Abstract
Tea tree oil (TTO) is a yellow liquid extracted from Melaleuca alternifolia. Although the antimicrobial activity of TTO has been known for a long time, its specific antimicrobial effects and mechanism underlying these remain poorly characterized. The present study investigated the chemical composition of TTO and the dynamics and mechanism of its antimicrobial activities in two bacterial and two fungal strains. Gas chromatography–mass spectrometry analysis identified alkenes and alcohols as the main constituents of TTO. Terpinen-4-ol was the most abundant individual component, accounting for approximately 23 % of the TTO. Poisoned food technique assessment showed that the minimum inhibitory concentrations of TTO for bacterial strains (Escherichia coli and Staphylococcus aureus) and fungal strains (Candida albicans and Aspergillus niger) were 1.08 and 2.17 mg/mL, respectively. Antimicrobial dynamic curves showed that with increasing concentrations of TTO, the rate of cell killing and the duration of growth lag phase increased correspondingly. These data indicated that TTO produced concentration and time-dependent antimicrobial effects. The minimum bactericidal and fungicidal concentrations of TTO were 2.17, 4.34, and 4.34 against E. coli, S. aureus, and C. albicans, respectively. However, A. niger conidia were not completely eradicated, even after 3 days in the presence of 17.34 mg/mL TTO. Transmission electron microscopy images indicated that TTO penetrated the cell wall and cytoplasmic membrane of all the tested bacterial and fungal strains. TTO may also penetrate fungal organelle membrane. These findings indicated that TTO maybe exerts its antimicrobial effects by compromising the cell membrane, resulting in loss of the cytoplasm and organelle damage, which ultimate leads to cell death.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tea tree oil (TTO) is a yellow liquid extracted from Melaleuca alternifolia, an evergreen native Australian tree commonly referred to as the “tea tree” or “paperbark” (Homer et al. 2000). TTO has been used in medicine for at least 80 years (Carson and Riley 1993; Hammer et al. 1996; Hammer et al. 1999; Arweiler et al. 2000). The European Pharmacopeia (EDQM 2006) and the International standard (ISO 4730) (1996) require TTO to be obtained from the foliage and terminal branchlets of M. alternifolia Cheel by steam distillation. TTO consists of about 100 different compounds, and the major components are terpenes and sesquiterpenes, such as terpinen-4-ol, α- and γ-terpinene, 1,8-cineole, and terpinolene (Brophy et al. 1989; Kwieciński et al. 2009; Hammer et al. 2012). The concentrations of the components of TTO can vary significantly between different preparations, and this may influence their antimicrobial activities (Arweiler et al. 2000). Moreover, there is natural variation in the TTO content of M. alternifolia Cheel. Six distinct oil chemotypes were identified based on TTO compositions and yields from 615 trees. One chemotype is dominated by terpinen-4-ol, one by 1,8-cineole, and one by terpinolene; the remaining three chemotypes are all dominated by 1,8-cineole and differ in either terpinen-4-ol or terpinolene content (Homer et al. 2000). ISO 4730 (1996) requires commercial tea tree oil to have a minimum content of 30 % terpinen-4-ol and a maximum content of 15 % 1,8-cineole. Terpinen-4-ol is the major TTO component, and this has shown strong antimicrobial and anti-inflammatory properties (Kwieciński et al. 2009; Mondello et al. 2006).
The efficacy of TTO against bacteria (Carson et al. 1995; Nelson 1997; Natalie et al., 2013; Lim and Hammer 2015; Shi et al. 2016), fungi (Hammer et al. 1997; Hammer et al. 1998; Hammer et al. 2000; Li et al., 2016a, b), virus (Bishop 1995; Garozzo et al. 2011; Evgeny et al. 2013), and protozoa (Campli et al. 2012; James and Callander 2012; Pazinato et al. 2014) has gained the attention of scientists, physicians, and consumers. TTO efficiently killed all tested clinical strains of Staphylococcus aureus, both as planktonic cells and as biofilms, and its effective concentration was never higher than 1 % v/v (Kwieciński et al. 2009). In an in vivo assay, TTO showed antifungal activity against Trichophyton equinum (Pisseri et al. 2009), with a minimum inhibitory concentration (MIC) of 2.5 % (v/v) while 4 % (v/v) yielded a fungicidal effect (Nardoni et al. 2010). TTO and its nanoparticles were active against both bacteria and fungi, with MICs ranging from 0.002 to 2.5 % (Souza et al. 2014). TTO showed strong antiviral activity against the influenza A virus and the Escherichia coli phage, M13, and was capable of inactivating model viruses with an efficiency of more than 95 % within 5–15 min of exposure (Usachev et al. 2013).
Although many studies have reported the in vitro antimicrobial activity and in vivo efficacy of TTO, little is known about its antimicrobial dynamics and mechanisms underlying these. This has limited the wider application of TTO as an antimicrobial agent and further research into these aspects is therefore urgent. The present study explored these aspects of TTO activity using four test microorganism strains (two bacterial and two fungal): E. coli ATCC 8739, S. aureus ATCC 6538, Candida albicans ATCC 10231, and Aspergillus niger ATCC 16404.
Materials and methods
Chemical reagents, microorganisms, mediums, and cultivation
TTO, extracted from the foliage and terminal branchlets of M. alternifolia Cheel, was provided by Guangdong Fuyang Biotechnology Company Ltd. (Heyuan city, Guangdong province, China). This TTO was nearly 100 % in purity, with a density of 0.867 g/mL. (−)-Terpinen-4-ol was purchased from Aldrich (purity >95 %, # BCBM5331V), with a density of 0.934 g/mL. The microorganism strains (E. coli ATCC 8739, S. aureus ATCC 6538, C. albicans ATCC 10231, and A. niger ATCC 16404) were purchased from the American Type Culture Collection (ATCC) and stored in our laboratory. The media used to culture these strains were described previously (Li et al. 2010, 2013, 2016a, b). These were Mueller-Hinton (MH) medium and Mueller-Hinton Agar (MHA) medium for aerobic culture of E. coli and S. aureus, Sabouraud dextrose broth (SDB) medium and Sabouraud dextrose agar (SDA) medium for the aerobic culture of C. albicans, and potato dextrose water (PDW) medium and potato dextrose agar (PDA) medium for aerobic culture of A. niger. All solvents and reagents were of analytical grade.
Gas chromatography-mass spectrometry (GC-MS) analyses of TTO
The constituents of TTO were analyzed using a similar approach to that employed in our previous studies (Li et al. 2013, 2014a). A Thermo Finnigan Trace GC ultra-gas chromatograph with a DB 5-ms column was used (30 m length × 0.25 mm ID × 0.25 μm particle diameter), equipped with a Finnigan Trace DSQ mass spectrometer. Briefly, the operating conditions were programmed from 60 to 220 °C at 10 °C/min, with an injection port temperature of 70–250 °C. Compounds were tentatively identified by comparing their mass spectra with those of authentic samples in the NIST MS library.
Antimicrobial activities of TTO and terpinen-4-ol
Antimicrobial activities were measured using the poisoned food technique described in our previous study, with slight modification (Li et al. 2013, 2014b). The test concentrations of TTO were 0 (as control), 0.14, 0.27, 0.54, 1.08, 2.17, or 4.34 mg/mL. The test concentrations of terpinen-4-ol were 0 (as control), 0.15, 0.29, 0.58, 1.17, 2.34, or 4.67 mg/mL. Around 106 colony-forming units (CFU) of E. coli or S. aureus were employed/plate or around 105 CFU of C. albicans cells or A. niger conidia/plate. The cell concentrations of E. coli, S. aureus, and C. albicans were determined by measuring optical density (OD) at 600 nm; an OD600 of 0.1 corresponded to 108 CFU/mL of the two bacterial strains, or 106 CFU/mL of C. albicans. The concentration of A. niger conidia was determined by hemocytometer. The bacterial and fungal plates were sealed using Parafilm and incubated at 37 or 28 °C, respectively, in an incubator for either 7 or 14 days. The MIC values for TTO against these bacterial and fungal strains were determined as those that showed no visible growth after incubated for 1 or 7 days separately. Two separate experiments were carried out, each with triplicate evaluations.
Antimicrobial dynamics of TTO
The antimicrobial dynamics of TTO were determined as described in our previous study (Li et al. 2013), with slight modification. The test concentrations of TTO were 0 (as control), 0.14, 0.27, 0.54, 1.08, 2.17, or 4.34 mg/mL. The cell concentrations of E. coli or S. aureus were both about 106 CFU/mL, and the cell or conidia concentrations of C. albicans or A. niger were both about 105 CFU/mL. The bacterial and fungal preparations were incubated with shaking at 150 rpm in water baths at 37 or 28 °C, respectively. Samples of bacteria were taken after incubation for 0, 2, 4, 6, 8, 10, 12, 24, 48, and 72 h; gradient dilutions of these samples were then cultured on MHA Petri dishes for 1 or 2 days at 37 °C. The bacterial colonies were counted after this incubation period, and the total number of live cells per milliliter was calculated. Similarly, fungal samples were taken and cultured on SDA and PDA Petri dishes for 2 or 3 days at 28 °C. The fungal colonies were counted after this incubation period, and the total number of live cells per milliliter was calculated. The antimicrobial dynamic curves for TTO against these four strains were developed using these data. The experiments were carried out in triplicate.
Transmission electron microscopy (TEM) of bacterial and fungal strains exposed to TTO
This was investigated as described in our previous study (Li et al. 2011). The test concentrations of TTO were 0 (as control) or 2.17 mg/mL. The bacterial and fungal cell concentrations were 108 and 107 CFU/mL, respectively. Before adding TTO, A. niger had been incubated at 28 °C for 15 h and the mycelium was well established. TTO or an equal volume of phosphate-buffered saline was then added into the TTO treatment cultures or control cultures, respectively. These were incubated with shaking at 150 rpm in water baths at 37 or 28 °C, as appropriate, for 5 h. The cell cultures were then sampled and prepared for TEM (Hitachi H-7650). These experiments were carried out in triplicate.
Results
The main constituents of TTO
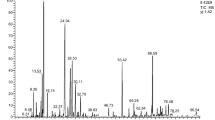
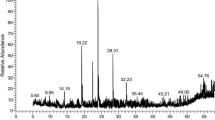
The major TTO constituents identified by GC-MS are presented in Table 1 and their chemical formulae are shown in Fig. 1. The main chemical components of TTO identified in the present study were alkenes and alcohols. Terpenes (γ-terpinene, α-terpinene, terpinolene, β-pinene, α-pinene, and β-thujene) and terpene alcohols (terpinen-4-ol, carveol, and α-terpineol) were the first and second major categories of constituents, accounting for approximately 38.5 and 32.2 %, respectively, of the TTO. The level of terpinen-4-ol was particularly high, representing approximately 23 % of the whole. The third major constituent category was sesquiterpenes (isoledene, α-gurjunene, caryophyllene, alloaromadendrene, ledene, and δ-cadinene), followed by sesquiterpene alcohol (globulol, viridifloro, and cubenol); these accounted for approximately 15.4 and 4.9 %, respectively, of the TTO.
The activity of TTO against four microbe strains
Images of the poisoned food determinations of antimicrobial activity are shown in Fig. 2. E. coli (Fig. 2 (A)), colonies were formed in the presence of 0, 0.14, 0.27, or 0.54 mg/mL TTO after incubation for 1 day, but no colonies were found in the presence of 1.08 or 2.17 mg/mL TTO. These results were almost unchanged after incubation for 7 days. For S. aureus (Fig. 2 (B)), many colonies were formed after incubation for 1 day in the presence of 0, 0.14, or 0.27 mg/mL TTO, while dozens of colonies developed in the presence of 0.54 mg/mL TTO, and none were observed in the plates with 1.08 or 2.17 mg/mL TTO. This pattern was also observed after the 7-day incubation. Therefore, the MIC of TTO against both E. coli and S. aureus was 1.08 mg/mL (1.25 μL/mL). For the fungal strains, numerous C. albicans (Fig. 2 (C)) colonies developed, in the presence of 0, 0.14, 0.27, or 0.54 mg/mL TTO after incubation for 2 days, while none developed in the plates containing 1.08 or 2.17 mg/mL TTO. However, after incubation for 7 days, many colonies grew in the plates with 1.08 mg/mL TTO, while none developed in the presence of 2.17 mg/mL TTO. This indicated that the MIC of TTO against C. albicans was 2.17 mg/mL (2.5 μL/mL). The plates containing 0, 0.27, or 0.54 mg/mL TTO were full of white mycelium colonies of A. niger (Fig. 2 (D)) after 1 day, while none developed in the presence of 1.08, 2.17, or 4.34 mg/mL TTO. After 7 days, the 0, 0.27, 0.54, or 1.08 mg/mL TTO plates were all full of mycelia and were covered with black A. niger conidia, while none developed in the 2.17 or 4.34 mg/mL TTO plates. After incubation for 14 days, the plates containing 2.17 mg/mL TTO were also full of mycelia and had small quantities of black conidia. These findings indicated that the MIC of TTO against A. niger conidia was 2.17 mg/mL (2.5 μL/mL) for a 7-day incubation.
Images of the poisoned food determinations of the antimicrobial activity of TTO against four microbial strains. E. coli (A), S. aureus (B), and C. albicans (C) are shown after treatment for 7 days with (a) no TTO (control), (b) 0.14 mg/mL TTO, (c) 0.27 mg/mL TTO, (d) 0.54 mg/mL TTO, (e) 1.08 mg/mL TTO, (f) 2.17 mg/mL TTO; (D) A. niger conidia treated for 14 days with the concentrations of TTO (a) no TTO (control), (b) 0.27 mg/mL TTO, (c) 0.54 mg/mL TTO, (d) 1.08 mg/mL TTO, (e) 2.17 mg/mL TTO, (f) 4.34 mg/mL TTO
Images of the poisoned food determinations of terpinen-4-ol antimicrobial activity are shown in Fig. S1. The MICs of terpinen-4-ol were 1.17 (1.25 μL/mL), 1.17 (1.25 μL/mL), 2.34 (2.5 μL/mL), and 2.34 mg/mL (2.5 μL/mL) against E. coli (Fig. S1-A), S. aureus (Fig. S1-B), C. albicans (Fig. S1-C), and A. niger (Fig. S1-D), respectively.
The dynamics of TTO against four microbe strains
The antimicrobial dynamic curves of TTO against the four tested strains are shown in Fig. 3. Under control conditions (no TTO), E. coli (Fig. 3a) and S. aureus (Fig. 3b) strains showed typical growth curves, including a 2-h lag phase, an exponential phase, a stationary phase after 10 h, and a decline phase. E. coli cultured with 0.27 mg/mL TTO showed a longer lag phase (4 h) and a delayed stationary phase (12 h). In the presence of 0.54 mg/mL TTO, a sharp decline in the quantity of live E. coli cells was observed after 2 h and the small number of tolerant E. coli cells gradually entered into lag phase and reached the stationary phase after 24 h. The E. coli cells cultured with 1.08 mg/mL TTO showed a sharper decline after 2 h, and the growth of the few remaining cells began to grow slowly after 48 h. No live E. coli cells were observed in the presence of 2.17 mg/mL TTO from 2 to 72 h. As shown in Fig. 3b, S. aureus showed a greater tolerance to TTO than did E. coli. A gradual time-dependent decline in the quantity of live S. aureus cells, without obvious growth, was observed in the presence of 1.08, 2.17, 4.34, or 8.67 mg/mL TTO. However, no living cells were observed after incubation of S. aureus with 4.34 or 8.67 mg/mL TTO for 12 or 24 h.
The effects of TTO on the growth of four microbial strains. a E. coli, the concentrations (mg/mL) of TTO were 0 (black sqaure), 0.27 (black circle), 0.54 (black triangle), 1.08 (white square), 2.17 (white circle); b S. aureus, the concentrations (mg/mL) of TTO were 0 (black square), 1.08 (black circle), 2.17 (black triangle), 4.34 (white square), 8.67 (white circle); c C. albicans, the concentrations (mg/mL) of TTO were 0 (black square), 0.54 (black circle), 1.08 (black triangle), 2.17 (white square), 4.34 (white circle); d A. niger conidia, the concentrations (mg/mL) of TTO were 0 (black square), 2.17 (black circle), 4.34 (black triangle), 8.67 (white square), 17.34 (white circle)
Figure 3c shows that under control condition, C. albicans showed a 3-h lag phase, followed by an exponential growth phase, and the stationary phase occurred after 12 h. In the presence of 0.54 mg/mL TTO, the number of surviving cells was reduced by three orders of magnitude after 3 h and reduced by two orders of magnitude after 6 h. No further decrease was observed at later time-points. The few remaining cells entered an exponential growth phase when incubated for 12 h. Cell survival declined sharply in the presence of 1.08, 2.17, or 4.34 mg/mL TTO and no surviving cells were observed after 6, 3, and 3 h, respectively. However, a small number of live cells were detected after 72-h incubation with 1.08 or 2.17 mg/mL TTO; none were identified after 72 h in the presence of 4.34 mg/mL TTO.
As shown in Fig. 3d, A. niger grown under control conditions showed no obvious decline in the number of conidia until 6 h. At this time-point, the conidia germinated and the mycelium grew after incubation for 9 h. The number of surviving conidia in the control group was not therefore determined after the 9-h time-point. Only minor decreases in the numbers of surviving conidia were observed after 72-h incubations with 2.17, 4.34, 8.67, or 17.34 mg/mL TTO. The survival conidia in 2.17 mg/mL TTO germinated and grew mycelia after incubation for 48 h. The number of surviving conidia in the 2.17 mg/mL TTO group was not therefore determined after the 48-h time-point. No germination mycelia were observed after the 72-h incubation with 4.34, 8.67, or 17.34 mg/mL TTO.
The effects of TTO against four microbe strains observed by TEM
Figure 4 shows TEM images of the internal morphology of TTO-treated microbe strains. E. coli cells cultured under control conditions (Fig. 4 (A—a, b)) showed a typical rod shape. The cell wall, membrane, and cytoplasm were all regular and visible. The cell wall and cytoplasm membrane were close together and the cells showed homogeneous electron density. In contrast, the internal morphology of E. coli cells cultured with 2.17 mg/mL TTO (Fig. 4 (A—c, d)) was altered. There was a large gap between the cytoplasm membrane and the cell wall. In addition, plasmolysis and a partial disappearance of the cytoplasmic membrane were observed. Moreover, some cells appeared to be at the final stage of cell disruption, with some cytoplasm spilling out of the cells.
The S. aureus cells grown in control medium (Fig. 4 (B—a, b)) showed the normal characteristics of coccal bacteria. Their cell walls and membranes were intact, with a normal peptidoglycan layer and cytoplasmic membrane. Furthermore, the cytoplasm showed homogeneous electron density. Significant morphological changes were observed in S. aureus cells treated with 2.17 mg/mL TTO (Fig. 4 (B—c, d)). The cytoplasm of these cells showed heterogeneous electron density and some of it appeared to have escaped from some cells. Localized separation of the cytoplasmic membrane from the cell wall was also observed.
The regular internal structure of C. albicans was observed in cells grown under control condition (Fig. 4 (C—a, b)). These cells showed homogeneous electron density in the cytoplasm. Their cell walls, membranes, and organelles were intact and visible. However, some irreversible alterations were observed in the internal morphology of C. albicans cells treated with 2.17 mg/mL TTO (Fig. 4 (C—c, d)). Some vacuoles and electron-dense areas were observed within the cells, although the cell wall and membrane appeared intact.
The hyphae of A. niger grown under control conditions showed a regular healthy structure (Fig. 4 (D—a, b)), with intact cell walls, homogeneous electron density in the cytoplasm, and organelles that were intact, clear, and showed a regular arrangement. After treatment with 2.17 mg/mL TTO, the internal structures of the A. niger hyphae were also altered (Fig. 4 (D—c, d)). Cytoplasmic loss appeared to have occurred, and the organelles were deformed, damaged, and arranged irregularly.
Discussion
The results of the present study indicated that TTO had antimicrobial activity against four microbe strains, with MIC values of 1.08 mg/mL against both E. coli and S. aureus bacteria and 2.17 mg/mL against both C. albicans and A. niger fungi. We previously reported that the MIC of citronella oil against A. niger was 1.08 mg/mL (Li et al. 2013), the MICs of garlic oil against P. funiculosum and C. albicans were 0.69 mg/mL (Li et al. 2014a) and 0.35 mg/mL (Li et al., 2016a, b), respectively, and the MIC of Litsea cubeba oil against E. coli was 1.08 mg/mL (Li et al. 2014b). Compared to these essential oil antimicrobial activities, TTO showed moderate antibacterial and antifungal activity. Furthermore, the bacterial strains employed in the present study were more sensitive to TTO than the fungal strains. Some essential oils such as citronella oil (Delespaul et al. 2000) and garlic oil (Avato et al. 2000) have strong antifungal activities but weak antibacterial activities. This reflects the varied chemical compositions of essential oils, which produce different antibacterial and antifungal activities. The present GC-MS analysis showed that the main components of TTO were alkenes and alcohols. It also identified terpinen-4-ol as the major individual component, which accounted for approximately 23 % of the TTO. Terpinen-4-ol has previously shown strong antimicrobial and anti-inflammatory properties (Kwieciński et al. 2009). Mondello et al. (Mondello et al. 2006) found that terpinen-4-ol was as effective as TTO for accelerating vaginal clearance of all the examined Candida strains. The present study also showed that terpinen-4-ol had MICs of 1.17, 1.17, 2.34, and 2.34 mg/mL against E. coli, S. aureus, C. albicans, and A. niger, respectively; these values were equivalent to those of TTO against these four strains. These data strengthened the conclusion that terpinen-4-ol was a major component and a contributor to the antimicrobial activity of TTO.
The dynamic curves showed that the minimum bactericidal concentrations of TTO against E. coli and S. aureus were 2.17 and 4.34 mg/mL, respectively, and the minimum fungicidal concentration against C. albicans was 4.34 mg/mL; however, the A. niger conidia were not completely eradicated by exposure to 17.34 mg/mL TTO for 3 days. E. coli was thus the most sensitive to TTO, while the A. niger conidia were the most resistant to TTO. The numbers of surviving A. niger conidia only have minor decreases after 72-h incubation with TTO. The survival conidia in 2.17 mg/mL TTO germinated and grew mycelia after incubation for 48 h, but the germination of conidia was inhibited after the 72-h incubation with 4.34 mg/mL and beyond TTO. Furthermore, a trend was observed that with increasing concentrations of TTO, the rate of cell killing and the duration of growth lag phase increased correspondingly. These data indicated that TTO had time- and concentration-dependent antibacterial and antifungal effects; this finding was consistent with previous studies of citronella oil (Li et al. 2013), garlic oil (Li et al. 2016a, b; Li et al. 2014a), and Litsea cubeba oil (Li et al. 2014b).
TEM analysis of E. coli, S. aureus, C. albicans, and A. niger showed irreversible TTO-induced changes, as compared with the control experimental groups. Gaps were observed between the bacterial cell wall and cytoplasmic membrane. Carson et al. (Carson et al. 2002) also found that TTO altered the cellular morphology of S. aureus and compromised its cytoplasmic membrane. In contrast, no significant changes were observed in the fungal cell wall. The cytoplasmic electron densities showed heterogeneity in all the TTO-treated bacterial and fungal strains, with some cytoplasmic loss from the cells. The organelles of fungal cells were damaged, deformed, and irregularly arranged. This was consistent with the reported antifungal effects of garlic oil (Li et al., 2016a, b) and citronella oil (Li et al. 2013), which destroyed some organelles such as mitochondria in C. albicans and A. niger hyphae. However, it differed from our previous report of the antibacterial effects of silver nanoparticles (Li et al. 2011), which cause DNA a tense state in S. aureus. The present results indicated that TTO shared some antimicrobial effects observed with other essential oils, but differed from other types of antimicrobial materials. Taken together, these studies suggest that TTO penetrates through the cell wall and cytoplasmic membrane of the tested bacterial and fungal strains, causing damage to these structures and the subsequent loss of cytoplasmic material. Essential oils can penetrate the cytoplasmic membrane due to their lipophilicity (Nogueira et al. 2010; Rassoli and Owlia 2005). In addition, TTO may penetrate fungal organelle membranes, and induce organelle damage. Finally, these irreversible TTO-mediated changes lead to cell death.
In summary, the main components of TTO were alkenes and alcohols, with the major individual component (terpinen-4-ol) accounting for approximately 23 % of the TTO as a whole. TTO showed moderate antibacterial and antifungal activities, with MICs of 1.08, 1.08, 2.17, and 2.17 mg/mL against E. coli, S. aureus, C. albicans, and A. niger, respectively. This activity was mainly attributed to the presence of terpinen-4-ol, which showed equivalent antimicrobial activity against these bacterial and fungal strains. Bacterial strains were more sensitive to TTO than fungal strains. TTO showed good antimicrobial dynamics, with a concentration- and time-dependent effect. The minimum bacteriacidal and fungicidal concentrations of TTO against E. coli, S. aureus, and C. albicans were 2.17, 4.34, and 4.34 mg/mL, respectively, while A. niger conidia were not completely eradicated by 17.34 mg/mL TTO. TTO may penetrate and damage the cell wall and cytoplasmic membrane of all four tested bacterial and fungal strains, leading to cytoplasmic loss. Additionally, TTO could penetrate fungal organelle membranes, inducing deformation and damage. Ultimately, these irreversible damages result in microbe cell death.
References
Arweiler NB, Donos N, Netuschil L, Reich E, Sculean A (2000) Clinical and antibacterial effect of tea tree oil—a pilot study. Clin Oral Investig 4:70–73
Avato P, Tursi F, Vitali C, Miccolis V, Candido V (2000) Allylsulfide constituents of garlic volatile oil as antimicrobial agents. Phytomedicine 7(3):239–223
Bishop CD (1995) Antiviral activity of the essential oil of Melaleuca alternifolia (maiden and Betche) Cheel (tea tree) against tobacco mosaic virus. J Essent Oil Res 7:641–644
Brophy JJ, Davies NW, Southwell IA, Stiff IA, Williams LR (1989) Gas chromatographic quality control for oil of Melaleuca terpinen-4-ol type (Australian tea tree). J Agric Food Chem 37:1330–1335
Campli ED, Bartolomeo SD, Pizzi PD, Giulio MD, Grande R, Nostro A, Cellini L (2012) Activity of tea tree oil and nerolidol alone or in combination against Pediculus capitis (head lice) and its eggs. Parasitol Res 111(5):1985–1992
Carson CF, Cookson BD, Farrelly HD, Riley TV (1995) Susceptibility of methicillin-resistant Staphylococcus aureus to the essential oil of Melaleuca alternifolia. J Antimicrob Chemother 35:421–424
Carson CF, Mee BJ, Riley TV (2002) Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob Agents Chemother 46(6):1914–1920
Carson CF, Riley TV (1993) Antimicrobial activity of the essential oil of Melaleuca alternifolia. Lett Appl Microbiol 16:49–55
Delespaul Q, Billerbeck VG, Roques CG, Michel G (2000) The antifungal activity of essential oils as determined by different screening methods. J Essent Oil Res 12:256–266
European Directorate for the Quality of Medicines (2006): the 5th Edition of European Pharmacopoeia and its subsequent supplements. 5.5 CD-Rom.
Evgeny VU, Oleg VP, Olga VU (2013) Antiviral activity of tea tree and eucalyptus oil aerosol and vapour. J Aerosol Sci 59:22–30
Garozzo A, Timpanoro R, Stivala A, Bisignano G, Castro A (2011) Activity of Melaleuca alternifolia (tea tree) oil on influenza virus a/PR/8: study on the mechanism of action. Antivir Res 89:83–88
Hammer KA, Carson CF, Riley TV (1996) Susceptibility of transient and commensal skin flora to the essential oil of Melaleuca alternifolia (tea tree oil). Am J Infect Control 24:186–189
Hammer KA, Carson CF, Riley TV (1997) In vitro susceptibility of Malassezia furfur to the essential oil of Melaleuca alternifolia. J Med Vet Mycol 35:375–377
Hammer KA, Carson CF, Riley TV (1998) In vitro activity of essential oils, in particular Melaleuca alternifolia (tea tree) oil and tea tree oil products, against Candida spp. J Antimicrob Chemother 42:591–595
Hammer KA, Carson CF, Riley TV (1999) In vitro susceptibilities of Lactobacilli and organisms associated with bacterial vaginosis to Melaleuca alternifolia (tea tree) oil. Antimicrob Agents Chemother 43(1):196–196
Hammer KA, Carson CF, Riley TV (2000) In vitro activities of ketoconazole, econazole, miconazole, and Melaleuca alternifolia (tea tree) oil against Malassezia species. Antimicrob Agents Chemother 44(2):467–469
Hammer KA, Carson CF, Riley TV (2012) Effects of Melaleuca alternifolia (tea tree) essential oil and the major monoterpene component terpinen-4-ol on the development of single- and multistep antibiotic resistance and antimicrobial susceptibility. Antimicrob Agents Chemother 56(2):909–915
Homer LE, Leach DN, Lea D, Lee LS, Henry RJ, Baverstock PR (2000) Natural variation in the essential oil content of Melaleuca alternifolia Cheel (Myrtaceae). Biochem Syst Ecol 28:367–382
International Organization for Standardization. ISO 4730 (1996) : Oil of Melaleuca, Terpinen-4-ol type (Tea Tree Oil) Geneva: International Organization for Standardization.
James PJ, Callander JT (2012) Dipping and jetting with tea tree (Melaleuca alternifolia) oil formulations control lice (Bovicola ovis) on sheep. Vet Parasitol 189:338–343
Kwieciński J, Eick S, Wójcik K (2009) Effects of tea tree (Melaleuca alternifolia) oil on Staphylococcus aureus in biofilms and stationary growth phase. Int J Antimicrob Agents 33:343–347
Li M, Zhu LF, Liu BM, Du LN, Jia XD, Han L, Jin YG (2016b) Tea tree oil nanoemulsions for inhalation therapies of bacterial and fungal pneumonia. Colloids Surf B 141:408–416
Li WR, Shi QS, Dai HQ, Liang Q, Xie XB, Huang XM, Zhao GZ, Zhang LX (2016a) Antifungal activity, dynamics and molecular mechanism of action of garlic oil against Candida albicans. Sci Rep 6:22805. doi:10.1038/srep22805
Li WR, Shi QS, Liang Q, Huang XM, Chen YB (2014a) Antifungal effect and mechanism of garlic oil on Penicillium funiculosum. Appl Microbiol Biotechnol 98:8337–8346
Li WR, Shi QS, Liang Q, Xie XB, Huang XM, Chen YB (2014b) Antibacterial activity and kinetics of Litsea cubeba oil on Escherichia coli. PLoS One 9(11):e110983. doi:10.1371/journal.pone.0110983
Li WR, Shi QS, Ouyang YS, Chen YB, Duan SS (2013) Antifungal effects of citronella oil against Aspergillus niger ATCC 16404. Appl Microbiol Biotechnol 97:7483–7492
Li WR, Xie XB, Shi QS, Duan SS, Ouyang YS, Chen YB (2011) Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Biometals 24(1):135–141
Li WR, Xie XB, Shi QS, Zeng HY, Ouyang YS, Chen YB (2010) Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl Microbiol Biotechnol 85(4):1115–1122
Lim EL, Hammer KA (2015) Adaptation to NaCl reduces the susceptibility of Enterococcus faecalis to Melaleuca alternifolia (tea tree) oil. Curr Microbiol 71(4):429–433
Mondello F, Bernardis FD, Girolamo A, Cassone A, Salvatore G (2006) In vivo activity of terpinen-4-ol, the main bioactive component of Melaleuca alternifolia Cheel (tea tree) oil against azole-susceptible and -resistant human pathogenic Candida species. BMC Infect Dis 6:158–166
Nardoni S, Bertoli A, Pinto L, Mancianti F, Pisseri F, Pistelli L (2010) In vitro effectiveness of tea tree oil against Trichophyton equinum. J Mycol Med 20:75–79
Natalie AT, Katherine AH, Thomas VR, Belkum AV, Carson CF (2013) Effect of habituation to tea tree (Melaleuca alternifolia) oil on the subsequent susceptibility of Staphylococcus spp. to antimicrobials, triclosan, tea tree oil, terpinen-4-ol and carvacrol. Int J Antimicrob Agents 41:343–351
Nelson RRS (1997) In-vitro activities of five plant essential oils against methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium. J Antimicrob Chemother 40:305–306
Nogueira JHC, Goncalez E, Galleti SR, Facanali R, Marques MOM, Felício JD (2010) Ageratum conyzoides Essential oil as aflatoxin suppressor of Aspergillus flavus. Int J Food Microbiol 137:55–60
Pazinato R, Klauck V, Volpato A, Tonin AA, Santos RC, Souza ME, Vaucher RA, Raffin R, Gomes P (2014) Influence of tea tree oil (Melaleuca alternifolia) on the cattle tick Rhipicephalus microplus. Exp Appl Acarol 63(1):77–83
Pisseri F, Bertoli A, Nardoni S, Pinto L, Pistelli L, Guidi G, Mancianti F (2009) Antifungal activity of tea tree oil from Melaleuca alternifolia against Trichophyton equinum: an in vivo assay. Phytomedicine 16:1056–1058
Rassoli I, Owlia P (2005) Chemoprevention by thyme oils of Aspergillus parasiticus growth and aflatoxin production. Phytochemistry 66:2851–2856
Shi C, Zhao XC, Yan HY, Meng RZ, Zhang Y, Li WL, Liu ZH, Guo N (2016) Effect of tea tree oil on Staphylococcus aureus growth and enterotoxin. Food Control 62:257–263
Souza ME, Lopes LQS, Vaucher RA, Mário DN, Alves SH, Agertt VA, Bianchini BV, Felicidade SI, Campus MMA, Boligon AA, Athayde ML, Santos CG, Raffin RP, Gomes P, Santos RCV (2014) Antimycobacterial and antifungal activities of Melaleuca alternifolia oil nanoparticles. J Drug Deliv Sci Technol 24(5):559–560
Usachev EV, Pyankov OV, Usacheva OV (2013) Antiviral activity of tea tree and eucalyptus oil aerosol and vapour. J Aerosol Sci 59:22–30
Acknowledgments
We thank all partners and laboratory members for their kind help. We are obliged to the anonymous reviewers of Applied Microbiology and Biotechnology for their constructive comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors gratefully acknowledge the support of the Nature Science of Foundation of China (No. 31500113), Guangdong Provincial Nature Science of Foundation (No. 2016A030313800), Guangdong Provincial Science and Technology Project (No. 2013B010102014), and Guangzhou Municipal Science and Technology Research Project (No. 201607020020).
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical statements
This paper is our original work. It has not been submitted elsewhere, and it is not under consideration in any other journal. This article does not contain any studies with human participants or animals performed by any of the authors. All the authors have seen the manuscript and approved its submission to applied microbiology and biotechnology.
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s00253-017-8119-6.
Electronic supplementary material
ESM 1
(PDF 699 kb)
Rights and permissions
About this article
Cite this article
Li, WR., Li, HL., Shi, QS. et al. The dynamics and mechanism of the antimicrobial activity of tea tree oil against bacteria and fungi. Appl Microbiol Biotechnol 100, 8865–8875 (2016). https://doi.org/10.1007/s00253-016-7692-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7692-4