Abstract
Lactic acid bacteria (LAB) can interfere with pathogens through different mechanisms; one is the production of biosurfactants, a group of surface-active molecules, which inhibit the growth of potential pathogens. In the present study, biosurfactants produced by Lactobacillus reuteri DSM 17938, Lactobacillus acidophilus DDS-1, Lactobacillus rhamnosus ATCC 53103, and Lactobacillus paracasei B21060 were dialyzed (1 and 6 kDa) and characterized in term of reduction of surface tension and emulsifying activity. Then, aliquots of the different dialyzed biosurfactants were added to Streptococcus mutans ATCC 25175 and Streptococcus oralis ATCC 9811 in the culture medium during the formation of biofilm on titanium surface and the efficacy was determined by agar plate count, biomass analyses, and flow cytometry. Dialyzed biosurfactants showed abilities to reduce surface tension and to emulsifying paraffin oil. Moreover, they significantly inhibited the adhesion and biofilm formation on titanium surface of S. mutans and S. oralis in a dose-dependent way, as demonstrated by the remarkable decrease of cfu/ml values and biomass production. The antimicrobial properties observed for dialyzed biosurfactants produced by the tested lactobacilli opens future prospects for their use against microorganisms responsible of oral diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biosurfactants (BSFs) are surface-active compounds synthesized by diverse groups of microorganisms, which exist in a wide variety of chemical compounds, such as fatty acids, neutral lipids, phospholipids, glycolipids, and lipopeptides. Biosurfactants have attracted much interest as hydrocarbon dissolution agents in late 1960s (Francy et al. 1991), but over the past five decades, they have greatly extended their applications in food, pharmaceutical, and oil industries, thanks to their peculiar properties. For instance, they have been proposed as an improved alternative to synthetic surfactants, such as carboxylates, sulphonates, and sulfate acid esters.
Some advantages of biosurfactants are represented by their specific action, lower toxicity, higher biodegradability, and effectiveness at a wide range of temperatures, pH, and salinity (Rodrigues et al. 2006a; Saharan et al. 2012).
The most commonly isolated biosurfactants are represented by glycolipids and lipopeptides, but rhamnolipids produced by Pseudomonas aeruginosa (Nitschke et al. 2005), sophorolipids released from Candida species (Daverey and Pakshirajan 2009), and surfactin and iturin from Bacillus subtilis (Ahimou et al. 2000) are also included. The biosurfactants production yields by these microorganisms are relatively high (2–10 g/l), and their ability to reduce the water surface tension to values below 30 mN/m was reported (Ahimou et al. 2000; Daverey and Pakshirajan 2009; Nitschke et al. 2005).
On the other hand, biosurfactants were found to be also released by different species of lactobacilli, also called lactic acid bacteria (LAB). They are classified as “Generally Recognized As Safe” (GRAS) bacteria and are commensal microorganism involved in several homeostatic processes in different body districts. Lactobacilli produced biosurfactants in lower amounts (20–100 mg/l) (Rodrigues et al. 2006b) and are generally less effective to lower the air-water surface tension (around 36–40 mN/m). Such biosurfactants have been characterized as complex mixtures, whose composition has been only partially identified. However, several authors reported the interesting properties of biosurfactants produced by lactobacilli to inhibit the adhesion of pathogens to biomaterial and/or cell surfaces, highlighting their key role in the biofilm formation process (Saravanakumari and Mani 2010; Gudiña et al. 2010; Sharma et al. 2014; Sharma et al. 2015).
A biofilm is a thin layer of microorganisms adhering to the surface of an organic or inorganic structure, together with their secreted “extracellular polymeric substances” (EPS). Biofilms represent the predominant phenotype of nearly all bacteria in their natural habitat, whether pathogenic or environmental. For instance, biofilm formation by oral pathogens, such as Streptococci spp., on the solid surface of the enamel or the root of the teeth is considered to play a major role in the pathogenesis of dental caries (Socransky and AD 2002). Moreover, the presence of bacterial biofilm on the dental implants can cause peri-implantitis inflammatory disease and/or implant loss (Fürst et al. 2007; Elter et al. 2008). The application of antimicrobial agents seems to be a useful tool for controlling the formation and growth of oral biofilms (Ntrouka et al. 2011). However, bacteria self-organized in a cooperating biofilm can be up to 1000 times more resistant to the common antibiotics and antimicrobials than the same bacteria circulating in a planktonic state (Reffuveille et al. 2013). For this reason, in the last decade, the attention was paid to new class of antimicrobial agents, which have demonstrated antimicrobial activity against cariogenic bacteria (Jeon et al. 2011), or new strategies to control biofilm formation. Among these, the study of the effects of probiotics in the pathogenesis of oral diseases is a relatively new research area in oral health (Haukioja et al. 2008; Söderling et al. 2011). Particularly, in the recent years, the attention of researchers has turned up to the ability of lactobacilli to interfere with the adhesion of oral pathogens to abiotic surfaces (Teanpaisan et al. 2011; Söderling et al. 2011; Marttinen et al. 2013). The inhibition of pathogen adhesion and, consequently, of biofilm formation is generally attributed to the activity of biosurfactants produced by different species of bacteria such as lactobacilli (Gudiña et al. 2010; Tahmourespour et al. 2011).
Data in literature are mostly referred to the so called “cell-bound biosurfactants,” which are commonly extracted at the end of the fermentation by washing the centrifuged bacteria cells with phosphate buffer under gentle stirring at room temperature (Rodrigues et al. 2006b; Gudiña et al. 2010; Tahmourespour et al. 2011). However, few data are available for the so called “excreted biosurfactants,” which are released in the culture media during the fermentation process (Sharma and Saharan 2014). For this reason, the aim of the present study was to characterize in terms of surface tension reduction and emulsifying activity, the “excreted biosurfactants,” produced by selected Lactobacillus spp., purified through dialysis using membranes at two different molecular weight cut-off (1 and 6 kDa). Moreover, the anti-biofilm effect of each dialyzed biosurfactant against Streptococcus mutans ATCC 25175 and Streptococcus oralis ATCC 9811 was investigated by plate count agar, biomass analyses, and flow cytometry (FCM).
Materials and methods
Bacteria and culture conditions
Seven Lactobacillus spp. were used in this study: Lactobacillus reuteri DSM 17938 (Reuflor, Italchimici, Italy), Lactobacillus acidophilus DDS-1 (Nutratec, Urbino, Italy), Lactobacillus paracasei B21060 (Floratec, Bracco, Italy), Lactobacillus rhamnosus ATCC 53103, Lactobacillus rhamnosus ATCC 7469, Lactobacillus casei ATCC 15008, and Lactobacillus salivarius ATCC 11741. All these strains were grown in Man Rogosa and Shape agar (MRS) (Oxoid, UK) at 37 °C for 24–48 h under microaerophilic conditions (5 % O2; 10 % CO2; 85 % N2).
In addition, S. mutans ATCC 25175 and S. oralis ATCC 9811, two reference human oral pathogens, were used as pathogen bacteria model. These strains were routinely grown on Sheep blood agar base (Oxoid, UK) with 5 % of Sheep blood (Oxoid, UK) at 37 °C for 24 h.
Stock cultures of each strain were keep at −80 °C in Nutrient broth (Oxoid, UK) with 15 % of glycerol.
Biosurfactants preparation and assessment of the antimicrobial activity by time-killing studies
LAB strains overnight cultures (15 ml) were inoculated to 600 ml of MRS broth (Oxoid, UK) and incubated at 37 °C for 48 h under microaerophilic conditions. For the recovery of the crude excreted biosurfactants (BSF), bacterial cultures were centrifuged at 17,000 rpm for 15 min at 4 °C, and the supernatants were filtered through a 0.22-μm pore size filter (Millipore).
To test the antimicrobial effect of the crude excreted biosurfactants, S. mutans ATCC 25175 and S. oralis ATCC 9811 were grown in brain-heart infusion broth (BHI) (Oxoid, UK) supplemented with 1 % yeast extract at 37 °C for 18 h. Then, 500 μl of each bacterial culture (about 108 cfu/ml) and 500 μl of each biosurfactant were combined in 24-well-polystyrene plates and incubated at 37 °C. At the baseline time (0 h) and after 3, 6, and 24 h, different aliquots were aseptically removed, serially diluted in physiological saline solution, and plated on Sheep agar base with 5 % sheep blood at 37 °C for 24–48 h. At the end of incubation, plates were observed to calculate the colony-forming unit (cfu/ml). Each experiment was performed in duplicate.
Dialysis of biosurfactants
Four Lactobacillus spp. (L. reuteri DSM 17938, L. acidophilus DDS-1, L. rhamnosus ATCC 53103, and L. paracasei B21060) were selected on the basis of the antimicrobial activity determined by time-killing studies, and their excreted biosurfactants were purified through dialysis. The dialyses were performed against demineralized water at room temperature using Spectra/Por® membranes at two different molecular weight cut-offs, 1 and 6 kDa (Spectrum® Laboratories, Inc.). The solutions containing the biosurfactants were finally freeze-dried.
Characterization of dialyzed biosurfactant surface properties
The following experiments were conducted to determine biosurfactant activities of dialyzed/freeze-dried biosurfactants in terms of reduction in air-water surface tension and oil-water emulsion ability.
The reduction in air-water surface tension (ST) of biosurfactants was determined by the Ring method (Kim et al. 2000) using a tensiometer (DCA-100 Contact Angle Tensiometer—First Ten Angstroms, Inc., USA) equipped with a 1.9-cm De Noüy platinum ring at room temperature. About 15 ml of each sample was withdrawn, and surface tension was measured at 0.5, 1, 2.5, 5, and 10 mg/ml. MRS broth was also analyzed at the same concentrations for comparison.
The emulsification activity of biosurfactants is expressed as emulsification index (E24) (Kuiper et al. 2004). Equal volumes of the aqueous phase containing the dialyzed biosurfactant (1 and 10 mg/ml) and paraffin oil were vigorously mixed with a vortex for 2 min, and allowed 24 h to settle. The emulsification index was calculated by the following equation:
Each test was performed in duplicate.
Biofilm formation
Biofilm formation on titanium surface of S. mutans ATCC 25175 and S. oralis ATCC 9811 was obtained as described by Ciandrini et al. (Ciandrini et al. 2014) with some modification. First, sterilized titanium disks were placed on 24-well-polystyrene cell culture plates (Cellstar®, Greiner Bio-One, Germany) and incubated for 4 h by gentle shaking at room temperature with human saliva, collected from four healthy volunteers (with their informed consent). Saliva was previously clarified by centrifugation (15,000×g for 15 min at 4 °C) and sterilized by 0.22-μm filtration. The disks were finally washed with 10 mM PBS at pH 7. At this point, titanium disks were covered with 1.6 ml of BHI and infected with 200 μl (106 cell/ml) of each bacterial suspension after incubation in BHI broth for S. mutans ATCC 25175 and S. oralis ATCC 9811. Plates were, then, incubated at 37 °C for 24 h. The bacteria adherent to titanium disks were harvested by vigorous vortexing for 2 min in physiological saline solution, then serially diluted and plated on Sheep agar base with 5 % sheep blood (Oxoid, UK). The plates were incubated at 37 °C under the adequate conditions for 24–48 h, and the colony-forming units per milliliter (cfu/ml) were counted.
To assess biofilm formation, biomass analysis was carried out using Crystal Violet (CV) staining. Briefly, at each time point, titanium disks were removed, washed with PBS, and immersed in a new well containing 0.1 % (v/v) of CV (Sigma, Italy) for 15 min. Then, disks were washed again with PBS, air-dried, and the remaining CV was dissolved in 85 % ethanol for 15 min at room temperature. Finally, 200 μl from each well was transferred into a 96-well plate to be spectrophotometrically analyzed at 570 nm, using a Multiscan Ex Microplate Reader (Thermo Scientific). Data point was averaged from at least eight replicate per well, and each experiment was performed three times using independent cultures.
Anti-biofilm effect of dialyzed biosurfactants
To evaluate the anti-biofilm activity of excreted biosurfactants of L. reuteri DSM 17938, L. acidophilus DDS-1, L. rhamnosus ATCC 53103, and L. paracasei B21060 strains, each dialyzed biosurfactant (1 and 6 kDa) was tested at two concentrations (1 and 10 mg/ml) against S. mutans ATCC 25175 and S. oralis ATCC 9811 during biofilm formation on titanium surface.
For this purpose, saliva-conditioned titanium disks, once positioned into a 24-wells polystyrene cell culture plate, were covered with 200 μl (106 cell/ml) of S. mutans ATCC 25175 and S. oralis ATCC 9811 cultures and 1 ml of each dialyzed biosurfactant, at the mentioned concentrations. For each microorganism, wells, inoculated with 200 μl (106 cell/ml) of S. mutans ATCC 25175 and S. oralis ATCC 9811 in BHI broth, were also included as controls. Plates were incubated at 37 °C for 24 h to allow biofilm formation and, at the end of the incubation, adherent bacteria were harvested as described above for plate count enumerations.
Two additional series of 24-wells polystyrene cell culture plates were prepared using the same described procedure, to perform biomass analysis and flow cytometry (FCM), respectively. For biomass analysis, after S. mutans ATCC 25175 and S. oralis ATCC 9811 biofilm formation, wells were washed twice in PBS and stained by CV 0.1 % (v/v) (Sigma, Milan, Italy) for 15 min. Disks were PBS washed and air-dried; at this point, the remaining CV was dissolved in 85 % ethanol for 15 min at room temperature and 200 μl from each well was transferred into a 96-well plate and analyzed by a Multiscan Ex Microplate Reader spectrophotometrically at 570 nm.
For FCM analysis, the titanium adherent bacteria were harvested by vigorous vortexing for 2 min in physiological saline solution. Each sample, diluted in the same buffer, was labeled with SYBR Green I (1/10,000 v/v) and Propidium Iodide (PI) (10 μg/ml) (Barbesti et al. 2000), incubated in the dark for 15 min at room temperature and immediately processed by FACSCalibur flow cytometer (BD Biosciences, USA) equipped with a 488 nm laser. All experiments were performed in triplicates. Multi-parametric analyses were performed on both scattering signals as forward-scattered light (FSC) and side-scattered light (SSC) and fluorescence emission in FL1/FL3 channels. In particular, the green fluorescence of SYBR Green I was detected in the FL1 (530/30) channel while PI red fluorescence in the FL3 (>670) channel. Threshold levels were set on FSC in order to eliminate noise, due to the presence of cellular debris which contribute much smaller than intact cells to the overall signal. Bacterial cells were gated according to FSC/SSC parameters. The data were analyzed using CellQuest™ Pro software (BD Biosciences, USA).
Statistical analysis
Statistical analysis was performed using Prism version 5.0 (GraphPad Inc., USA). All experiments were performed in duplicate, and the standard error of the mean was calculated from the combined measurements. Data points were analyzed through one-way analysis of variance (ANOVA) with Bonferroni post hoc test unless the assumptions for the parametric test were not respected. In this case, Kruskal-Wallis non-parametric test with Dunnett’s multiple comparison test was applied. P values <0.05 were considered to be statistically significant.
Results
Antimicrobial activity of crude excreted biosurfactants in killing studies
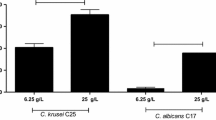
The antimicrobial activities of all Lactobacillus spp. crude excreted biosurfactants against oral streptococci were determined by time-kill assays (Fig. 1). In general, the results show that BSFs were able to reduce the growth of S. mutans ATCC 25175 and S. oralis ATCC 9811 up to 24 h with variable ability depending on the producing LAB strain. In detail, the biosurfactants from L. acidophilus DDS-1, L. paracasei B21060, and L. rhamnosus ATCC 53103 induced 1-log reduction in cfu/ml values after 3 h of incubation for S. mutans ATCC 25175, and these values remained essentially unchanged after 6 h reaching 2-log reduction after 24 h of incubation. Biosurfactant from L. reuteri DSM 17938 showed the greatest antimicrobial activity, with a cfu/ml reduction of 2-log after 3 h, 3-log after 6 h, and even 8-log after 24 h (Fig. 1a).
Reduction of cfu/ml values also occurred toward S. oralis ATCC 9811, but, in this case, biosurfactants of L. acidophilus DDS-1, L. paracasei B21060, and L. rhamnosus ATCC 53103 induced a reduction of 2-log after 3 and 6 h of incubation, reaching 5.4-log after 24 h. The biosurfactant of L. reuteri DSM 17938 showed the greatest antimicrobial activity, with a decrease of 3-, 4-, and 8-log after 3, 6, and 24 h, respectively (Fig. 1b). Biosurfactants excreted by L. rhamnosus ATCC 7469, L. casei ATCC 15008, and L. salivarius ATCC 11741 showed to be less active in reducing the growth of S. mutans ATCC 25175 and S. oralis ATCC 9811.
For this reason, only the BSFs of L. reuteri DSM 17938, L. acidophilus DDS-1, L. paracasei B21060, and L. rhamnosus ATCC 53103 were chosen for subsequent dialysis procedure.
Screening assays for dialyzed biosurfactants production
Dialyzed (1a and 6 kDa) biosurfactants excreted by L. reuteri DSM 17938, L. acidophilus DDS-1, L. rhamnosus ATCC 53103, and L. paracasei B21060 strains were tested using qualitative and quantitative methods (Table 1).
All dialyzed biosurfactants were able to decrease air-water surface tension (Fig. 2). The reduction in surface tension was quite linear in the range of the tested concentrations (1–10 mg/ml) and clearly more pronounced for the biosurfactants with respect to the growth medium (MRS broth), analyzed for comparison. In fact, the measured surface tension for MRS broth was 48 mN/m at 10 mg/ml, different from the biosurfactants, which showed lower values (35–40 mN/m) (Table 1). No marked differences among biosurfactants can be observed despite, in general, a slightly higher surface activity has been shown by the 1 kDa dialyzed compounds.
Effect of dialyzed (1 and 6 kDa) biosurfactants on the air/water surface tension (mN/m) at different concentrations, obtained from L. reuteri DSM 17938, L. acidophilus DDS-1, L. rhamnosus ATCC 53103, L. paracasei B21060, and the starting MRS broth used. The reference surface tension value was 72.2 mN/m. Results represent the average of three independent measurements, and error bars represent standard deviations of the mean values
The estimation of emulsification activity (E24) against paraffin oil was used, together with the reduction of air-water surface tension to assess the biosurfactant activity. Data obtained from the differently dialyzed biosurfactants (Table 1 ) were compared with those of distilled water, as negative control, and 1 % SDS, a common chemical surfactant, as positive control. All the dialyzed biosurfactants showed dose-dependent emulsifying activities, with the highest E24 index of 50 and 61.11 % for the 1 and 6 kDa dialyzed biosurfactants from L. paracasei B21060, respectively. However, all the 6 kDa dialyzed biosurfactants, with the exception of that from L. paracasei B21060, showed E24 values higher than those of the correspondent 1 kDa dialyzed biosurfactants.
Effect of dialyzed biosurfactants on streptococci biofilm formation
The anti-biofilm activity of dialyzed biosurfactants (1 and 6 kDa) against streptococci was assessed by the plate counts agar and biomass analysis. Data are presented in Fig. 3. As shown, all 1 and 6 kDa dialyzed BSFs were effective to inhibit the biofilm growth of S. mutants ATCC 25175 and S. oralis ATCC 9811. In particular, BSFs from L. acidophilus DDS-1, L. paracasei B21060, and L. rhamnosus ATCC 53103, tested at 10 mg/ml, induced a 3-log reduction in S. mutans ATCC 25175 cfu/ml values, while that from L. reuteri DSM 17938 determined a reduction less than 2-log. Moreover, the lower logarithmic decrease of the cfu/ml values of S. mutans ATCC 25175 treated with 1 kDa dialyzed BSFs at the concentration 1 mg/ml highlighted their dose-dependent effect (Fig. 3a). In the case of S. oralis ATCC 9811 biofilm, the 1 kDa dialyzed BSF from L. acidophilus DDS-1 (10 mg /ml) was the most effective, showing a cfu/ml reduction of 3-log, while those of L. reuteri DSM 17938, L. rhamnosus ATCC 53103, and L. paracasei B21060 caused a modest decrease of cfu/ml values in S. oralis ATCC 9811 (Fig. 3a). Regarding anti-biofilm activity of 6 kDa dialyzed biosurfactants, BSFs from L. reuteri DSM 17938, L. acidophilus DDS-1, and L. paracasei B21060, tested at 10 mg/ml, induced 1-log reduction in cfu/ml values of S. mutans ATCC 25175, while that of L. rhamnosus ATCC 53103 determined 3-log reduction (Fig. 3b). Against S. oralis ATCC 9811, the BSFs 6 kDa of L. acidophilus DDS-1 and L. rhamnosus ATCC 53103 (10 mg/ml) exhibited good antimicrobial activity with a 3-log reduction, while those of L. reuteri DSM 17938 and L. paracasei B21060, at the same concentration, induced only 1-log reduction in cfu/ml values (Fig. 3b). A dose-dependent effect was also confirmed for the 6 kDa BSFs against S. mutans ATCC 25175 and S. oralis ATCC 9811.
Activity of dialyzed biosurfactants (1 and 6 kDa) produced by L. reuteri DSM 17938, L. acidophilus DDS-1, L. rhamnosus ATCC 53103, and L. paracasei B21060 at two concentrations (10 and 1 mg/ml) against S. mutans ATCC 25175 and S. oralis ATCC 9811 biofilm formation assessed by plate counts agar (a, b) and CV staining at 570 nm (c, d)
In general, biomass analysis on biofilms treated with 1 and 6 kDa dialyzed BSFs confirmed the data previously obtained by plate count agar. In particular, the most effective dialyzed 1 kDa BSFs against S. mutans ATCC 25175 were those from L. acidophilus DDS-1 and L. rhamnosus ATCC 53103 (10 mg/ml) with optical density of 0.423 (±0.022) and 0.514 (±0.022), respectively, in comparison to 1.024 (±0.034) of the control (Fig. 3c). Dialyzed 1 kDa biosurfactants (10 mg/ml) were also active toward S. oralis ATCC 9811 biofilm, with optical density of 0.259 (±0.012) for BSF of L. acidophilus DDS-1, 0.243 (±0.011) for L. rhamnosus ATCC 53103, and 0.254 (±0.011) for L. paracasei B21060; lower activity was observed for L. reuteri DSM 17938 BSF (0.347 ± 0.023) (Fig. 3d). The 6 kDa dialyzed BSFs also possess anti-biofilm activity against S. mutans ATCC 25175 and S. oralis ATCC 9811, although more noticeable in the case of S. oralis ATCC 9811 with optical density of 0.329 (±0.026) for BSF of L. reuteri DSM 17938, 0.280 (±0.009) for L. acidophilus DDS-1, 0.356 (±0.025) for L. rhamnosus ATCC 53103, and 0.261 (±0.012) for L. paracasei B21060. The biosurfactant showing the most relevant decrease in optical density (0.312 ± 0.008) against S. oralis ATCC 9811 at low concentration (1 mg/ml), in comparison to the control (0.603 ± 0.060), was the BSF from L. acidophilus DDS-1 (Fig. 3d). Biomass analysis highlighted the dose-dependent effect of all the dialyzed biosurfactants (1 and 6 kDa), particularly remarkable in the case of biofilm formation inhibition of S. oralis ATCC 9811.
FCM analysis, assessed by double staining with SYBR Green I and PI, showed a remarkable biofilm inhibition percentages in term of total viable cells in each treated sample (Table 2). In particular, all the 1 kDa dialyzed biosurfactants 10 mg/ml showed the higher inhibition percentages of biofilm formation against S. mutans ATCC 25175, with values ranging from 98.25 % with BSF from L. acidophilus DDS-1 to 99.18 % with that from L. reuteri DSM 17938. In the case of S. oralis ATCC 9811, the percentages of biofilm formation inhibition ranged from 92.32 % with BSF from L. paracasei B21060 to 98.19 % with BSF from L. reuteri DSM 17938. As regards 6 kDa biosurfactants, biofilm inhibition percentages against S. mutans ATCC 25175 ranged from 92.52 % with BSF from L. rhamnosus ATCC 53103 to 96.43 % with BSF from L. acidophilus DDS-1. Similarly, biofilm inhibition percentages toward S. oralis ATCC 9811 varied from 94.79 % with BSF from L. reuteri DSM 17938 to 95.52 % with that from L. acidophilus DDS-1. FCM analysis also registered the dose-dependent effect of 1 and 6 kDa dialyzed biosurfactants with less activity at 1 mg/ml concentration against S. mutans ATCC 25175 and S. oralis ATCC 9811.
Discussion
Several studies have shown the beneficial properties of lactobacilli for humans in various anatomic districts, including the gastrointestinal and the genito-urinary tracts (Gomaa 2013). In addition, these bacteria have been recognized as fundamental in the maintenance of homeostasis in the oral cavity (Meurman and Stamatova 2011). Oral diseases, in fact, represent important human infections and are widespread in all age groups with the least success to control the actual infection. The ability, showed by streptococci, to steadily adhere to the surfaces of salivary protein-coated teeth and to dental implants, and to create a biofilm is fundamental for the promotion of the oral lesion development leading, at least, to dental or implant loss.
An important protective role was demonstrated for lactobacilli in the biofilm process (Meurman and Stamatova 2011; Teanpaisan et al. 2011). Lactobacilli were demonstrated to reduce the growth in the oral cavity of pathogens, such as streptococci (Çaglar et al. 2006; Çaglar et al. 2008), and to inhibit the biofilm formation (Jalasvuori et al. 2012). Lactobacilli are also involved in the process of biofilm formation on oral surfaces, by exerting an antimicrobial and anti-adhesive effect against pathogen bacteria through the production of specific substances, such as bacteriocins and biosurfactants and, at the same time, by competing with them for the colonization (Söderling et al. 2011).
In this work, the antimicrobial activity of the crude excreted biosurfactants from different Lactobacillus spp. toward S. mutans ATCC 25175 and S. oralis ATCC 9811 was initially investigated in time-killing studies. The obtained data showed a time-dependent decrease of S. mutans ATCC 25175 and S. oralis ATCC 9811 growth, particularly remarkable in the presence of the supernatant from L. reuteri DSM 17938 that is able to completely inhibit the growth of both streptococci up to 24 h. Conversely, Söderling et al. (2011) found that the antimicrobial effect of two L. reuteri strains was weaker and less efficient in reducing the viability (6.25 log cfu/ml) of tested S. mutans strains after 60 min of exposure. Our results suggest that L. reuteri, reuterin-producer in optimal conditions, showed antimicrobial activity also against oral streptococci other than against gut microorganisms (Spinler et al. 2008).
Since biosurfactants produced by different lactobacilli are complex biological mixtures, in this study, crude excreted biosurfactants, selected on the basis of their antimicrobial activity in killing studies, were dialyzed. The two obtained fractions (1 and 6 kDa cut-off), after freeze-drying, were characterized in terms of surface tension reduction and emulsifying ability and demonstrated to possess surface-active characteristics, such as the ability to bring down the air-water surface tension or to emulsify a water/oil mixture. Data published in the literature reported that bacteria with high surface activity and emulsifying properties represent promising microbial candidates for biosurfactant production (Banat et al. 2000).
Regarding the surface tension observed for all 1 and 6 kDa dialyzed, obtained after 48 h of incubation, a reduction of the interfacial tension from 47.92 to 34.81 mN/m was observed when compared to the surface tension of MRS broth (53.0 mN/m), with a slight higher surface activity in the 1 kDa fractions. This decrease of surface tension confirmed the production of biosurfactants by the isolates and accumulation within the media. These data are in agreement with those reported by Sharma and Saharan (Sharma and Saharan 2014), showing similar values of ST for excreted biosurfactants produced by a different Lactobacillus strains, with a surface tension reduction of the production media to 40.8 mN/m from the initial value of 53 mN/m. Moreover, in this study, another approach for screening potential biosurfactant-producing lactobacilli was the determination of the emulsification activity (% E24). The highest emulsifying activity was obtained from the dialyzed 6 kDa biosurfactant of L. paracasei B21060 (61.11 %), comparable with 58 and 57 % referred by Sharma and Saharan.
Another characteristic of a biosurfactant is the ability to reduce pathogen adhesion and subsequent biofilm formation on different surfaces, such as plastic materials or glass (Rodrigues et al. 2006a; Gudiña et al. 2010; Tahmourespour et al. 2011; Gomaa 2013). For this, in the present study, the antimicrobial activity of dialyzed biosurfactants produced by selected Lactobacillus spp. was evaluated during biofilm formation of S. mutans ATCC 25175 and S. oralis ATCC 9811 on titanium surface through three different techniques as agar plate count, biomass analysis, and flow cytometry. Our results indicate an inhibition of the biofilm produced by both the examined oral pathogens when the dialyzed BSFs were added in the culture medium, as demonstrated by decrease of cfu/ml, biomass production, as well as FCM values. The inhibitory effect showed a dose-dependence for both the 1 and 6 kDa BSFs against S. mutans ATCC 25175 and S. oralis ATCC 9811. These findings are in agreement with data reported by Tahmourespour et al. (Tahmourespour et al. 2011), which referred that biosurfactant of L. acidophilus interfered in the adhesion and biofilm formation of S. mutans to glass slide. Moreover, anti-adhesive effect depending on L. paracasei biosurfactant concentration toward several bacteria, including S. mutans and S. oralis, on plastic tissue culture plate was reported by Gudiña et al. (Gudiña et al. 2010).
The precise mechanisms of biosurfactant antimicrobial effect have not yet been explained, even if, as observed in our study, it seems to be highly dependent on biosurfactant type and the target bacteria. In fact, the antimicrobial activity of biosurfactants has not been observed in all cases (Rodrigues et al. 2006b; Walencka et al. 2008). Some authors have observed anti-adhesive activity with biosurfactants against several pathogenic microorganisms such as Staphylococcus aureus, Staphylococcus epidermidis, and Streptococcus agalactiae, but not against Escherichia coli, Candida albicans, and P. aeruginosa (Gudiña et al. 2010).
In conclusion, surface interactions are mediated by the amphiphilic nature of molecules with hydrophilic (acid, peptide cations, anions, or polysaccharides) and hydrophobic regions (unsaturated or saturated hydrocarbon chains or fatty acids), allowing them to act as surfactants at the interfaces (Myers 2005; Banat et al. 2010). Biosurfactants can be excreted in the culture broth or remain attached to the cell wall of bacteria, but most data in literature are referred to cell-bound biosurfactants (Sharma et al. 2014; Sharma et al. 2015), and few reports are available on biosurfactants contained in culture media (Saravanakumari and Mani 2010; Sharma and Saharan 2014). This research represents the first work in which the excreted biosurfactants of different LAB strains were dialyzed and characterized for their surface ability prior to be tested for their antimicrobial activity. In addition, we have demonstrated that active biosurfactant molecules are released by LAB in the culture media, from which can be separated by a simple dialysis method, and the obtained dialyzed fractions, in particular those with 6 kDa molecular weight, possess antimicrobial and anti-biofilm activities against oral streptococci.
Our results confirm that LAB strains are biosurfactant producers and, since these microorganisms are considered as GRAS, their biosurfactants are safe for human consumption and biomedical applications. In particular, biosurfactants of LAB origin, reducing the ability of streptococci to adhere and develop biofilm on oral surfaces, may contribute to prevent oral diseases. These findings are encouraging and could suggest the application of lactobacilli excreted biosurfactants as surface active agents in oral hygiene formulations, or as suitable alternative to conventional antimicrobials. Further studies are undergoing to better understand the chemical structural characterization of these excreted biosurfactants.
References
Ahimou F, Jacques P, Deleu M (2000) Surfactin and iturin a effects on Bacillus subtilis surface hydrophobicity. Enzym Microb Technol 27:749–754. doi:10.1016/S0141-0229(00)00295-7
Banat I, Franzetti A, Gandolfi I, Bestetti G, Martinotti M, Fracchia L, Smyth T, Marchant R (2010) Microbial biosurfactants production, applications and future potential. Appl Microbiol Biotechnol 87:427–444. doi:10.1007/s00253-010-2589-0
Banat IM, Makkar RS, Cameotra SS (2000) Potential commercial applications of microbial surfactants. Appl Microbiol Biotechnol 53:495–508
Barbesti S, Citterio S, Labra M, Baroni MD, Neri MG, Sgorbati S (2000) Two and three-color fluorescence flow cytometric analysis of immunoidentified viable bacteria. Cytometry 40:214–218. doi:10.1002/1097-0320(20000701)40:3<214::AID-CYTO6>3.0.CO;2-M
Çaglar E, Cildir SK, Ergeneli S, Sandalli N, Twetman S (2006) Salivary mutans streptococci and lactobacilli levels after ingestion of the probiotic bacterium Lactobacillus reuteri ATCC 55730 by straws or tablets. Acta Odontol Scand 64:314–318. doi:10.1080/00016350600801709
Çaglar E, Kuscu OO, Cildir SK, Kuvvetli SS, Sandalli N (2008) A probiotic lozenge administered medical device and its effect on salivary mutans streptococci and lactobacilli. Int J Paediatr Dent 18:35–39. doi:10.1111/j.1365-263X.2007.00866.x
Ciandrini E, Campana R, Federici S, Manti A, Battistelli M, Falcieri E, Papa S, Baffone W (2014) In vitro activity of Carvacrol against titanium-adherent oral biofilms and planktonic cultures. Clin Oral Investig 18:2001–2013. doi:10.1007/s00784-013-1179-9
Daverey A, Pakshirajan K (2009) Production, characterization, and properties of sophorolipids from the yeast Candida bombicola using a low-cost fermentative medium. Appl Biochem Biotechnol 158:663–674. doi:10.1007/s12010-008-8449-z
Elter C, Heuer W, Demling A, Hannig M, Heidenblut T, Bach F-W, Stiesch-Scholz M (2008) Supra- and subgingival biofilm formation on implant abutments with different surface characteristics. Int J Oral Maxillofac Implants 23:327–334
Francy DS, Thomas JM, Raymond RL, Ward CH (1991) Emulsification of hydrocarbons by subsurface bacteria. J Ind Microbiol 8:237–245. doi:10.1007/BF01576061
Fürst MM, Salvi GE, Lang NP, Persson GR (2007) Bacterial colonization immediately after installation on oral titanium implants. Clin Oral Implants Res 18:501–508. doi:10.1111/j.1600-0501.2007.01381.x
Gomaa EZ (2013) Antimicrobial and anti-adhesive properties of biosurfactant produced by lactobacilli isolates, biofilm formation and aggregation ability. J Gen Appl Microbiol 59:425–436. doi:10.2323/jgam.59.425
Gudiña EJ, Teixeira JA, Rodrigues LR (2010) Isolation and functional characterization of a biosurfactant produced by Lactobacillus paracasei. Colloids Surf B Biointerfaces 76:298–304. doi:10.1016/j.colsurfb.2009.11.008
Haukioja A, Loimaranta V, Tenovuo J (2008) Probiotic bacteria affect the composition of salivary pellicle and streptococcal adhesion in vitro. Oral Microbiol Immunol 23:336–343. doi:10.1111/j.1399-302X.2008.00435.x
Jalasvuori H, Haukioja A, Tenovuo J (2012) Probiotic Lactobacillus reuteri strains ATCC PTA 5289 and ATCC 55730 differ in their cariogenic properties in vitro. Arch Oral Biol 57:1633–1638. doi:10.1016/j.archoralbio.2012.07.014
Jeon J-G, Rosalen PL, Falsetta ML, Koo H (2011) Natural products in caries research: current (limited) knowledge, challenges and future perspective. Caries Res 45:243–263
Kim SH, Lim EJ, Lee SO, Lee JD, Lee TH (2000) Purification and characterization of biosurfactants from Nocardia sp. L-417. Biotechnol Appl Biochem 31:249–253
Kuiper I, Lagendijk EL, Pickford R, Derrick JP, Lamers GEM, Thomas-Oates JE, Lugtenberg BJJ, Bloemberg GV (2004) Characterization of two Pseudomonas putida lipopeptide biosurfactants, putisolvin I and II, which inhibit biofilm formation and break down existing biofilms. Mol Microbiol 51:97–113. doi:10.1046/j.1365-2958.2003.03751.x
Marttinen A, Haukioja A, Keskin M, Söderling E (2013) Effects of Lactobacillus reuteri PTA 5289 and L. paracasei DSMZ16671 on the adhesion and biofilm formation of Streptococcus mutans. Curr Microbiol 67:193–199. doi:10.1007/s00284-013-0352-3
Meurman JH, Stamatova I (2011) Lactic acid bacteria in oral health. in: lactic acid bacteria in oral health, lactic acid bacteria: microbiological and functional aspects. CRC Press, pp 403–422
Myers D (2005) Surfactant science and technology, 3rd edn. Wiley, Newyork
Nitschke M, Costa SGVAO, Contiero J (2005) Rhamnolipid surfactants: an update on the general aspects of these remarkable biomolecules. Biotechnol Prog 21:1593–1600. doi:10.1021/bp050239p
Ntrouka VI, Slot DE, Louropoulou A, Van der Weijden F (2011) The effect of chemotherapeutic agents on contaminated titanium surfaces: a systematic review. Clin Oral Implants Res 22:681–690. doi:10.1111/j.1600-0501.2010.02037.x
De la Fuente-Núñez C, Reffuveille F, Fernández L, Hancock REW (2013) Bacterial biofilm development as a multicellular adaptation: antibiotic resistance and new therapeutic strategies. Curr Opin Microbiol 16:580–589. doi:10.1016/j.mib.2013.06.013
Rodrigues L, Banat IM, Teixeira J, Oliveira R (2006a) Biosurfactants: potential applications in medicine. J Antimicrob Chemother 57:609–618. doi:10.1093/jac/dkl024
Rodrigues LR, Teixeira JA, van der Mei HC, Oliveira R (2006b) Isolation and partial characterization of a biosurfactant produced by Streptococcus thermophilus a. Colloids Surf B Biointerfaces 53:105–112. doi:10.1016/j.colsurfb.2006.08.009
Saharan BS, Sahu RK, Sharma D (2012) A review on biosurfactants: fermentation, current developments and perspectives. Genet Eng Biotechnol J 1:1–14
Saravanakumari P, Mani K (2010) Structural characterization of a novel xylolipid biosurfactant from Lactococcus lactis and analysis of antibacterial activity against multi-drug resistant pathogens. Bioresour Technol 101:8851–8854. doi:10.1016/j.biortech.2010.06.104
Sharma D, Saharan BS (2014) Simultaneous production of biosurfactants and bacteriocins by probiotic Lactobacillus casei MRTL3. Int J Microbiol Article ID 698713 . doi:10.1155/2014/6987137 pages
Sharma D, Saharan B, Chauhan N, Procha S, Lal S (2015) Isolation and functional characterization of novel biosurfactant produced by Enterococcus faecium. SpringerPlus 4:4
Sharma D, Saharan BS, Chauhan N, Bansal A, Procha S (2014) Production and structural characterization of Lactobacillus helveticus derived biosurfactant. Sci World J Article ID:493548 . doi:10.1155/2014/4935489 pages
Socransky SS, AD H (2002) Dental biofilms: difficult therapeutic targets. Periodontol 2000 28:12–55
Söderling E, Marttinen A, Haukioja A (2011) Probiotic lactobacilli interfere with Streptococcus mutans biofilm formation in vitro. Curr Microbiol 62:618–622. doi:10.1007/s00284-010-9752-9
Spinler JK, Taweechotipatr M, Rognerud CL, Ou CN, Tumwasorn S, Versalovic J (2008) Human-derived probiotic Lactobacillus reuteri demonstrate antimicrobial activities targeting diverse enteric bacterial pathogens. Anaerobe 14:166–171. doi:10.1016/j.anaerobe.2008.02.001
Tahmourespour A, Salehi R, Kermanshahi RK (2011) Lactobacillus acidophilus-derived biosurfactant effect on gtfb and gtfc expression level in Streptococcus mutans biofilm cells. Braz J Microbiol 42:330–339
Teanpaisan R, Piwat S, Dahlén G (2011) Inhibitory effect of oral Lactobacillus against oral pathogens. Lett Appl Microbiol 53:452–459. doi:10.1111/j.1472-765X.2011.03132.x
Walencka E, Różalska S, Sadowska B, Różalska B (2008) The influence of Lactobacillus acidophilus-derived surfactants on staphylococcal adhesion and biofilm formation. Folia Microbiol (Praha) 53:61–66. doi:10.1007/s12223-008-0009-y
Acknowledgments
This work was supported by partial grants from PIO SODALIZIO dei PICENI Foundation (Rome, Italy).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
ᅟ
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Eleonora Ciandrini and Raffaella Campana contributed equally to the research.
Rights and permissions
About this article
Cite this article
Ciandrini, E., Campana, R., Casettari, L. et al. Characterization of biosurfactants produced by Lactobacillus spp. and their activity against oral streptococci biofilm. Appl Microbiol Biotechnol 100, 6767–6777 (2016). https://doi.org/10.1007/s00253-016-7531-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7531-7