The sequence of gE-up consisted of 35–537 aa of full-length gE, while the sequence of gE-down consisted of 73–537 aa of full-length. Segments marked in green indicate high probability of coverage (> 95 %), while segments marked in grey indicate non-coverage
Glycosylation analysis was performed to analyse different forms of rgE. Both gE-up and gE-down showed no change in the SDS-PAGE before and after digestion with glycosidases (Fig. 1b, c), but the molecular weight of RNase B (control protein) significantly decreased (Fig. 1d). These results implied that the two forms of rgE may not be caused by glycosylation. The two bands were excised and sequenced by mass spectroscopy, and the results verified that both bands were gE proteins. The sequence of gE-up consisted of 35–537 aa of the full-length gE, and the sequence of gE-down consisted of 73–537 aa of the full-length gE (Fig. 1e).
Performance of gpELISA, gE ELISA, and double gE antigen sandwich ELISA in the detection of human VZV-positive and VZV-negative serum
In this study, three assays, including gpELISA, gE ELISA, and double gE antigen sandwich ELISA, were used to evaluate human VZV-positive and VZV-negative sera, which were verified with the FAMA test. In a typical detection, human serum was tested with a twofold serial dilution series starting from 1:20. The results showed that all three methods could distinguish VZV-positive serum from VZV-negative serum with gpELISA and gE ELISA showing greater sensitivity than the double gE antigen sandwich ELISA. However, when detecting negative serum with a low dilution, gpELISA and gE ELISA showed a degree of non-specific reaction, while the double gE antigen sandwich ELISA showed no non-specific reaction (Fig. 2). Traditionally, the non-specific reaction could be reduced by deducting the reaction of the serum with tissue culture control (TCC), which was prepared using the identical procedure for VZV gps preparation (Hammond et al. 2006). However, there was no need to consider the non-specific reaction even with un-diluted serum detection using the double gE antigen sandwich ELISA.
Comparison of gpELISA, gE ELISA and gE antigen sandwich ELISA in the detection of human serum. a Detection of VZV-positive and VZV-negative serum using the gpELISA. VZV-positive and VZV-negative sera were initially diluted 1:20 and subsequently twofold serially diluted, and the diluted sera were detected using the gpELISA. b Detection of VZV-positive and VZV-negative serum using the double gE antigen sandwich ELISA. VZV-positive and VZV-negative sera were initially diluted 1:20 and subsequently twofold serially diluted, and the diluted sera were detected using the double gE antigen sandwich ELISA. c Detection of VZV-positive and VZV-negative serum using the gE ELISA. VZV-positive and VZV-negative sera were initially diluted 1:20 and subsequently two-fold serially diluted, and the diluted sera were detected using the gE ELISA
Characteristics of the double gE antigen sandwich ELISA in human serum detection
Three combinations of double gE antigen sandwich ELISA (gE-total/gE-total-HRP, gE-up/gE-up-HRP and gE-down/gE-down-HRP) were developed to detect positive and negative human serum. All three combinations showed good sensitivity and specificity in serum detection (Fig. 3a). In the following experiments, we used the gE-down to establish this assay. The sensitivity of the double antigen sandwich ELISA was tested with the twofold serial dilutions of the reference serum, ranging from 11.25 to 720 m IU/mL. The results showed that the double gE-down antigen sandwich ELISA could detect reference serum at a concentration of 11.25 m IU/mL (Fig. 3b). The linear interval of the antigen sandwich ELISA ranged from 11.25 to 360 m IU/mL (R 2 = 0.9985) (Fig. 3c). Thus, the established double antigen ELISA can be used to quantitatively determine the level of VZV-specific antibodies in vaccinated or infected subjects.
Characteristics of the double gE antigen sandwich ELISA in human serum detection. a Detection of VZV-positive and VZV-negative sera by three combinations of the double gE antigen sandwich ELISA. The results showed that the double gE antigen sandwich ELISA had no non-specific reaction with a low dilution of negative serum, while the three combinations showed similar sensitivities in the detection of VZV-positive serum. b Analysis of the sensitivity of the double antigen sandwich ELISA. Two-fold serial dilutions of the reference sera were detected, and the result showed that the established double antigen sandwich ELISA could detect reference serum at a concentration of 11.25 m IU/mL. c Determination of the linearity interval in serum detection. Serial dilutions of the reference serum were detected, and the correlation between OD450 and concentration of the antibody was analysed. The results showed that OD450 correlated well with the concentrations of antibody in the range from 11.25 to 360 m IU/mL (R 2 = 0.9985)
Evaluation of human serum using the double gE antigen sandwich ELISA compared to the FAMA test and a commercial ELISA kit
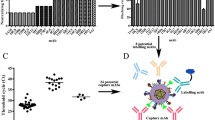
To evaluate the performance of the established double antigen sandwich ELISA in serum detection, the study included two comparison experiments. In one experiment, a collection of 125 human sera was evaluated by the established double gE antigen sandwich ELISA and the FAMA test. The cutoff value of the FAMA test was set at 1:8, while the cutoff value of the double gE antigen sandwich was set at 40 m IU/mL (calculated by 2.1 × mean OD of negative pool + standard deviation). The result of the FAMA test showed that the collection of 125 sera included 67 positive sera and 58 negative sera, and 3 positive sera showed a negative result when detected by the double gE antigen sandwich ELISA (Fig. 4a). Thus, with the FAMA test as the reference, the double gE antigen sandwich ELISA showed a sensitivity of 95.08 % and a specificity of 100 %, and the two assays showed a coincidence rate of 97.60 % (Table 1).
Evaluation of human serum using the double gE antigen sandwich ELISA compared to the FAMA test and a commercial ELISA kit. a Evaluation of human serum using the double gE antigen sandwich ELISA compared to the FAMA test. The result of the FAMA test showed that the collection of 125 sera included 67 positive sera and 58 negative sera, and 3 positive sera (marked as a blue square) showed negative results when detected by the double gE antigen sandwich ELISA. b ROC analysis of the double gE antigen sandwich ELISA and the commercial ELISA kit in the evaluation of neutralizing antibodies in sera. With the commercial ELISA kit as a reference, the double gE antigen sandwich ELISA performed excellently in the detection of the other collection of 240 sera (AUC = 0.9907)
In the second comparison experiment, another collection of 240 adult sera was evaluated by the established double antigen sandwich ELISA and a commercial ELISA kit. The commercial ELISA kit was obtained from Beijing Wantai Biological Pharmacy Enterprise and has been certificated by China Food and Drug Administration (CFDA). With the results of the commercial ELISA kit as a reference, receive operating characteristic (ROC) curve analysis was performed to evaluate the detection performance of the double gE antigen sandwich ELISA, and the area under the curve was 0.9907 (Fig. 4b). Using ROC analysis with a cutoff value of 75.2 m IU/mL, the gE antigen sandwich ELISA showed a sensitivity of 100 % and specificity of 94.74 % (Table 2).
Quantitative evaluation of population immunity to VZV using the established double gE antigen sandwich ELISA
In this study, a serum calibrated with the international anti-varicella zoster serum W1044 was used as a reference and the VZV-specific antibody concentration of the collection of 240 adult sera was quantitatively determined using the established double gE antigen sandwich ELISA. There were 18 sera (18/240) with antibody concentrations <80 m IU/mL, which were interpreted as negative (Sauerbrei et al. 2012). The antibody concentration ranges of the sera were as follows: 27 sera (27/240) had concentration ranges from 80 to 1000 m IU/mL; 44 sera (44/240) had concentration ranges from 1000 to 2000 m IU/mL; and 151 sera (151/240) had concentrations greater than 2000 m IU/mL (Fig. 5). Thus, the established double gE antigen sandwich ELISA can be used as a rapid and high-throughput method to evaluate population immunity to VZV.
Determination of the concentration of VZV-specific antibodies in human sera. With the reference serum as the standard, this study quantitatively determined the concentration of VZV-specific antibodies in a collection of 240 adult sera. According to the results, the collection of sera was sorted into four groups as follows: the VZV-negative group (antibody concentration < 80 m IU/mL) containing 18 sera (18/240 = 7.5 %); the weak positive group (80–1000 m IU/mL) containing 27 sera (27/240 = 11.25 %); the moderate positive group (1000–2000 m IU/mL) containing 44 sera (44/240 = 18.33 %); and the strong positive group (>2000 m IU/mL) containing 151 sera (151/240 = 62.92 %)
Discussion
The VZV Oka vaccine strain was obtained in 1974 and subsequently used to develop safe and effective live attenuated vaccines (Takahashi et al. 1975). Immunization with varicella vaccine has dramatically reduced the incidence of varicella (Guris et al. 2008). However, only a few developed countries have currently included varicella vaccination in their childhood immunization schedules, and global vaccine coverage remains limited. Measurement of population immunity to VZV could help in developing proper immunization strategies. Furthermore, Varicella might be severe in specific populations. Serological confirmation of VZV-specific immunity has been recommended for healthcare workers, immunocompromised populations, and pregnant women.
The FAMA test and gpELISA have been most frequently used to evaluate VZV-specific immunity. The FAMA test is the classical method and the best laboratory indication for measuring immunity to varicella. Antibodies to VZV measured by the FAMA test are correlated with protection from varicella after household exposure (Michalik et al. 2008). However, the FAMA test is labour-intensive, has low throughput and is not amenable to automation. Moreover, the interpretation of FAMA test results is subjective, and the test requires special equipment. The gpELISA is used to measure the seroconversion rate in varicella vaccine inoculators, but it is difficult to prepare VZV gps and control gps. Furthermore, lentil lectin Sepharose cannot purify VZV glycoproteins specifically, and the effective antigen in VZV gps varies from batches and should be standardized with an in-house panel serum (Wasmuth and Miller 1990). Thus, both the FAMA test and gpELISA remain to be improved.
VZV gE is the major and most immunogenic membrane protein. VZV-positive sera have been shown to contain a high titre of gE-specific antibodies (Keller et al. 1986), and these antibodies could be used as a candidate indication for serological evaluation of VZV-specific immunity. In this study, rgE was expressed by a baculovirus expression system, and two forms of rgE were purified. Glycosylation analysis and mass spectroscopy were performed to identify these proteins. The results showed that gE-up consisted of 35–537 aa of full-length gE and that gE-down consisted of 73–537 aa of full-length gE, and the two rgE forms did not result from glycosylation. According to the information from a protein database (UniProt), VZV gE is a type I transmembrane protein, and it contains an N-terminal signal peptide (1–30 aa, predicted by sequence analysis). When the signal peptide is removed, it results in the loss of N-terminal sequences. In addition, there may be other digestion processes that result in greater losses of N-terminal sequences of gE-down.
VZV gE was used to establish an assay for serological evaluation. Firstly, indirect ELISA (gE ELISA) was used to detect human serum, but a non-specific reaction occurred when detecting negative sera with low dilutions. Subsequently, HRP-conjugated rgE was introduced to establish a double gE antigen sandwich ELISA, which showed a high specificity. In a parallel comparison, the double gE antigen sandwich ELISA showed lower sensitivity than gpELISA and gE ELISA. However, the established double gE antigen sandwich ELISA could detect at a concentration of 11.25 m IU/mL when detecting the reference serum, and concentrations <80 m IU/mL were interpreted as negative according to a previous report (Sauerbrei et al. 2012). Thus, the sensitivity of the established double gE antigen sandwich ELISA was sufficient to distinguish positive serum from negative serum. Furthermore, the double gE antigen sandwich ELISA showed good linearity in the detection of serial dilutions of the reference serum ranging from 11.25 to 360 m IU/mL. Thus, the double gE antigen sandwich ELISA can be used to quantitatively determine the concentration of VZV-specific antibodies in serum.
In this study, the detection performance of the double gE antigen sandwich ELISA was further evaluated by comparison to the FAMA test and a commercial ELISA kit. A collection of 125 human sera was tested by both the FAMA test and the double gE antigen sandwich ELISA. The double gE antigen sandwich ELISA showed a sensitivity of 95.08 % and specificity of 100 % in the qualitative analysis. In evaluation of another collection of 240 human sera, the established double antigen sandwich ELISA showed excellent performance and high coincidence when compared with the commercial ELISA kit. Because there are no consensus quantitative assays and the cutoff value for positive/negative determination is obscure (Krah et al. 1997; Michalik et al. 2008; Provost et al. 1991; Sauerbrei et al. 2012), we did not set a consensus cutoff value for all the experiments in this study. In the comparison experiments, we determined the cutoff value according to the method and raw results (Table 1) or the algorithm design of the ROC program (Table 2). In Fig. 5, we determined the cutoff value according to a previous publication (Sauerbrei et al. 2012). All of these experimental results demonstrated a satisfying performance for the qualitative evaluation of human sera using the established double antigen sandwich ELISA.
The coated antigens of conventional ELISAs are derived from VZV-infected cells, including VZV-infected cell lysate supernatants (Shehab and Brunell 1983), individual glycoproteins (Keller et al. 1986) or VZV gps (Wasmuth and Miller 1990). Current commercial tests adopt a similar indirect ELISA format but use different coating antigens (Sauerbrei et al. 2012). In a previous comparison experiment of three commercial assays with the FAMA test in the evaluation of human sera, the assay using a coating consisting of VZV envelop gps shows significantly lower specificity (89.4 %), and the assay using a coating consisting of highly purified VZV proteins shows significantly lower specificity (90.5 %). Moreover, this previous study showed that the assay using a coating consisting of extract from VZV-infected cells exhibited the best performance (Sauerbrei et al. 2012). In these conventional ELISAs, control antigens are needed for background reaction subtraction. Furthermore, the component of this coating antigen is complicated, and the effective antigen varies among batches (Keller et al. 1986; Wasmuth and Miller 1990). In this study, rgE was obtained by secretory expression and was expressed at high levels (>10 mg/L) using the baculovirus expression system. The purity of purified rgE can reach greater than 90 %, and it is much easier to obtain large amounts of highly purified rgE than to purify a specific antigen from VZV-infected cells. The established double gE antigen sandwich ELISA was shown to be highly sensitive and specific. Conventional ELISAs used for serological evaluation adopt the indirect ELISA format. In this study, the indirect ELISA based on highly purified rgE still had a background reaction when detection was performed on low dilutions of negative sera. Conventional ELISAs usually detect sera at specific dilutions, and they utilize a control gps to increase the specificity of detection. The employment of HRP-conjugated rgE and elimination of the use of a secondary antibody may improve the specificity of the conventional ELISA. In this study, the double gE antigen sandwich ELISA was demonstrated to be highly specific to detect negative sera and to have an advantage to detect weakly positive sera. Thus, the established double gE antigen sandwich ELISA can be used as a sensitive, specific, easy and high-throughput method to quantitatively evaluate population immunity to VZV.
References
Baba K, Yoshida M, Tawa A, Yabuuchi H, Maeda K, Takahashi M (1984) A simplified immunofluorescence technique for antibody to varicella-zoster membrane antigen (FAMA. Biken J 27(1):23–29. doi:10.1016/0167-7012(95)00066-6
Baiker A, Haase R, Eberle J, Vizoso Pinto MG, Pfrepper KI, Petrich A, Deml L, Campe H, Nitschko H, Jaeger G (2010) Early detection of varicella-zoster virus (VZV)-specific T-cells before seroconversion in primary varicella infection: case report. Virol J 7:54. doi:10.1186/1743-422X-7-54
Bourre B, Lefaucheur R, Ahtoy P, Travers F, Fetter D (2013) Varicella-zoster virus acute myelitis in a patient with MS treated with natalizumab. Neurology 81(22):1966–1967. doi:10.1212/01.wnl.0000439052.05262.1d
Caunt AE, Shaw DG (1969) Neutralization tests with varicella-zoster virus. J Hyg (Lond) 67(2):343–352
Choo PW, Donahue JG, Manson JE, Platt R (1995) The epidemiology of varicella and its complications. J Infect Dis 172(3):706–712. doi:10.1093/infdis/172.3.706
Davison AJ, Edson CM, Ellis RW, Forghani B, Gilden D, Grose C, Keller PM, Vafai A, Wroblewska Z, Yamanishi K (1986) New common nomenclature for glycoprotein genes of varicella-zoster virus and their glycosylated products. J Virol 57(3):1195–1197
Drolet M, Brisson M, Schmader KE, Levin MJ, Johnson R, Oxman MN, Patrick D, Blanchette C, Mansi JA (2010) The impact of herpes zoster and postherpetic neuralgia on health-related quality of life: a prospective study. Can Med Assoc J 182(16):1731–1736. doi:10.1503/cmaj.091711
Gershon AA, Gershon MD (2010) Perspectives on vaccines against varicella-zoster virus infections. Curr Top Microbiol Immunol 342:359–372. doi:10.1007/82_2010_12
Gershon AA, Larussa P, Steinberg S (1994) Detection of antibodies to varicella-zoster virus using a latex agglutination assay. Clin Diagn Virol 2(4–5):271–277. doi:10.1016/0928-0197(94)90051-5
Grose C, Edmond BJ, Brunell PA (1979) Complement-enhanced neutralizing antibody response to varicella-zoster virus. J Infect Dis 139(4):432–437. doi:10.1093/infdis/139.4.432
Guris D, Jumaan AO, Mascola L, Watson BM, Zhang JX, Chaves SS, Gargiullo P, Perella D, Civen R, Seward JF (2008) Changing varicella epidemiology in active surveillance sites—United States, 1995-2005. J Infect Dis 197(Suppl 2):S71–S75. doi:10.1086/522156
Hammond O, Wang Y, Green T, Antonello J, Kuhn R, Motley C, Stump P, Rich B, Chirmule N, Marchese RD (2006) The optimization and validation of the glycoprotein ELISA assay for quantitative varicella-zoster virus (VZV) antibody detection. J Med Virol 78(12):1679–1687. doi:10.1002/jmv.20754
Han SB, Kang KR, Huh DH, Lee HC, Kim JH, Kang JH, Ma SH (2014) Seroepidemiology of varicella-zoster virus in Korean adolescents and adults using fluorescent antibody to membrane antigen test. Epidemiol Infect 143:1643–1650. doi:10.1017/S0950268814002441
Han X, Ping B, Morita M, Ebi R, Inoue J, Tanaka Y, Chen Y, Wan X, Yang W, Chang B, Wu X (2015) A novel highly sensitive and specific flow cytometry system for cervical cancer screening. Gynecol Oncol 139(1):52–58. doi:10.1016/j.ygyno.2015.07.102
Keller PM, Lonergan K, Neff BJ, Morton DA, Ellis RW (1986) Purification of individual varicella-zoster virus (VZV) glycoproteins gpI, gpII, and gpIII and their use in ELISA for detection of VZV glycoprotein-specific antibodies. J Virol Methods 14(2):177–188. doi:10.1016/0166-0934(86)90048-0
Kimura H, Straus SE, Williams RK (1997) Varicella-zoster virus glycoproteins E and I expressed in insect cells form a heterodimer that requires the N-terminal domain of glycoprotein I. Virology 233(2):382–391. doi:10.1006/viro.1997.8625
Krah DL, Cho I, Schofield T, Ellis RW (1997) Comparison of gpELISA and neutralizing antibody responses to Oka/Merck live varicella vaccine (Varivax) in children and adults. Vaccine 15(1):61–64
Larussa P, Steinberg S, Waithe E, Hanna B, Holzman R (1987) Comparison of five assays for antibody to varicella-zoster virus and the fluorescent-antibody-to-membrane-antigen test. J Clin Microbiol 25(11):2059–2062
Liu J, Zhu R, Ye X, Yang L, Wang Y, Huang Y, Wu J, Wang W, Ye J, Li Y, Zhao Q, Zhu H, Cheng T, Xia N (2015) A monoclonal antibody-based VZV glycoprotein E quantitative assay and its application on antigen quantitation in VZV vaccine. Appl Microbiol Biot 99(11):4545–4553. doi:10.1007/s00253-015-6602-5
Maple PA, Gray J, Breuer J, Kafatos G, Parker S, Brown D (2006) Performance of a time-resolved fluorescence immunoassay for measuring varicella-zoster virus immunoglobulin G levels in adults and comparison with commercial enzyme immunoassays and Merck glycoprotein enzyme immunoassay. Clin Vaccine Immunol 13(2):214–218. doi:10.1128/CVI.13.2.214-218.2006
Marin M, Meissner HC, Seward JF (2008) Varicella prevention in the United States: a review of successes and challenges. Pediatrics 122(3):e744–e751. doi:10.1542/peds.2008-0567
Michalik DE, Steinberg SP, Larussa PS, Edwards KM, Wright PF, Arvin AM, Gans HA, Gershon AA (2008) Primary vaccine failure after 1 dose of varicella vaccine in healthy children. J Infect Dis 197(7):944–949. doi:10.1086/529043
Park R, Hwang JY, Lee KI, Namkoong S, Choi SK, Park S, Park H, Park J (2015) Measurement of antibodies to varicella-zoster virus using a virus-free fluorescent-antibody-to-membrane antigen (FAMA) test. J Microbiol Biotech 25:268–273. doi:10.4014/jmb.1408.08048
Provost PJ, Krah DL, Kuter BJ, Morton DH, Schofield TL, Wasmuth EH, White CJ, Miller WJ, Ellis RW (1991) Antibody assays suitable for assessing immune responses to live varicella vaccine. Vaccine 9(2):111–116
Sauerbrei A, Wutzler P (2006) Serological detection of varicella-zoster virus-specific immunoglobulin G by an enzyme-linked immunosorbent assay using glycoprotein antigen. J Clin Microbiol 44(9):3094–3097. doi:10.1128/JCM.00719-06
Sauerbrei A, Farber I, Brandstadt A, Schacke M, Wutzler P (2004) Immunofluorescence test for sensitive detection of varicella-zoster virus-specific IgG: an alternative to fluorescent antibody to membrane antigen test. J Virol Methods 119(1):25–30. doi:10.1016/j.jviromet.2004.02.012
Sauerbrei A, Schafler A, Hofmann J, Schacke M, Gruhn B, Wutzler P (2012) Evaluation of three commercial varicella-zoster virus IgG enzyme-linked immunosorbent assays in comparison to the fluorescent-antibody-to-membrane-antigen test. Clin Vaccine Immunol 19(8):1261–1268. doi:10.1128/CVI.00183-12
Sawanyawisuth K, Phuttharak W, Tiamkao S, Boonpila A (2007) MRI findings in acute disseminated encephalomyelitis following varicella infection in an adult. J Clin Neurosci 14(12):1230–1233. doi:10.1016/j.jocn.2006.09.001
Shehab Z, Brunell PA (1983) Enzyme-linked immunosorbent assay for susceptibility to varicella. J Infect Dis 148(3):472–476. doi:10.1093/infdis/148.3.472
Takahashi M, Okuno Y, Otsuka T, Osame J, Takamizawa A (1975) Development of a live attenuated varicella vaccine. Biken J 18(1):25–33
Vazquez M, LaRussa PS, Gershon AA, Steinberg SP, Freudigman K, Shapiro ED (2001) The effectiveness of the varicella vaccine in clinical practice. N Engl J Med 344(13):955–960. doi:10.1056/NEJM200103293441302
Wasmuth EH, Miller WJ (1990) Sensitive enzyme-linked immunosorbent assay for antibody to varicella-zoster virus using purified VZV glycoprotein antigen. J Med Virol 32(3):189–193. doi:10.1002/jmv.1890320310
Williams V, Gershon A, Brunell PA (1974) Serologic response to varicella-zoster membrane antigens measured by direct immunofluorescence. J Infect Dis 130(6):669–672. doi:10.1093/infdis/130.6.669
Acknowledgments
We thank members of the Analysis and Testing Centre (School of Life Sciences, Xiamen University) for their mass spectrometry work. Editors at NPG Language Editing provided editing assistance to the authors during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by a grant from the National High Technology Research and Development Programme of China (No. 2012AA02A408) and the National Science and Technology Major Project of Infectious Diseases (No. 2012ZX10004503-005). The funding sources had no involvement in study design, data collection and decision for publication.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the Independent Ethics Committee approval obtained from the Ethics Committee of the National Institute of Diagnostics and Vaccine Development in Infectious Diseases.
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Jian Liu and Chunye Chen contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, J., Chen, C., Zhu, R. et al. Evaluation of immunity to varicella zoster virus with a novel double antigen sandwich enzyme-linked immunosorbent assay. Appl Microbiol Biotechnol 100, 9321–9329 (2016). https://doi.org/10.1007/s00253-016-7821-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7821-0