Abstract
This study was designed to investigate the cooperative effect of selected Lactobacillus gasseri strains and Cudrania tricuspidata (CT) leaf extract in enhancing the health-promoting activities of fermented milk. Addition of CT increased total bacterial counts and proteolysis during fermentation of milk with L. gasseri strains. Antioxidant capacities were determined by measuring the ABTS, DPPH, and peroxyl radical scavenging activities and ferric reducing power. The antioxidant capacity of CT-supplemented milk was greater than that of milk without supplementation; moreover, the antioxidant activity of CT-supplemented milk was synergistically improved by fermentation with L. gasseri strains. In particular, CT-supplemented milk fermented by L. gasseri 505 showed the highest antioxidant activity. The phenolic compounds in CT, such as neo-chlorogenic, chlorogenic, and caffeic acid, were metabolized during fermentation with L. gasseri strains, and 3,4-dihydroxy-hydrocinnamic acid was produced as a fermentation metabolite. Moreover, the liberation of bioactive peptides of fermented milk was increased by the proteolytic activity of L. gasseri strains. In particular, six peptides, which were mainly derived from β-casein, were newly identified in this study. These findings suggest that L. gasseri strains metabolize the phenolic acids in the CT and the bioactive peptides released through this interaction improve the antioxidant activity of the fermented milk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lactic acid bacteria (LAB), including members of the genera Lactobacillus, Lactococcus, Leuconostoc, Pediococcus, and Streptococcus, are generally recognized as important beneficial members of the gastrointestinal (GI) microbiota of humans and animals (Axelsson 2004). Lactobacillus gasseri, a LAB, is abundant in the GI tract, oral cavity, and vagina in humans (Selle and Klaenhammer 2013). Lactobacillus gasseri is used as a starter strain in the production of various fermented dairy products and has potential for probiotic application (Selle and Klaenhammer 2013). Many researchers have reported that using L. gasseri as a probiotic in various fermented foods enhances the functionality of the foods by producing antihypertensive, immunomodulating, and antimicrobial peptides and bacteriocins (Kadooka et al. 2010; Kawai et al. 2000). Some L. gasseri strains produce antibacterial peptides, namely, bacteriocins (Arakawa et al. 2010). However, previous studies indicated that L. gasseri grows poorly in milk and milk-based media without any supplementation, although L. gasseri can grow in semisynthetic media for LAB strains, such as MRS broth (Arakawa et al. 2008). However, synthetic media cannot be used in food products because they include ingredients that are unauthorized for use as food additives. Therefore, new natural food ingredients that enhance the growth of L. gasseri are needed to allow their use as probiotics.
Fructooligosaccharide, galactooligosaccharide, and isomaltooligosacharide are widely used prebiotics that enhance the activity of gut microorganisms (Manning and Gibson 2004). Recently, the concept of prebiotic has emerged, and research efforts have been focused on finding prebiotics from plant extracts. However, only few studies have shown the functional and prebiotic properties of plant extracts in yogurt processing. In addition, the interest in plant-derived food additives has grown in recent years because herbal extracts have been shown to possess health-promoting properties such as antimicrobial and antioxidant activity. In a search for new natural prebiotic sources, we used plant extracts, such as Cudrania tricuspidata (CT) leaf extract, as supplements in the production of fermented milk. C. tricuspidata is a ubiquitous traditional herbal remedy in Asia. There are extensive reports on the antibacterial efficacy of the essential oil of C. tricuspidata fruit (Bajpai et al. 2013) and the inhibitory effect of water-soluble C. tricuspidata leaf extract on lipid peroxidation (Jae-Young 2000).
Recently, milk proteins and their peptides have drawn attention as potential bioactives. Bioactive peptides are released from milk via the following ways: (a) GI digestion (Kilara and Panyam 2003), (b) fermentation with proteolytic starter cultures (Fuglsang et al. 2003; Gobbetti et al. 2000), or (c) hydrolysis by proteolytic enzymes from microorganisms and plants (Yamamoto et al. 1994). During production of fermented milk, the LAB used as starter culture may be responsible for the release of bioactive milk peptides. Several peptides in milk fermented with various LAB, with known sequence and location, have been shown to possess a wide range of nutritional, functional, and biological activities, such as antihypertensive, antibacterial, and antioxidative effects (Hernández-Ledesma et al. 2004; Matar and Goulet 1996; Nakamura et al. 1995). However, the release of antioxidant peptides in milk fermented with L. gasseri strains has not been fully investigated.
The purpose of the present study was to investigate the synbiotic interaction of selected L. gasseri strains newly isolated from infant feces as probiotics and C. tricuspidata as a potential natural prebiotic source. The change in pH, viable cell counts, and proteolytic and antioxidant properties of CT-supplemented milk during fermentation with selected L. gasseri strains was evaluated. In addition, the degradation and synthesis of phenolic compounds in CT were investigated and the profile of the bioactive peptides in the fermented milk was analyzed using MALDI-TOF/MS.
Materials and methods
Preparation of CT
C. tricuspidata leaves were obtained from the local market (Sunchang, Jeollabuk-Do, South Korea). Leaves (100 g) were washed and then soaked in distilled water (1000 mL) in a water bath (100 °C) with occasional shaking for 9 h for extraction. Then, the leaf extract was filtered through a filter paper. The clear solution was concentrated by evaporation to dryness under vacuum at temperatures not higher than 50 °C. The concentrated herbal extracts were freeze-dried before use in yogurt production.
Bacterial strains
Four probiotic Lactobacillus isolates of human origin, i.e., L. gasseri 505, L. gasseri 545, L. gasseri 559, and L. gasseri 575 (Korean Culture Center of Microorganisms, Seoul, Korea; KCCM 11766P, 11767P, 11768P, and 11769P, respectively), were selected for this study, because these strains exhibited probiotic potential. Preliminary, for the selection of probiotic starter culture candidate, Lactobacillus strains were isolated from infant feces. Briefly, the feces were weighed and homogenized for 30 s in saline and diluted. Aliquots of serial dilutions were plated on de Man, Rogosa, and Sharpe (MRS) (Difco Laboratories, MI, USA) agar and incubated at 37 °C for 48–72 h. In total, 32 strains were isolated and were purified by streaking on MRS agar. Our research group evaluated these bacterial isolates for their probiotic potential by various tests, such as acid and bile tolerance, bacterial adhesion capacity, antibacterial activity, and cholesterol-reducing ability (data not shown) (Argyri et al. 2013; Huang et al. 2013). Strain identity was confirmed prior to use by 16S rRNA sequencing. The 16S rRNA gene sequence data for KCCM 11766P, 11767P, 11768P, and 11769P have been deposited in the NCBI GenBank (http://www.ncbi.nlm.nih.gov/genbank/) and are accessible through accession numbers KU517710, KU517711, KU517712, and KU517713, respectively.
Preparation of fermented milk
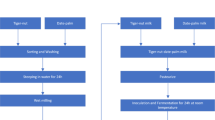
CT-supplemented milk fermented by Lactobacillus strains was prepared on the same day. The powdered herbal extracts (0.2 % [g/g]) were added to pre-warmed (60 °C) milk, and the mixtures were pasteurized (85 °C, 15 min) and cooled to 41 °C. Milks with and without CT supplementation were inoculated with a 3 % suspensions of Lactobacillus strains (approximately 107 CFU/mL). The mixtures were incubated at 41 °C for 48 h. Fermented milk without added CT was used as control. All samples were lyophilized and stored at −20 °C until use.
Measurement of pH
The changes in pH in the fermented milk were measured after calibration of the pH meter with standardized pH buffer solutions of 4.0, 7.0, and 10.0 (Fisher Scientific).
Determination of viable cell counts and proteolytic activity
For each experiment, samples were analyzed immediately after fermentation. Lactobacillus strains were enumerated on MRS after aerobic incubation at 37 °C for 72 h.
The proteolytic activity of milk fermented with Lactobacillus strains was assessed by measuring the total amount of released peptides by the o-phthaldialdehyde (OPA) method described by Nielsen et al. (2001). The peptide concentration was calculated from a standard curve prepared using tryptone (0.5–10 mg/mL). The samples, standard, or blank (deionized water) (30 μL) were mixed with 1 mL of OPA reagent and then allowed to react at room temperature for 2 min. The absorbance was measured with a Synergy H1 plate reader (Bio-Tek Instruments Inc.) at 340 nm.
Determination of antioxidant activity
The antioxidative compounds in fermented milk were extracted with methanol using the method of Hui et al. (2004) with slight modifications. Lyophilized fermented milk samples (500 mg) were extracted three times with 5 mL of methanol. The methanol extracts were filtered through a 0.2-μm pore size syringe filter. The solvent was dried in speed vacuum and the pellet was redissolved in 5 mL of methanol. The antioxidant activity of fermented milk was determined based on the DPPH radical scavenging activity, the results of ferric reducing antioxidant power assay, and ABTS radical scavenging activity using the method of Oh et al. (2013). The oxygen radical absorbance capacity (ORAC) of the sample was measured using the procedures described by Roy et al. (2010). Further, the total flavonoid content (TFC) of the samples was determined using a modified version of the colorimetric method of Zhishen et al. (1999). Quercetin (Sigma Aldrich Co., St. Louis, MO, USA) was used as a standard, and the results were expressed as micrograms of quercetin equivalent. Total phenolic compounds were determined by the method of Maksimovic et al. (2005). Total phenolic contents (TPC) were expressed as micrograms of gallic acid equivalent using a regression of known concentrations of gallic acid, which was determined every time total phenolic assay was carried out.
Identification of phenolic compounds by UPLC–MS/MS
For extraction of phenolic compounds from the fermented milk, lyophilized samples (2 g) were homogenized with 14 mL of 50 % ethanol containing 0.05 M H3PO4 in water. The extracts were sonicated in an ultrasonic bath at room temperature for 20 min and centrifuged at 5000 rpm for 30 min. The supernatant was filtered through a 0.2-μm pore size membrane filter into HPLC vials for analysis.
UPLC–MS/MS analyses were carried out using an ACQUITY™ Ultra Performance Liquid Chromatography system (Waters, Milford, MA, USA) equipped with a Z-spray electrospray ionization (ESI) source and ZEVO TQ iontrap (MS/MS) (Waters, Milford, MA, USA) operating in the negative mode. MassLynx™ software (version 4.0, Waters, Milford, MA, USA) was used to control the instruments and for data acquisition and processing. Sample solutions were injected into a reversed phase column (BEH C18, 1.7 μm, 2.1 × 150 mm, Waters, Milford, MA, USA), which was maintained at 30 °C. The separation was executed with a mobile phase consisting of 0.1 % formic acid in water (mobile phase A) and 0.1 % formic acid in acetonitrile (mobile phase B) with linear gradient elution performed as follows: 0–9.8 min, 8 % B; 9.8–21.80 min, 15 % B; 21.8–23.8 min, 22 % B; 23.8–27.8 min, 40 % B; 27.8–28.2 min, hold on 40 % B; and 28.2–29.8 min, back to 8 % B. The linear binary gradient was set to a flow rate of 0.2 mL/min and total run time was 29.8 min. Ten microliters of sample was injected into the electrospray source (source temperature 150 °C, desolvation temperature 360C°, capillary voltage 2.5 kV, cone voltage 25 V). Argon was used as collision gas (collision energy 25 eV at the start).
Identification of peptides derived from fermented milk by MALDI-TOF/MS/MS
Fermented milk peptide extraction was conducted by the method from Ebner et al. (2015). For peptide analysis, the peptide extracts mixed with an equal volume of matrix solution (HCCA) and 1 μL was spotted onto MALDI target. MALDI-TOF/MS experiments were performed using a Bruker Autoflex (Bruker Datonics, Bremen, Germany) equipped with a nitrogen laser (337 nm). Laser-desorbed positive ions in the peptide were analyzed after acceleration at 19 kV in the reflector mode. External calibration was performed using a mix of angiotensins I and II, substance P, bombesin, ACTH clips 1–17 and 18–39, and somatostatin 28. For each displayed mass spectrum, at least 500 laser shots from several positions on the spots were collected.
Statistical analysis
All data were expressed as means ± SD. Statistical significance for the differences between the groups was assessed using Duncan’s multiple range tests. SAS software version 9.2 (SAS Institute Inc., Cary, NC, USA) was used to perform all statistical tests. P < 0.05 was considered statistically significant.
Results
Strain selection and fermentation
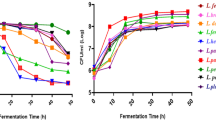
Among the 32 strains, 19 potential probiotic strains, Lactobacillus spp. were selected on the basis of the in vitro screening assays for probiotic potential (data not shown). The further screening of Lactobacillus strains was done based on the acidification of CT-supplemented fermented milk (Fig. 1). The pH reduced after fermentation, ranging from 3.83 ± 0.2 to 6.66 ± 0.33. In particular, strains that caused the highest decrease were L. gasseri 505, 545, 559, and 575 (3.83 ± 0.2, 4.27 ± 0.15, 4.32 ± 0.16, and 4.39 ± 0.22, respectively), which were used for the production of CT-supplemented fermented milk in further analysis.
Viability of probiotic bacteria and proteolytic activities
The growth of the four selected L. gasseri strains was evaluated by determining the viable cell counts (Table 1). After 48 h of incubation, the pH of the control decreased slightly from 6.36 ± 0.1 to 6.29 ± 0.05. In addition, bacterial counts decreased slightly at the end of incubation. On the other hand, the bacterial counts in CT-supplemented fermented milk were higher than that in the control, suggesting that the addition of CT increased growth of bacteria while promoting acidification.
The proteolytic activity of selected L. gasseri strains in fermented milk was assessed by the OPA method (Table 1). The result showed a significant (P < 0.05) improvement of proteolytic activity in milks fermented by all L. gasseri strains in the presence of CT; especially, the proteolytic activity in CT-supplemented milk fermented by L. gasseri 505 was 19.4-fold than in milk fermented with the other strains. On the other hand, no significant (P < 0.05) changes were observed during fermentation with L. gasseri strains at 41 °C without CT supplementation. The proteolytic activity in CT-supplemented fermented milk was consistent with the results of the bacterial counts.
Antioxidant capacity of fermented milk
The antioxidant activities were evaluated based on the results of DPPH, ABTS, ORAC, and FRAP assays. Initially, free radical scavenging activity was evaluated by reacting with stable ABTS and DPPH free radicals. The antioxidant activity in CT-supplemented fermented milk was described as free radical scavenging activity (Fig. 2a–b). Fermentation with L. gasseri strains in the presence of CT significantly (P < 0.05) increased both DPPH and ABTS radical scavenging activities in a concentration-dependent manner. In particular, the ABTS radical scavenging activity of CT-supplemented milk fermented by L. gasseri 505 was the highest among the samples. After 48 h of fermentation, the addition of CT led to an increase in DPPH radical scavenging activity, ranging from 90.8 ± 1.7 to 92.8 ± 0.2 %, without significant differences (P < 0.05) between the milks fermented by the four selected Lactobacillus strains. The results of ORAC (Fig. 2c) were similar to those of ABTS and DPPH radical scavenging activities. The addition of CT into milk resulted in 48 % increase of the ORAC value, and the ORAC value of fermented milk increased by 2.03–2.69 times relative to that in unfermented milk. In addition, the FRAP assay was performed to determine the reducing power of the fermented milk (Fig. 2d). Compared to unfermented milk, CT-supplemented fermented milk had significantly higher reducing power. The highest reducing power was observed in the CT-supplemented milk fermented by L. gasseri 505 (4.21 ± 0.06 mM M FeSO4·7H2O), which had 1.99 times the reducing power of unfermented milk.
ABTS (a) and DPPH radical scavenging activity (b) and ORAC (c) and FRAP (d) values of CT-supplemented milk fermented with selected L. gasseri strains. The results are presented as the mean ± SD (n = 3). Different letters indicate statistically significant differences among the different groups (P < 0.05)
Total flavonoid content (TFC) and total polyphenol content (TPC) in CT-supplemented fermented milk were also determined (Fig. 3). Compared to unfermented milk, CT-supplemented fermented milk had significantly (P < 0.05) higher TFC and TPC. TFC in the CT-supplemented fermented milk ranged from 19.18 ± 0.32 to 20.21 ± 0.67 μg quercetin/100 mg dry matter of milk. TPC also increased by 1.9~2.4 times relative to that in unfermented milk (Fig. 4).
Determination of phenolic compound contents in fermented milk
The phenolic compound content in the fermented milk was evaluated throughout fermentation. Figure 5 shows the UPLC–MS total ion current chromatograms of a mixed standard and CT-supplemented milk fermented by L. gasseri 505. The retention times, mass spectral characteristics, and individual multiple reaction monitoring (MRM) transitions used for quantifying are specified in Table 2. Differences in the type and quantity of phenolic compounds were detected in the CT-supplemented fermented milk (Fig. 4). The phenolic compound profiles of the unfermented and fermented CT-supplemented milk were similar; however, the amount of individual compounds differed. In total, ten phenolic compounds were detected in CT-supplemented fermented milk. CT-supplemented milk contained a variety of phenolic acids, and the predominant compound was neo-chlorogenic acid (72.23 ± 0.97 μg/g), followed by chlorogenic acid (52.30 ± 1.28 μg/g). In fermented milk, the relative distribution of phenolic acids was different from that in unfermented milk. Through the fermentation process, up to 47.6~58.5 % and 76.4~84.9 % losses were observed for neo-chlorogenic and chlorogenic acid, respectively. In particular, caffeic acid was the most affected phenolic acid during the fermentation process and decreased by 99 % relative to that in unfermented milk. On the other hand, 3,4-dihydroxy-hydrocinnamic acid significantly (P < 0.05) increased after fermentation, ranging from 82.62 ± 8.21 to 108.13 ± 0.37 μg/g.
UPLC-MS total ion current chromatograms of phenolic compounds of unfermented CT-supplemented milk (a) and CT-supplemented milk fermented with L. gasseri 505 (b). 1, Neo-chlorogenic acid; 2, chlorogenic acid; 3, 3,4-dihydroxyhydrocinnamic acid; 4, caffeic acid; 5, rutin hydrate; 6, quercetin-3-galactoside; 7, quercetin-3-glucoside; 8, kaempferol-3-galactoside; 9, kaempferol-3-rutinoside; 10, kaempferol-3-glucoside. Mass fragmentation patterns of identified phenolic compounds (c–f)
Additionally, the predominant flavonoid was quercetin-3-glucoside followed by rutin hydrate in CT-fermented milk. Quercetin-3-glucoside slightly reduced after fermentation, whereas rutin hydrate significantly (P < 0.05) increased, with the exception of the CT-supplemented milk fermented by L. gasseri 575. In this study, flavonoids in CT-supplemented milk were more stable compared with phenolic acids.
Peptide profiles of fermented milk
A detailed peptide analysis of fermented milk was performed by direct MALDI-TOF/MS/MS in the m/z range from 500 to 4500 Da. The peptide profiles are presented in Table 3. The release of bioactive peptides from CT-supplemented milk fermented by selected L. gasseri strains increased. A total of 16–20 peptide fragments were identified in the milk fermented with four selected L. gasseri strains. Most peptides originated from β-casein (15), followed by αs1-casein (4), and αs2-casein (2); however, fragments from κ-casein were not detected. The peptide content was the highest in the CT-supplemented milk fermented by L. gasseri 505, which is consistent with the result of proteolytic activity. Importantly, the peptides derived from β-casein originated from the C-terminal region of β-casein. An N-terminal αs1-casein fragment (f1–14, 1–19, 1–20, and 1–23) was also identified in the fermented milk. In particular, peptides derived from the C-terminal of β-casein, f190–209 and f197–209; center of β-casein, f165–189; and N-terminal of αs1-casein, f1–19 were only observed in milk fermented with L. gasseri 505.
Discussion
Phenolic compounds are important constituents of food products of plant origin. CT, which possesses a variety of phenolic compounds, has beneficial properties such as antimicrobial, anti-inflammatory, and antitumor activities and α-glucosidase inhibitory activity (Choi et al. 2009; Park et al. 2006; Seo et al. 2007; Zou et al. 2004). It has been reported that the water extracts of C. tricuspidata leaves, stems, root, and fruits contain a variety of phenolic compounds, such as flavonoids and phenolic acids; in particular, C. tricuspidata leaves exhibit high phenolic compound content (Jeong et al. 2009). Although the chemical and nutritional properties of C. tricuspidata are well investigated, the potential of CT leaf extract for use as a prebiotic ingredient, which selectively promotes the growth of probiotic bacteria such as lactobacilli and bifidobacteria, has not been reported. In recent years, the concept of prebiotics has emerged. Multiple studies have reported that various commercial prebiotics such as inulin, maltodextrin, oligofructose, and polydextrose accelerated the acidification of yogurt and reduced the fermentation time (Jaya and Das 2004). According to Bindels et al. (2015), the prebiotic concept should not be restricted to carbohydrates. It has been reported that polyphenols were metabolized and bioconverted by intestinal microorganisms (Hassaninasab et al. 2011; Van Duynhoven et al. 2011). In addition, it has been reported that red wine polyphenol extracts and cocoa-derived flavanols increased the growth of gut microbiota, indicating that these natural polyphenols have prebiotic potential (Queipo-Ortuño et al. 2012; Tzounis et al. 2011). Further, Vodnar and Socaciu (2012) reported that microencapsulation of LAB with green tea extract enhanced the viability and stability of the bacteria.
Recently, several studies suggested that the levels of bioactive or biogenic substances are enhanced through fermentation with appropriate selected starter cultures (Gobbetti et al. 2010). The incorporation of suitable starters and functional ingredients may contribute to the enhancement of properties such as flavor, texture, and sensory attributes and improved the nutritional value of foods. Therefore, in-depth research on the production of fermented foods is required to adequately manage the starter culture and plant extracts as new functional food ingredients.
This study aimed at investigating the utility of CT and L. gasseri strains for the production of fermented milk and their synbiotic effect. We produced CT-supplemented milk fermented by Lactobacillus strains to determine the action of Lactobacillus strains on CT. The antioxidant properties, metabolism of phenolic compounds, and peptide profiles of the CT-supplemented milk fermented by the selected L. gasseri strains were evaluated. We isolated and purified LAB strains from infant feces, and the isolates were identified by tests for acid and bile tolerance, adhesion capacity, antibacterial activity, and cholesterol-reducing ability. Based on these preliminary investigations, 19 Lactobacillus strains were selected for production of fermented milk. Based on the changes in pH of CT-supplemented milk, four strains, L. gasseri 505, L. gasseri 545, L. gasseri 559, and L. gasseri 575, were found to be well adapted to growth in CT-supplemented milk. L. gasseri is widely used as a probiotic in fermented milk products. However, it has been reported that L. gasseri is unable to grow in milk and milk-based culture media (Arakawa et al. 2008). This problem was overcome by supplementation of milk with CT. In milk without CT supplementation, the viable cell count and proteolytic activity did not increase during 48 h fermentation. Conversely, the four selected L. gasseri strains grew well in CT-supplemented milk, with cell count increase from 1.03 to 1.56 log CFU/mL during the 48-h fermentation. The proteolytic activity of CT-supplemented fermented milk was also higher than that of control after 48 h. The breakdown of large milk proteins into smaller peptides and amino acids is due to the proteolytic activity of LAB (Christensen et al. 1999). Hence, the increase in peptides and amino acids is related to the proteolytic activity of the potential probiotic L. gasseri strains, which was improved in the presence of CT.
The methods for measuring the antioxidant capacity can be classified primarily into electron transfer (ET)-based and hydrogen atom transfer (HAT)-based assays (Rodriguez-Amaya 2010). The ET-based assay includes DPPH assay for determining free radical scavenging activity, an ABTS assay for cation scavenging activity, and FRAP assay for determining ferric reducing capacity. The HAT-based assay includes the ORAC assay, in which antioxidants compete for thermally generated peroxyl radicals. ABTS, DPPH, and ORAC assays monitor the radical scavenging activity. The FRAP assay is different from these three assays as there are no free radicals, but the reduction of ferric ion to ferrous iron is monitored (Thaipong et al. 2006). Therefore, the antioxidant activity of CT-supplemented fermented milk was evaluated using four different measurement methods including DPPH, ABTS, ORAC, and FRAP assay, which are based on different reaction mechanisms. In our experiments, the CT-supplemented milk had higher antioxidant capacity than control (milk without CT). In addition, the antioxidant activity of CT-supplemented fermented milk increased significantly (P < 0.05) through following fermentation with the selected L. gasseri strains. This result was consistent with the enhancement of TFC and TPC during fermentation. According to Zainoldin and Baba (2009), the higher antioxidant activities of yogurts supplemented with plant extracts are likely due to the phytochemicals in plant extracts. Virtanen et al. (2007) investigated the antioxidant activity of milk fermented with various LAB strains. According to this study, antioxidant activity of fermented milk might be different based on the metabolic activity of different LAB species, even various strains of the same species. Hence, our results for the antioxidant activity of CT-supplemented fermented milk suggested that the addition of CT into fermented milk might enhance antioxidant activity.
CT contains appreciable levels of phenolic compounds and phenolic glycosides, such as chlorogenic acid, caffeic acid, and quercetin-3-glucoside (Jeong et al. 2009). These phenolic compounds are responsible for the antioxidative properties, and their contents are affected by microbial fermentation. In this study, fermentation of CT-supplemented milk by selected L. gasseri strains resulted in a significant difference (P < 0.05) in the contents of the phenolic compounds in CT. During 48 h of fermentation, neo-chlorogenic, chlorogenic, and caffeic acid content decreased in CT-supplemented fermented milk; however, 3,4-dihydroxy-hydrocinnamic acid levels increased significantly (P < 0.05). Degradation of chlorogenic acid by cleavage of the ester bond between caffeic acid and quinic acid results in the production of 3,4-dihydroxy-hydrocinnamic acid, suggesting that caffeic acid was reduced at the double bond (Couteau et al. 2001). This result indicated that the intrinsic phenolic compounds, such as neo-chlorogenic, chlorogenic, and caffeic acid, in CT were utilized by the L. gasseri strains during fermentation and metabolized into other compounds. Another study has also reported the degradation of phenolic compounds, such as anthocyanins, in blueberry yogurt (Ścibisz et al. 2012). The results showed that anthocyanin follows a first-order reaction kinetic during bacterial culture.
In the current study, the fermentation of CT-supplemented milk by selected L. gasseri strains resulted in the microbial and chemical changes, including in proteolytic activity, and improvement of antioxidant activity. In particular, the enhancement of antioxidant activity of CT-supplemented fermented milk might be due to the generation of peptides during fermentation by L. gasseri. Although many studies investigated the antioxidant activity of fermented milk, studies to identify putative bioactive compounds in products fermented with L. gasseri strains have not been conducted. To obtain the bioactive peptide profiles of fermented milk, crude peptide extracts of fermented milk were analyzed by MALDI-TOF/MS. The most frequently identified peptide was derived from β-casein, followed by αs1-casein and αs2-casein. Among the identified peptides, most peptides have been previously described to possess antimicrobial, antihypertensive, and antioxidative activities and ACE inhibitory activity (Lahov and Regelson 1996; Miguel et al. 2006; Yamamoto et al. 1994). Various bioactive peptides have been identified in dairy products, such as different types of yogurt and cheese. However, six peptides were newly identified in this study. The number of bioactive peptides increased during the fermentation of CT-supplemented milk, and the difference in the peptide contents of the different fermented milks was likely due to the difference in the proteolytic action of the four L. gasseri strains. The strain-dependent variation in the production of fermented milk may influence the peptides released and their bioactivity (Osuntoki and Korie 2010). The ability of LAB to grow to high cell densities in fermented milk is also correlated with proteolytic activity associated with the release of casein-derived peptides (Christensen et al. 1999). Casein-derived peptides of CT-supplemented fermented milk could be a rich source of functional dairy food ingredients, and the interest in using milk peptides as food supplements is also increasing (Meisel and FitzGerald 2003).
In conclusion, we found the use of four selected strains of L. gasseri (505, 545, 559, and 575) as potential functional dairy starter culture and the effect of CT on milk fermented with the L. gasseri strains. L. gasseri strains grew well in milk supplemented with CT, and all strains showed higher proteolytic activity in the CT-supplemented milk than in milk without supplementation. The proteolytic activity was strain dependent, with L. gasseri 505 showing the highest proteolytic activity. These results show that CT may promote fermentation. After 48 h of fermentation, CT-supplemented milk fermented by selected L. gasseri strains showed increased radical scavenging activity and reducing power relative to that of the control. The total flavonoid and polyphenol contents increased during fermentation, and the increase correlated with the antioxidant activity. In addition, L. gasseri strains utilized and metabolized the phenolic compounds, mainly neo-chlorogenic, chlorogenic, and caffeic acid, whereas 3,4-dihydroxy-hydrocinnamic acid was generated during fermentation. Moreover, fermentation with L. gasseri strains in CT-supplemented milk released potential bioactive peptides. Collectively, these findings suggest that CT may serve as a good source of prebiotics, which promote the growth of probiotic bacteria, and this synbiotic combination of CT and L. gasseri strains can serve as potential probiotics in fermented milk to improve the antioxidant activity of dairy products.
References
Arakawa K, Kawai Y, Fujitani K, Nishimura J, Kitazawa H, Komine K, Kai K, Saito T (2008) Bacteriocin production of probiotic Lactobacillus gasseri LA39 isolated from human feces in milk-based media. Anim Sci J 79(5):634–640
Arakawa K, Kawai Y, Ito Y, Nakamura K, Chujo T, Nishimura J, Kitazawa H, Saito T (2010) HPLC purification and re-evaluation of chemical identity of two circular bacteriocins, gassericin A and reutericin 6. Lett Appl Microbiol 50(4):406–411
Argyri AA, Zoumpopoulou G, Karatzas K-AG, Tsakalidou E, Nychas G-JE, Panagou EZ, Tassou CC (2013) Selection of potential probiotic lactic acid bacteria from fermented olives by in vitro tests. Food Microbiol 33(2):282–291
Axelsson L (2004) Lactic acid bacteria: classification and physiology. Food Science and Technology-New York-Marcel Dekker 139:1–66
Bajpai VK, Sharma A, Baek K-H (2013) Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control 32(2):582–590
Bindels LB, Delzenne NM, Cani PD, Walter J (2015) Towards a more comprehensive concept for prebiotics. Nat Rev Gastro Hepat 12(5):303–310
Chang OK, Roux É, Awussi AA, Miclo L, Jardin J, Jameh N, Dary A, Humbert G, Perrin C (2014) Use of a free form of the Streptococcus thermophilus cell envelope protease PrtS as a tool to produce bioactive peptides. Int Dairy J 38(2):104–115
Choi S-R, You D-H, Kim J-Y, Park C-B, Kim D-H, Ryu J, Choi D-G, Park H-M (2009) Antimicrobial activity of methanol extracts from Cudrania tricuspidata bureau according to the parts harvested and time. Korean J Med Crop Sci 17(5):335–340
Christensen JE, Dudley EG, Pederson JA, Steele JL (1999) Peptidases and amino acid catabolism in lactic acid bacteria. Antonie Van Leeuwenhoek 76(1–4):217–246
Couteau D, McCartney A, Gibson G, Williamson G, Faulds C (2001) Isolation and characterization of human colonic bacteria able to hydrolyse chlorogenic acid. J Appl Microbiol 90(6):873–881
Ebner J, Arslan AA, Fedorova M, Hoffmann R, Küçükçetin A, Pischetsrieder M (2015) Peptide profiling of bovine kefir reveals 236 unique peptides released from caseins during its production by starter culture or kefir grains. J Proteome 117:41–57
Fuglsang A, Nilsson D, Nyborg NC (2003) Characterization of new milk-derived inhibitors of angiotensin converting enzyme in vitro and in vivo. J Enzym Inhib Med Ch 18(5):407–412
Gobbetti M, Cagno RD, De Angelis M (2010) Functional microorganisms for functional food quality. Crit Rev Food Sci 50(8):716–727
Gobbetti M, Ferranti P, Smacchi E, Goffredi F, Addeo F (2000) Production of angiotensin-I-converting-enzyme-inhibitory peptides in fermented milks started by Lactobacillus delbrueckii subsp. bulgaricus SS1 and Lactococcus lactis subsp. cremoris FT4. App Environ Microb 66(9):3898–3904
Hassaninasab A, Hashimoto Y, Tomita-Yokotani K, Kobayashi M (2011) Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism. P Natl Acad Sci USA 108(16):6615–6620
Hayes M, Stanton C, Slattery H, O'Sullivan O, Hill C, Fitzgerald G, Ross R (2007) Casein fermentate of Lactobacillus animalis DPC6134 contains a range of novel propeptide angiotensin-converting enzyme inhibitors. App Environ Microb 73(14):4658–4667
Hernández-Ledesma B, Amigo L, Ramos M, Recio I (2004) Angiotensin converting enzyme inhibitory activity in commercial fermented products. Formation of peptides under simulated gastrointestinal digestion. J Agr Food Chem 52(6):1504–1510
Huang Y, Wang X, Wang J, Wu F, Sui Y, Yang L, Wang Z (2013) Lactobacillus plantarum strains as potential probiotic cultures with cholesterol-lowering activity. J Dairy Sci 96(5):2746–2753
Hui YH, Meunier-Goddik L, Josephsen J, Nip W-K, Stanfield PS (2004) Handbook of food and beverage fermentation technology, vol 134. CRC, New York
Jae-Young C (2000) Effects of water-soluble extract from leaves of Morus alba and Cudrania tricuspidata on the lipid peroxidation in tissues of rats. J Korean Soc Food Sci Nutr 29(3):531–536
Jaya S, Das H (2004) Effect of maltodextrin, glycerol monostearate and tricalcium phosphate on vacuum dried mango powder properties. J Food Eng 63(2):125–134
Jeong C-H, Choi GN, Kim JH, Kwak JH, Heo HJ, Shim K-H, Cho B-R, Bae Y-I, Choi J-S (2009) In vitro antioxidative activities and phenolic composition of hot water extract from different parts of Cudrania tricuspidata. J Food Sci Nutr 14(4):283–289
Johansson A, Lugand D, Rolet-Répécaud O, Mollé D, Delage M-M, Peltre G, Marchesseau S, Léonil J, Dupont D (2009) Epitope characterization of a supramolecular protein assembly with a collection of monoclonal antibodies: the case of casein micelle. Mol Immunol 46(6):1058–1066
Kadooka Y, Sato M, Imaizumi K, Ogawa A, Ikuyama K, Akai Y, Okano M, Kagoshima M, Tsuchida T (2010) Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr 64(6):636–643
Kawai Y, Saitoh B, Takahashi O, Kitazawa H, Saito T, Nakajima H, Itoh T (2000) Primary amino acid and DNA sequences of gassericin T, a lactacin F-family bacteriocin produced by Lactobacillus gasseri SBT2055. Biosci Biotechnol Biochem 64(10):2201–2208
Kilara A, Panyam D (2003) Peptides from milk proteins and their properties. Crit Rev Food Sci 43(6):607–633
Kirilov N, Dimov S, Dalgalarrondo M, Ignatova T, Kambarev S, Stoyanovski S, Danova S, Iliev I, Haertlé T, Chobert J-M (2011) Characterization of enterococci isolated from homemade Bulgarian cheeses and katuk. Eur Food Res Technol 233(6):1029–1040
Lahov E, Regelson W (1996) Antibacterial and immunostimulating casein-derived substances from milk: casecidin, isracidin peptides. Food Chem Toxicol 34(1):131–145
Lignitto L, Cavatorta V, Balzan S, Gabai G, Galaverna G, Novelli E, Sforza S, Segato S (2010) Angiotensin-converting enzyme inhibitory activity of water-soluble extracts of Asiago d’allevo cheese. Int Dairy J 20(1):11–17
Møller KK, Rattray FP, Ardö Y (2013) Application of selected lactic acid bacteria and coagulant for improving the quality of low-salt Cheddar cheese: chemical, microbiological and rheological evaluation. Int Dairy J 33(2):163–174
Maksimovic Z, Malencic D, Kovacevic N (2005) Polyphenol contents and antioxidant activity of Maydis stigma extracts. Bioresource Technol 96(8):873–877
Manning TS, Gibson GR (2004) Prebiotics. Best Pract Res Clin Gastroenterol 18(2):287–298
Matar C, Goulet J (1996) β-casomorphin 4 from milk fermented by a mutant of Lactobacillus helveticus. Int Dairy J 6(4):383–397
Meisel H, FitzGerald R (2003) Biofunctional peptides from milk proteins: mineral binding and cytomodulatory effects. Curr Pharm Design 9(16):1289–1296
Miguel M, Recio I, Ramos M, Delgado M, Aleixandre M (2006) Antihypertensive effect of peptides obtained from Enterococcus faecalis-fermented milk in rats. J Dairy Sci 89(9):3352–3359
Nakamura Y, Yamamoto N, Sakai K, Takano T (1995) Antihypertensive effect of sour milk and peptides isolated from it that are inhibitors to angiotensin I-converting enzyme. J Dairy Sci 78(6):1253–1257
Nielsen PM, Petersen D, Dambmann C (2001) Improved method for determining food protein degree of hydrolysis. J Food Sci 66(5):642–646
Oh NS, Lee HA, Lee JY, Joung JY, Lee KB, Kim Y, Lee KW, Kim SH (2013) The dual effects of Maillard reaction and enzymatic hydrolysis on the antioxidant activity of milk proteins. J Dairy Sci 96(8):4899–4911. doi:10.3168/jds.2013-6613
Osuntoki A, Korie I (2010) Antioxidant activity of whey from milk fermented with Lactobacillus species isolated from Nigerian fermented foods. Food Technol Biotech 48(4):505–511
Park KH, Park Y-D, Han J-M, Im K-R, Lee BW, Jeong IY, Jeong T-S, Lee WS (2006) Anti-atherosclerotic and anti-inflammatory activities of catecholic xanthones and flavonoids isolated from Cudrania tricuspidata. Bioorg Med Chem Lett 16(21):5580–5583
Queipo-Ortuño MI, Boto-Ordóñez M, Murri M, Gomez-Zumaquero JM, Clemente-Postigo M, Estruch R, Diaz FC, Andrés-Lacueva C, Tinahones FJ (2012) Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am J Clin Nutr 95(6):1323–1334
Quirós A, Ramos M, Muguerza B, Delgado MA, Miguel M, Aleixandre A, Recio I (2007) Identification of novel antihypertensive peptides in milk fermented with Enterococcus faecalis. Int Dairy J 17(1):33–41
Recio I, Visser S (1999) Identification of two distinct antibacterial domains within the sequence of bovine α s2-casein. Biochim Biophys Acta-General Subjects 1428(2):314–326
Regazzo D, Mollé D, Gabai G, Tomé D, Dupont D, Leonil J, Boutrou R (2010) The (193–209) 17-residues peptide of bovine β-casein is transported through Caco-2 monolayer. Mol Nutr Food Res 54(10):1428–1435
Rodriguez-Amaya DB (2010) Quantitative analysis, in vitro assessment of bioavailability and antioxidant activity of food carotenoids—a review. J Food Compos Anal 23(7):726–740
Roy MK, Koide M, Rao TP, Okubo T, Ogasawara Y, Juneja LR (2010) ORAC and DPPH assay comparison to assess antioxidant capacity of tea infusions: relationship between total polyphenol and individual catechin content. Int J Food Sci Nutr 61(2):109–124
Ścibisz I, Ziarno M, Mitek M, Zaręba D (2012) Effect of probiotic cultures on the stability of anthocyanins in blueberry yoghurts. LWT-Food Sci Technol 49(2):208–212
Selle K, Klaenhammer TR (2013) Genomic and phenotypic evidence for probiotic influences of Lactobacillus gasseri on human health. FEMS Microbiol Rev 37(6):915–935
Seo EJ, Curtis-Long MJ, Lee BW, Kim HY, Ryu YB, Jeong T-S, Lee WS, Park KH (2007) Xanthones from Cudrania tricuspidata displaying potent α-glucosidase inhibition. Bioorg Med Chem Lett 17(23):6421–6424
Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Byrne DH (2006) Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compos Anal 19(6):669–675
Tzounis X, Rodriguez-Mateos A, Vulevic J, Gibson GR, Kwik-Uribe C, Spencer JP (2011) Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am J Clin Nutr 93(1):62–72
Van Duynhoven J, Vaughan EE, Jacobs DM, Kemperman RA, Van Velzen EJ, Gross G, Roger LC, Possemiers S, Smilde AK, Doré J (2011) Metabolic fate of polyphenols in the human superorganism. P Natl Acad Sci USA 108(Supplement 1):4531–4538
Virtanen T, Pihlanto A, Akkanen S, Korhonen H (2007) Development of antioxidant activity in milk whey during fermentation with lactic acid bacteria. J Appl Microbiol 102(1):106–115
Vodnar DC, Socaciu C (2012) Green tea increases the survival yield of Bifidobacteria in simulated gastrointestinal environment and during refrigerated conditions. Chem Cent J 6(1):61
Yamamoto N, Akino A, Takano T (1994) Antihypertensive effect of the peptides derived from casein by an extracellular proteinase from Lactobacillus helveticus CP790. J Dairy Sci 77(4):917–922
Zainoldin K, Baba A (2009) The effect of Hylocereus polyrhizus and Hylocereus undatus on physicochemical, proteolysis, and antioxidant activity in yogurt. World Acad Sci Eng Technol 60:361–366
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64(4):555–559
Zou Y-S, Hou A-J, Zhu G-F, Chen Y-F, Sun H-D, Zhao Q-S (2004) Cytotoxic isoprenylated xanthones from Cudrania tricuspidata. Bioorgan Med Chem 12(8):1947–1953
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the High Value-Added Food Technology Development Program of the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry, and Fisheries (iPET) and the Ministry for Food, Agriculture, Forestry, and Fisheries of Republic of Korea (313036-03-2-SB010) and the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2014R1A1A2008481 to S.O.)
Conflict of interest
The authors declare that they have no competing interests.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Nam Su Oh and Ji Young Lee contributed equally to this study.
Rights and permissions
About this article
Cite this article
Oh, N.S., Lee, J.Y., Oh, S. et al. Improved functionality of fermented milk is mediated by the synbiotic interaction between Cudrania tricuspidata leaf extract and Lactobacillus gasseri strains. Appl Microbiol Biotechnol 100, 5919–5932 (2016). https://doi.org/10.1007/s00253-016-7414-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7414-y