Abstract
The influence of different fermentation conditions on intracellular polysaccharide (IPS) production and activities of the phosphoglucomutase (PGM), UDPG-pyrophosphorylase (UGP), phosphoglucose isomerase (PGI), UDPG-dehydrogenase (UGD), and glucokinase (GK) implicated in metabolite synthesis in Cordyceps militaris was evaluated. The highest IPS production (327.57 ± 6.27 mg/100 mL) was obtained when the strain was grown in the optimal medium containing glucose (40 g · L−1), beef extract (10 g · L−1), and CaCO3 (0.5 g · L−1), and the initial pH and temperature were 7 and 25 °C, respectively. The activities of PGM, UGP, and PGI were proved to be influenced by the fermentation conditions. A strong correlation between the activities of these enzymes and the production of IPS was found. The transcription level of the pgm gene (encoding PGM) was 1.049 times and 1.467 times compared to the ugp gene and pgi gene (encoding UGP and PGI), respectively, in the optimal culture medium. This result indicated that PGM might be the highly key enzyme to regulate the biosynthesis of IPS of C. militaris in a liquid-submerged culture. Our study might be helpful for further research on the pathway of polysaccharide biosynthesis aimed to improve the IPS production of C. militaris.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cordyceps militaris or North cordyceps sinensis is an entomogenous fungus belonging to the Ascomycota, Pyrenomycetes, Sphaeriales, Clavicipitaceae (Fam) O.E. Erikss., Cordyceps (L. ex Fr.) Link. It was highly prized for its application in traditional Chinese medicine. It had also been increasingly studied and used in the West (Zheng et al. 2011; Paterson 2008; Zhou et al. 2009). Recently, artificial C. militaris has been increasingly viewed as a substitute for the natural Cordyceps because of their similar bioactive components (Dong et al. 2012; Huang et al. 2009; Cui 2015). As a new food material, C. militaris has multiple pharmacological activities such as anti-inflammatory, anti-hyperlipidemia, enhancing insulin resistance, and insulin secretion (Won and Park 2005; Choi et al. 2004). Furthermore, it has been indicated that the antioxidant activity of C. militaris is even stronger than that of Cordyceps sinensis and Cordyceps kyushuensis (Chen et al. 2004). Although much information has been accumulated on the culture, extraction, purification, and biological properties of C. militaris, the problems of biotechnological production and the novel medical applications of bioactive compositions of C. militaris remain ambiguous. Therefore, more investigations are required to optimize the modern culture techniques, the degeneration of isolates, the genes responsible for biosynthesis of bioactive components, the methods of efficient purification and isolation of active compounds, and the quality control of C. militaris (Cui 2015).
One of the major useful bioactive constituents of Cordyceps are the polysaccharides which are well-known for their roles as energy storage molecules in cells and structural elements of the cell walls of living organisms. Moreover, polysaccharides have been reported to have many interesting biological activities, including health-promoting effects, immuno-stimulating activity, antioxidant activity, antitumor activity, and so on (Yu et al. 2007; Hou et al. 2008; Lee and Hong 2011; Park et al. 2009). These unique bioactivities have resulted in the successful applications of polysaccharide in industrial fields such as pharmaceutical, cosmetics, food industries, and agronomy. In recent years, Delattre et al. (2015) have described the antioxidant activities of an oxidized polysaccharide by regioselective oxidation obtained through the reaction with NaOCl/NaBr by using 2,2,6,6-tetramethylpiperidine-1-oxy radical (TEMPO) as catalyst. Furthermore, several studies have demonstrated that many natural polysaccharides and polysaccharide-protein complexes have been applied or are under clinical trials as immune modifiers and drugs for cancer therapies (Yang and Zhang 2009; Zhang et al. 2007). More generally, the activities of polysaccharides are highly dependent on their chemical composition and structural characteristics such as glycosidic linkages, monosaccharides composition, and polymerization degrees (Methacanon et al. 2005). In fact, low molecular weight polysaccharides and oligosaccharides also possess a large variety of biological activities in numerous organisms. Therefore, there are still abundant references related to the therapeutic, prebiotic, and elicitor activities of probiotics and prebiotics in oligosaccharides (Saad et al. 2013; Kothari et al. 2015). Moreover, plentiful literature have focused more particularly on new enzymatic degradation routes of polysaccharides to produce specific oligosaccharides with high production yields and few purification steps (Delattre and Vijayalaksmi 2009; Mellal et al. 2008; Tavernier et al. 2008). Nevertheless, in most cases, the lack of industrial polysaccharide production is the main drawback limiting the biological applications of C. militaris. For this reason, there is an urgent need to improve the yield of polysaccharides from C. militaris. The limited natural resource and complex process of artificial cultivation make cultivation of the full fruit body of C. militaris very difficult in solid-state fermentation. Many attempts are being made to obtain useful cellular materials or to produce effective substances from a submerged mycelia culture (Park et al. 2001; Kwon et al. 2009; Cui and Zhang 2012). For instance, Park et al. (2002) reported that plant oils (vegetable, sunflower, and olive oil) and fatty acids (oleic, palmitic, and linoleic acid) have different stimulation effects on the exo-biopolymer production of C. militaris.
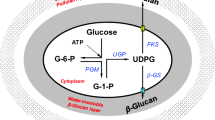
Based on the results of other research on polysaccharide biosynthesis in fungus: the carbohydrate source has an influence on the activities of enzymes involved in sugar metabolism in Pediococcus parvulus 2.6 (Velasco et al. 2007); Different sugar and nitrogen sources affect the activities of α-phosphoglucose mutase, UDPG-pyrophosphorylase, and glucosyltransferase involved in pullulan synthesis and pullulan production by Aureobasidium pullulans (Duan et al. 2008; Jiang et al. 2011). The pathway of polysaccharides biosynthesis in C. militaris is proposed based on the results of other researchers may be in Fig. 1.
It has been reported that UDP-glucose plays an important role in the biosynthesis of fungal polysaccharides, indicating UDP-glucose mainly as a pivotal precursor in the sugar dysplasia process (Shingel 2004). UDP-glucose is formed from glucose 1-phosphate and UTP in reverse reaction catalyzed by UDPG-pyrophosphorylase (UGP, EC 2.7.7.9) (Daran et al. 1995): UTP + glucose-1-phosphate → UDP-glucose + PPi. Then glucose-1-phosphate produced by catalyzed the reversible transfer between glucose-1-phosphate and glucose-6-phosphate via phosphoglucomutase (PGM, EC 2.7.5.1): UTP + glucose-6-phosphate → glucose-1-phosphate + PPi. These phosphorylated sugars are important sugar donors for the production of glucose-containing polysaccharides (Elisavet et al. 2011). Hence, PGM plays a key role in the pathways of metabolite synthesis. The phosphoglucose isomerase (PGI) leading to pyruvic acid formation and phosphoglucomutase (PGM) leading to polysaccharide formation are at the branch point between the Embden-Meyerhof-Parnas (EMP) pathway and the later part of glycogen biosynthesis (Tang and Zhong 2002). That is to say, the activity level of PGI will determine the glucose-6-phosphate conversion direction. Therefore, PGI may also control the polysaccharide synthesis to some extent. Glucokinase (GK) is the rate-limiting enzyme in the first step of glycolysis. In animal cells, GK promotes glucose phosphorylation; the phosphorylated glucose then produces lactic acid through glycolysis, which itself participates in the tricarboxylic acid cycle (TAC), or is converted into glycogen and stored in hepatocytes (Garcia et al. 2001).
Therefore, the activities of PGM, UGP, PGI, UGD, and GK the enzymes potentially involved in the production of precursors for polysaccharide biosynthesis in C. militaris have a close relationship with the production of Cordyceps polysaccharides. Although many investigators have reported the correlation of fermentation conditions with biomass and polysaccharide production of C. militaris (Kwon et al. 2009; Shih et al. 2007; Cui and Jia 2010), there are still few researches conducted on the mechanism analysis of the effect of fermentation conditions on the activities of relevant enzymes of polysaccharide biosynthesis. The objectives of this work were to investigate the effects of different fermentation times, different types of carbon source, nitrogen source, inorganic salt, different initial pH value, and temperature on the intracellular polysaccharide production and the activities of enzymes involved in metabolite synthesis of C. militaris.
Materials and methods
Fungi strain
Cordyceps militaris (CICC14015) were obtained from the China Center of Industrial Culture Collection (CICC), and stored in the Key Laboratory of Food Nutrition and Safety (Ministry of Education, China), College of Food Science and Biotechnology (Tianjin University of Science and Technology, Tianjin, China).
Media and fermentation conditions
The strain of C. militaris was maintained on potato dextrose agar (PDA) slant, it was stored at 4 °C after the slant was incubated at 25 °C for 7 days before was transferred to the seed culture medium by punching out about 5-mm diameter agar discs from a culture grown on PDA plates.
The seed culture was grown in a 250-mL flask containing 100 mL of seed culture medium (glucose 20 g L−1, peptone 10 g L−1, KH2PO4 1 g L−1, MgSO4 · 7 H2O 1 g L−1, distilled water, initially natural pH) at 25 °C on a rotary shaker incubator at 150 rpm for 3 days.
The flask culture experiments were set in a 250-mL flask containing 100 mL of the basal culture medium (glucose 40 g L−1, peptone 10 g L−1, KH2PO4 0.5 g L−1, MgSO4 · 7 H2O 0.5 g L−1, distilled water, initially natural pH) after being inoculated with 8 % (v/v) of the seed liquid. All experiments were performed in triplicate to ensure the reproducibility.
Extraction and measurement of intracellular polysaccharides
The cultured mycelia were separated from the liquid medium by centrifugation at 5000×g for 15 min. After repeated washing with distilled water, the mycelia were dried at 60 °C for a sufficient time to a constant weight. Intracellular polysaccharides (IPS) were extracted from dried mycelia power (400 mg) by suspending the mycelia in 8 mL distilled water at 80 °C for 2 h, and then repeating for two times. The extracts were gathered in one container and added with four volumes of 95 % (v/v) ethanol to overnight at 4 °C and centrifuged at 4500×g for 20 min to collect the precipitate. The crude polysaccharides used for measuring the polysaccharide yield were obtained from freeze drying. The sugar content was measured by a phenol sulfuric acid method (Dubois et al. 1956).
Enzyme extract
Mycelia was washed twice with cell extract buffer (pre-cooled at 4 °C) [20 mM potassium phosphate buffer: 0.425 % (m/v) KH2PO4, 0.0292 % (m/v) NaCl, 0.0952 % (m/v) MgCl2, 0.0154 % (m/v) DTT, pH 6.5], then it was putted in the mortar (pre-cooled at 4 °C) and grinded three times with liquid nitrogen. If not immediately processed, biomass was stored at −80 °C. 100 mg of mycelia power was suspended in 1.0 mL of 20 mM potassium phosphate buffer followed by centrifugation at 12,000×g for 20 min at 4 °C, and the supernatant was stored at −80 °C and used as a source of enzymes.
Enzymes assays
All in vitro enzyme assays were performed in a volume of 250 μL at 30 °C in a α-Heλios spectrophotometer (Thermoespectronic), and the formation or disappearance of NAD(P)H was monitored by measuring the absorbance at 340 nm (ε340 = 6.22 M−1 cm−1). The activities of the enzymes described below were expressed in nmol NAD(P)H (mg protein)−1 min−1. The protein content of the extracts was determined using the Bradford method (Bradford 1976) and was compared with a bovine serum albumin standard. All enzyme activity measurements were repeated three times.
The phosphoglucomutase (PGM) reaction mixture contained 50 mM of Triethanolamine buffer (pH 7.2), 5 mM MgCl2, 0.4 mM NADP, 0.05 μmol glucose-1,6-bisphosphatase, 50 μM glucose-6-phosphate, 4 U glucose-6-phosphate dehydrogenase, and 30 μL cell-free extracts. The reaction was started by adding 1.4 mM of glucose-1-phosphate (Qian et al. 1994).
The UDP-glucose pyrophosphorylase (UGP) forward reaction mixture contained 50 mM Tris–HCl (pH 7.8), 14 mM MgCl2, 0.3 mM NADP, 0.1 mM UDP-glucose, 2.1 U phosphoglucomutase, 4 U glucose-6-phosphate dehydrogenase, and 30 μL cell-free extracts. The reaction was started by adding 4 mM of inorganic pyrophosphate (Martínez et al. 2011).
The phosphoglucose isomerase (PGI) reverse reaction contained 50 mM potassium phosphate buffer (pH 6.8), 5 mM MgCl2, 0.4 mM NADP, 4 U glucose-6-phosphate dehydrogenase, and 30 μL cell-free extracts. The reaction was started by adding 5 mM fructose-6-phosphate (Grobben et al. 1996).
The reaction mixture for the glucokinase (GK) assay contained 100 mM Tris–HCl (pH 7.5), 5 mM MgCl2, 0.2 mM NADP, 0.2 U glucose-6-phosphate dehydrogenase, and 5 M ATP. The reaction was started by adding a cell-free extract (Petit et al. 1991).
The stated mixture for UDPG-dehydrogenase (UGD) in the assay contained 100 mM Tris–HCl (pH 7.5), 5 mM MgCl2, 1 mM NADP, 5 mM UDP-glucose, and 1 mM DTT. The reaction was started by adding a cell-free extract (Schiller et al. 1973).
Total RNA extraction
Total RNA of about 100 mg of fresh cells was ground into a fine powder and transferred into a 1.5-mL Eppendorf tube, and then 1 mL of Trizol solution (Invitrogen, Carlsbad, CA, USA) was added and rested for 30 s at room temperature followed by centrifugation at 12,000×g for 5 min at 4 °C. The supernatant was placed on the clean tube mixed with 200 μL chloroform and agitated vigorously, then rested for 30 s at room temperature followed by centrifugation at 12,000×g for 15 min at 4 °C. The supernatant was then mixed with 0.5 volume of chilled isopropanol and incubated at −20 °C for 20 min. The precipitated RNA was collected from the supernatant by centrifugation at 12,000×g, 4 °C for 20 min. The sediment was washed with of 75 % (v/v) ethanol and air-dried for about 10 min. The RNA was dissolved in 30 μL of DEPC treated water and stored at −80 °C. Total RNA solution was loaded on 1.2 % agarose gel to assess the integrity of total RNA bands. The DNA maker was DM 2000 (CWbio. Co. Ltd).
Primer designing and RT-PCR analysis
The transcriptional analysis of those three genes, i.e., pgm (encoding phosphoglucomutase), ugp (encoding UDPG-pyrophosphorylase), and pgi (encoding phosphoglucose isomerase) was carried out by real-time quantitative PCR (qRT-PCR) using Bio-Rad IQ5 (Bio-Rad, American). Specific primers were showed in Table 1. The cycling condition was 10 min at 95 °C, then 40 cycles of 5 s at 95 °C, 30 s at optimal annealing temperature 55 °C, followed by 81 cycles of 10 s at 55 ∼ 95 °C. The expression levels of different genes were normalized against GAPDH (as the internal control gene). For each gene, the reference sample (the basal group at each harvest time) was defined as the expression level 1.0, and results of the other samples were expressed as the fold of the messenger RNA (mRNA) level over the reference sample. The 2-△△Ct method was applied to analyze the qRT-PCR data (Livak and Schmittgen 2001).
Statistical analysis
All data obtained in this work were on a three-time basis, and the error bars indicated the corresponding standard deviation (SD).
Results
Effects of fermentation time on enzyme activities and IPS production
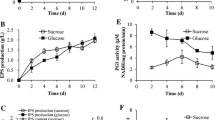
In order to identify optimal fermentation time of C. militaris, the effects of fermentation time on IPS production and the activity of enzymes involved in the pathway of IPS biosynthesis were studied at first. C. militaris cells were cultured in the basal medium for 9 days. Certain figures were calculated at the end of each day. The fermentations proceeded without pH control at 25 °C. The maximum production of IPS was obtained when cells were grown for 5 days. These results did not match up with the results of other investigators (Shih et al. 2010; Shih et al. 2007). Figure 2 also shows the profiles of the activity levels of PGI, UGP, UGD, PGM, and GK measured along different time points of the fermentation. The five enzymes all exhibited un-conspicuous activities during the incubation period (1–3 days). During the exponential growth phase (3–5 days), however, there was a sharp increase of PGM, UGP, and PGI activities, while a slight increase of UGD and GK activities was detected. In addition, the profile of the activities of the five enzymes remained similar in growth and decline trends with the IPS production profile during 4–6 days, and PGM, UGP, and PGI activities were obviously higher than that of UGD and GK. It indicated that there was a close relationship between the activities of PGM, UGP, and PGI with IPS biosynthesis, and the order of correlation was PGM > UGP > PGI. Entering the decline phase from the 6th day, the IPS production was falling and the activities of the five enzymes exhibited a similar state with the incubation period. Therefore, the 5th day was taken as the optimal fermentation time.
Effects of carbon source on enzyme activities and IPS production
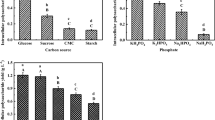
Carbohydrates provide an important nutrient and energy source for the growth and synthesis of secondary metabolites of higher fungi. To study the influence of different carbon sources on IPS production and the activity of enzymes involved in the pathway of IPS biosynthesis by C. militaris, the glucose component of basal medium was separately replaced by various carbon sources (such as sucrose, soluble starch, lactose, and soft sugar) at a concentration of 40 g L−1. The profiles of IPS production and enzyme activities were showed in Fig. 3a. The highest IPS production was observed in the medium containing glucose as a carbon source, and the lowest parameter was observed in the medium that contained soluble starch. This result was in accordance with the nutritional requirements of other species of fungi in submerged cultures. Duan et al. (2008) reported that glucose was the best carbon source for polysaccharide production by A. pullulans Y68. The activities of five enzymes had similar trend with IPS production, and the PGM obtained the maximum activity on glucose-grown medium, followed by PGI and UGD. However, compared to bacteria, the activity of PGM was higher on maltose-grown cultures than that on glucose or fructose (Velasco et al. 2007). Activity levels of UGD and GK were found irrespective of the carbon source, and they had no relationship with the synthesis of IPS. In conclusion, the higher level of IPS production and PGM activity of C. militaris were detected on the glucose-grown medium.
Effects of different carbon sources (a), nitrogen sources (b) and inorganic salts (c) on enzyme activities and IPS production in shake flask cultures of C. militaris. The initial carbon source, nitrogen source and inorganic salt concentrations in the media were 40 g L−1, 10 g L−1, and 0.5 g L−1, respectively. Data are given as means ± SD, n = 3
Effects of nitrogen source on enzyme activities and IPS production
Nitrogen is an essential component of proteins and nucleic acids, and nitrogen deficiency impairs fungal growth and metabolite production (Papagianni 2004). The effect of different nitrogen sources on enzyme activities and IPS production by C. militaris were shown in Fig. 3b. The highest IPS production (159.09 ± 4.12 mg/100 mL) and the highest activities of PGM, PGI and UGP [857, 550, and 281 nmol NAD(P)H (mg protein)−1 min−1, respectively] were observed in the media containing beef extract as a nitrogen source. Protein, although used as a universal nitrogen source for polysaccharide production, was proved as not the best nitrogen source. In terms of metabolite synthesis, the production of polysaccharides is higher with organic nitrogen sources than the inorganic sources (ammonium and nitrate). This was consistent with the previously suggested results of C. militaris growth in submerged cultures (Kim et al. 2003). Some other Cordyceps species obtained similar results (Kim et al. 2002; Xiao et al. 2004). These results clearly showed that the type of nitrogen source in the culture medium affected IPS production and activity of the five enzymes. In addition, the activity of PGM, UGP, and PGI were highly correlated with the amount of IPS produced. This result coincided with the previous reports of other fungi (Jiang et al. 2011). Therefore, beef extract was selected for further study.
Effects of inorganic salt on enzyme activities and IPS production
In addition to carbon and nitrogen sources, very low concentrations of some compounds including vitamins, fatty acids, and inorganic salt are known to stimulate fungal cell growth and metabolite formation (Xiao et al. 2010). Hence, various inorganic salts (ZnSO4, FeSO4, CaCO3, and CaCl2) were applied separately in cultivation medium to observe their effects on IPS production and enzyme activities of C. militaris. As shown in Fig. 3c, the type of inorganic salt had different effects on IPS biosynthesis. CaCO3 and CaCl2 addition obtained a similar and higher IPS production, while ZnSO4 and FeSO4 had lower impacts on IPS accumulation. The results suggested that SO4 2− may not be a stimulatory factor to enhance the metabolite production, which was similar to the report on the biosynthesis of cordycepin from C. militaris (Fan et al. 2012).
Effects of initial pH on enzyme activities and IPS production
The medium pH has been known to have a significant impact on the uptake of various nutrients and product biosynthesis (Yang et al. 2014) in all the environmental factors. As shown in Fig. 4a, the highest IPS production (316.15 ± 7.92 mg/100 mL) was obtained when the initial pH value of fermentation medium was 7.0, and the activities of PGM, PGI, and UGP were all higher than other samples of pH group. The activities of UGD and GK did not change in all the fermentation media of different initial pH values. This indicated that the activities of PGM, PGI, and UGP were affected by initial pH in submerged fermentation. Since the IPS production was highly correlated with these enzyme activities, IPS can be most efficiently synthesized when pH = 7.0. Another report (Fan et al. 2012) claimed that the medium pH was decreased during the period of fermentation, possibly because of the continuous consumption of carbon and nitrogen sources which would produce lots of acidic metabolites. The control of pH could improve metabolite content.
Effect of temperature on enzyme activities and IPS production
Using IPS production as maker, we tested three different fermentation temperatures (20, 25, and 30 °C) in shake flask cultures (Fig. 4b). The IPS obtained the highest production when the culture temperature was 25 °C. This result was coincided with the report in the literature that the best cultivation temperature for Cordyceps mycelial in submerged fermentation culture medium appeared to be 25 °C (Lin and Chiang 2008). The activities of PGM, PGI, and UGP reached the highest at the same culture temperature. However, the activities of UGD and GK which have no relationship with the synthesis of IPS and were not affected by different temperatures in the shake flask cultures.
The results indicated that the fermentation conditions had influences on the biosynthesis of intracellular polysaccharide and activities of some key enzymes involved in the polysaccharide biosynthetic pathway. The maximum IPS production was obtained on day 5, and the optimal carbon source, nitrogen source, inorganic salt, initial pH and temperature were glucose, beef extract, CaCO3, 7 and 25 °C, respectively. The PGM, UGP, and PGI were highly correlated with IPS biosynthesis.
Transcription levels of mRNA
The quality of extracted total RNA described in this study was judged in 1.2 % agarose gel electrophoresis. Figure 5 shows that 28S and 18S bands were intact and bright, which demonstrated that the total RNA, extracted from mycelium of the basal medium and the optimal medium, respectively, was not degraded.
To gain insight into the probable mechanism underlying the enhanced IPS production under optimal culture medium, the transcriptional levels of three genes, i.e., pgm (encoding phosphoglucomutase), ugp (encoding UDPG-pyrophosphorylase), and pgi (encoding phosphoglucose isomerase) were investigated by RT-PCR with samples grown in the optimal medium and the basal medium, respectively.
As shown in Fig. 6, the amplification curve of pgm gene (Fig. 6a), ugp gene (Fig. 6b), pgi gene (Fig. 6c), and GAPDH (Fig. 6d) were all smooth. It indicated that the primer of all genes could be used for RT-PCR, and the results were accurate and reliable. The melting curves of pgm gene (Fig. 6e), ugp gene (Fig. 6f), pgi gene (Fig. 6g), and GAPDH (Fig. 6h) all had a single peak. It indicated that all of them had the specific amplification of target products with good repeatability.
In order to determine the accuracy and repeatability of the real-time PCR reaction, all the reactions of these samples were repeated three times. The standard deviations (SD) of the Ct values were 0.03∼ 0.11 (Table 2). This indicated that the RT-PCR reaction for investigate gene expression differences had strong accuracy and repeatability. The relative quantitative results of the above genes in the optimal medium and the basal medium could be calculated by relative quantitative calculation formula 2-△△ct, the results were showed in Table 3. In the case of optimal group, all the three genes were highly expressed compared with the three genes of basal group. The expression levels of the pgm gene, ugp gene, and pgi gene of the optimal group were at around 2.094, 1.997, and 1.427 times compared to the basal group. This suggested that the fermentation conditions had a great influence on the transcription level of pgm gene, ugp gene, and pgi gene, which encoding PGM, UGP, and PGI, respectively. It indicated that all of the three enzymes were the key enzymes of the pathway of polysaccharide biosynthesis. In addition, the expression level of the pgm gene was the highest among the three genes of the optimal group, and it was 1.049 and 1.467 times compared to ugp gene and pgi gene, respectively. But because of a lack of similar works in the literature, we could not compare these results. The results implied that the pgm gene played a highly critical role in polysaccharide biosynthesis pathway, and the PGM was the highly key enzyme in this pathway.
Discussion
In the last decade, various efforts have been devoted to enhance further the bioactive composition production in C. militaris (Xu et al. 2002; Cui and Jia 2010; Shih et al. 2007). These efforts primarily focus upon the optimal submerged culture conditions for production of polysaccharides from C. militaris. There is no report of the relationship between fermentation conditions and activities of related enzymes involved in intracellular polysaccharides (IPS) biosynthesis of C. militaris. Therefore, the influence of different fermentation conditions on the production of IPS and activities of enzymes involved in pathway of polysaccharide synthesis of C. militaris in a liquid-submerged culture was explored in this work.
Our results show that the polysaccharide biosynthesis was significantly influenced by the nutritional requirements and environmental conditions in submerged cultures of C. militaris. It is in agreement with the previous report that culture media (carbon and nitrogen sources, metal ions, and duration of fermentation) are directly linked with cell proliferation and metabolite biosynthesis (Cui and Yuan 2011; Mao et al. 2005; Park et al. 2004). Moreover, the control of environmental conditions or the modification of media composition would be vital to enhance the production efficiency of polysaccharides (Yang et al. 2000; Yang and Liau 1998). The maximum IPS production (327.57 ± 6.27 mg/100 mL) was obtained on day 5 in the optimal medium containing glucose (40 g · L−1), beef extract (10 g · L−1), and CaCO3 (0.5 g · L−1), and the initial pH and temperature were 7 and 25 °C, respectively. Furthermore, all the fermentation conditions also significantly affect the activities of PGM, UGP, and PGI, while had slight relationship with the activities of UGD and GK. These results were in agreement with the previous found (Pan et al. 2013) that the controlled pH, type and concentration of nitrogen source, and fermentation time all affected the activity of UDPG-pyrophosphorylase and polysaccharide production. The results demonstrated that the higher activities of PGM, UGP, and PGI were desirable for the biosynthesis rate of IPS. It is similar to the report (Degeest and Vuyst 2000) that the levels of activity of a-phosphoglucomutase and UDP-glucose pyrophosphorylase were highly correlated with the amount of polysaccharide produced.
Previous studies indicated that the expression of genes is highly dependent on cultivation condition (Rachmawati et al. 2013). Therefore, the transcription levels of synthetic genes pgm, ugp, and pgi (encoding PGM, UGP, and PGI, respectively) were also achieved. Upregulation of pgm gene, ugp gene, and pgi gene transcription levels were noticed in the optimal medium compared to the basal medium. It could be concluded that pgm gene, ugp gene, and pgi gene were the key genes in C. militaris could regulate the polysaccharide biosynthesis. In addition, the expression level of pgm gene was higher than that of ugp gene and pgi gene in the optimal medium. It implied that pgm gene may be the highly key gene to control the biosynthesis of intracellular polysaccharide of C. militaris in a liquid-submerged culture. It also indicated that PGM might be the highly key enzyme to regulate the intracellular polysaccharide biosynthesis of C. militaris. This result was in agreement with the report (Degeest and Vuyst 2000) that PGM might play a controlling role in the flux from glucose 6-phosphate to polysaccharide biosynthesis by Streptococcus thermophilus LY03, an opposite conclusion was drawn from the report (Levander and Radstrom 2001) that PGM activity had no significant effect on exopolysaccharide production in glucose-growth medium of the same strain. In addition, it also similar to the investigative result of other higher fungus (Tang and Zhong 2002) that there exists a positive correlation between PGM activity and polysaccharide biosynthesis by Ganoderma lucidum. Overall, the information obtained in this study may be helpful to the further manipulation of the cell cultivation as well as to the molecular mechanism studies of polysaccharide biosynthesis by C. militaris.
References
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen C, Luo SS, Li Y, Sun YJ, Zhang CK (2004) Study on antioxidant activity of three Cordyceps sp. by chemiluminescence. Shanghai J Tradit Chin Med 38(7):53–55
Choi SB, Park CH, Choi MK, Jun DW, Park S (2004) Improvement of insulin resistance and insulin secretion by water extracts of Cordyceps militaris, Phellinus linteus, and Paecilomyces tenuipes in 90% pancreatectomized rats. Biosci Biotech Bioch 68:2257–2264
Cui JD (2015) Biotechnological production and applications of Cordyceps militaris, a valued traditional Chinese medicine. Crit Rev Biotechnol 35(4):475–484
Cui JD, Jia SR (2010) Optimization of medium on exopolysaccharides production in submerged culture of Cordyceps militaris [J]. Food Sci Biotechnol 19(6):1567–1571
Cui JD, Yuan LQ (2011) Optimization of culture conditions on mycelial grown in submerged culture of Cordyceps militaris. Int J Food Eng 7:1–11
Cui JD, Zhang YN (2012) Evaluation of metal ions and surfactants effect on cell growth and exopolysaccharide production in two-stage submerged culture of Cordyceps militaris. Appl Biochem Biotechnol 168:1394–1404
Daran JM, Dallies N, Thines-Sempoux D, Paquet V, Francois J (1995) Genetic and biochemical characterization of the UGP1 gene encoding the UDP-glucose pyrophosphorylase from Saccharomyces cerevisiae. Eur J Biochem 233:520–530
Degeest B, Vuyst LD (2000) Correlation of activities of the enzymes a-phosphoglucomutase, UDP-galactose 4-epimerase and UDP-pyrophosphorylase with exopolysaccharide biosynthesis by Streptococcus thermophilus LY03. Appl Environ Microb 66:3519–3527
Delattre C, Vijayalaksmi MA (2009) Monolith enzymatic microreactor at the frontier of glycomic toward a new route for the production of bioactive oligosaccharides. J Mol Catal B Enzym 60(3–4):97–105
Delattre C, Pierre G, Gardarin C, Traikia M, Eiboutachfaiti R, Isogai A, Michaud P (2015) Antioxidant activities of a polyglucuronic acid sodium salt obtained from TEMPO-mediated oxidation of xanthan. Carbohyd Polym 116:34–41
Dong JZ, Lei C, Ai XR, Wang Y (2012) Selenium enrichment on Cordyceps militaris Link and analysis on its main active components. Appl Biochem Biotechnol 166:1215–1224
Duan XH, Chi ZM, Wang L, Wang XH (2008) Influence of different sugars on pullulan production and activities of α-phosphoglucose mutase, UDPG-pyrophosphorylase and glucosyltransferase involved in pullulan synthesis in Aureobasidium pullulans Y68. Carbohyd Polym 73:587–593
Dubois M, Gilles KA, Hamilton JK (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–353
Elisavet K, George E, Diomi M, Evangelos T, Dimitris G, Paul C (2011) Constitutive expression, purification and characterization of a phosphoglucomutase from Fusarium oxysporum. Enzyme Microb Tech 48:217–224
Fan DD, Wang W, Zhong JJ (2012) Enhancement of cordycepin production in submerged cultures of Cordyceps militaris by addition of ferrous sulfate. Biochem Eng J 60:30–35
Garcia-Rocha M, Roca A, Iglesia NDL, Baba O, Fernandez-Novell JM, Ferrer JC (2001) Intracellular distribution of glycogen synthase and glycogen in primary cultured rat hepatocytes. Biochem J 357:17–24
Grobben GJ, Smith MR, Sikkema J, De Bont JAM (1996) Influence of fructose and glucose on the production of exopolysaccharides and the activities of enzymes involved in the sugar metabolism and the synthesis of sugar nucleotides in Lactobacillus delbrueckii subsp. bulgaricus NCFB 2772. Appl Microbiol Biot 46:279–284
Hou AI, Meng QF, An JS, Zhu K, Feng Y, Teng LR (2008) Isolation and purification of polysaccharides from Cordyceps minlitaris and its inhibition on the proliferation of rat glomerular mesangial cells. Chem Res Chinese U 5(5):584–587
Huang L, Li QZ, Chen YY, Wang XF, Zhou XW (2009) Determination and analysis of cordycepin and adenosine in the products of Cordyceps spp. Afr J Microbiol Res 3(12):957–961
Jiang LF, Wu SJ, Kim JM (2011) Effect of different nitrogen sources on activities of UDPG-pyrophosphorylase involved in pullulan synthesis and pullulan production by Aureobasidium pullulans. Carbohyd Polym 86:1085–1088
Kim SW, Hwang HJ, Xu CP, Na YS, Song SK, Yun JW (2002) Influence of nutritional conditions on mycelial growth and exopolysaccharide production in Paecilomyces sinclairii. Lett Appl Microbiol 34:389–393
Kim SW, Hwang HJ, Xu CP, Sung JM, Choi JW, Yun JW (2003) Optimization of submerged culture process for the production of mycelial biomass and exo-polysaccharides by Cordyceps militaris C738. J Appl Microbiol 94:120–126
Kothari D, Delatter C, Goyal A (2015) Bioactive isomalto-oligosaccharides synthesized from leuconostoc mesenteroides nrrl B-1426 dextransucrase with colon cancer cells inhibiting and functional food additive properties. Int J Food Nutr Sci 4(4):37–46
Kwon JS, Lee JS, Shin WC, Lee KE, Hong EK (2009) Optimization of culture conditions and medium components for the production of mycelial biomass and Exo-polysaccharides with Cordyceps militaris in liquid culture. Biotechnol Bioproc E 14:756–762
Lee JS, Hong EK (2011) Immunostimulating activity of the polysaccharides isolated from Cordyceps militaris [J]. Int J Immunopath Ph 11:1226–1233
Levander F, Radstrom P (2001) Requirement for phosphoglucomutase in exopolysaccharide biosynthesis in glucose- and lactose-utilizing Streptococcus thermophilus. Appl Environ Microbiol 67(6):2734–2738
Lin YW, Chiang BH (2008) Anti-tumor activity of the fermentation broth of Cordyceps militaris cultured in the medium of Radix astragali. Process Boichem 43:244–250
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using realtime quantitative PCR and the 2-△△Ct method. Methods 25:402–408
Mao XB, Eksriwong T, Chauvatcharin S, Zhong JJ (2005) Optimization of carbon source/nitrogen ratio for cordycepin production by submerged cultivation of medicinal mushroom Cordyceps militaris. Process Biochem 40:1667–1672
Martínez LI, Piattoni CV, Garay SA, Rodrígues DE, Guerrero SA, Iglesias AA (2011) Redox regulation of UDP-glucose pyrophosphorylase from Entamoeba histolytica. Biochimie 93:260–268
Mellal M, Jaffrin MY, Ding LH, Delattre C, Michaud P, Courtois J (2008) Separation of oligoglucuronans of low degrees of polymerization by using a high shear rotating disk filtration module. Sep Purif Technol 60(1):22–29
Methacanon P, Madla S, Kirtikara K, Prasitsil M (2005) Structural elucidation of bioactive fungi-derived polymers. Carbohyd Polym 60:199–203
Pan SK, Yao DR, Chen J, Wu SJ (2013) Influence of controlled pH on the activity of UDPG-pyrophosphorylase in Aureobasidium pullulans. Carbohyd Polym 92:629–632
Papagianni M (2004) Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol Adv 22:189–259
Park JP, Kim SW, Hwang HJ (2001) Optimization of submerged culture conditions for the mycelia growth and exo-biopolymer production by Cordyceps militaris [J]. Lett Appl Microbiol 33:76–81
Park JP, Kim SW, Hwang HJ, Cho YJ, Yun JW (2002) Stimulatory effect of plant oils and fatty acids on the exo-biopolymer production in Cordyceps militaris. Enzyme MicrobTech 31:250–255
Park JP, Kim SW, Hwang HJ, Yun JW (2004) Optimization of submerged culture conditions for the mycelial growth and exobiopolymer. Process Biochem 39:2241–2247
Park SE, Kim J, Lee YW (2009) Antitumor activity of water extracts from Cordyceps militaris in NCI-H460 cell xenografted nude mice [J]. J Acupunct Meridian Stud 2:294–300
Paterson RR (2008) Cordyceps: a traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry 69(7):1469–1495
Petit C, Grill JP, Maazouzi N, Marczak R (1991) Regulation of polysaccharide formation by Streptococcus thermophilus in batch and fed-batch cultures. Appl Microbiol Biot 36:216–221
Qian N, Stanley GA, Hahn-Hägerdal B, Rådström P (1994) Purification and characterisation of two phosphoglucomutases from Lactococcus lactis subsp. lactis and their regulation in maltose- and glucose-utilizing cells. J Bacteriol 176:5304–5311
Rachmawati R, Kinoshita H, Nihira T (2013) Establishment of transformation system in Cordyceps militaris by using integration vector with benomyl resistance gene. Procedia Environ Sci 17:142–149
Saad N, Delattre C, Urdaci M, Schmitter JM, Bressollier P (2013) An overview of the last advances in probiotic and prebiotic field. LWT-Food Sci Technol 50(1):1–16
Schiller JG, Bowser AM, David SF (1973) Partial purification and properties of UDPG dehydrogenase from Escherichia coli. BBA- Enzymology 293(1):1–10
Shih IL, Tsai KL, Hsieh C (2007) Effects of culture conditions on the mycelial growth and bioactive metabolite production in submerged culture of Cordyceps militaris. Biochem Eng J 33:193–201
Shih I, Chang S, Chen Y (2010) Cultivation of Cordyceps militaris in solid and liquid culture. J Am Diet Assoc 110(9):A51
Shingel KI (2004) Current knowledge on biosynthesis, biological activity, and chemical modification of the exopolysaccharide pullulan. Carbohyd Res 339:447–460
Tang YJ, Zhong JJ (2002) Exopolysaccharide biosynthesis and related enzyme activities of the medicinal fungus, Ganoderma lucidum, grown on lactose in a bioreactor. Biotechnol Lett 24:1023–1026
Tavernier ML, Petit E, Delattre C, Courtois B, Courtois J, Strancar A, Michaud P (2008) Production of oligoglucuronans using a monolithic enzymatic microreactor. Carbohyd Res 343(15):2687–2691
Velasco SE, Yebra MJ, Monedero V, Ibarburu I, Dueñas MT, Irastorza A (2007) Influence of the carbohydrate source on β-glucan production and enzyme activities involve d in sugar metabolism in Pediococcus parvulus 2.6. Int J Food Microbiol 115:325–334
Won SY, Park EH (2005) Anti-inflammatory and related pharmacological activities of cultured mycelia and fruiting bodies of Cordyceps militaris. J Ethnopharmacol 96:555–561
Xiao JH, Chen DX, Liu JW, Liu ZL, Wan WH, Fang N, Xiao Y, Qi Y, Liang ZQ (2004) Optimization of submerged culture requirements for the production of mycelial growth and exo-polysaccharide by Cordyceps jiangxiensis JXPJ 0109. J Appl Microbiol 96:1105–1116
Xiao JH, Xiao DM, Xiong Q, Liang ZQ, Zhong JJ (2010) Nutritional requirements for the hyperproduction of bioactive exopolysaccharides by submerged fermentation of the edible medicinal fungus Cordyceps taii. Biochem Eng J 49:241–249
Xu CP, Kim SW, Hwang HJ, Yun JW (2002) Application of statistically based experimental designs for the optimization of exopolysaccharide production by Cordyceps militaris NG3. Biotechnol Appl Biochem 36:127–131
Yang FC, Liau CB (1998) The influence of environmental conditions on polysaccharide formation by Ganoderma lucidum in submerged cultures. Process Biochem 33(5):547–553
Yang L, Zhang L (2009) Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohyd Polym 76:349–361
Yang FC, Ke YF, Kuo SS (2000) Effect of fatty acids on the mycelial growth and polysaccharide formation by Ganoderma lucidum in shake flask cultures. Enzyme Microb Tech 27:295–301
Yang S, Jin L, Ren XD, Lu JH, Meng QF (2014) Optimization of fermentation process of Cordyceps militaris and antitumor activities of polysaccharides in vitro. J Food Drug Anal 22(4):468–476
Yu R, Yang W, Song L (2007) Structural characterization and antioxidant activity of a polysaccharide from the fruiting bodies of cultured Cordyceps militaris [J]. Carbohyd Polym 70:430–436
Zhang M, Cui SW, Cheung PCK, Wang Q (2007) Antitumor polysaccharides from mushrooms: a review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci Tech 18:4–19
Zheng P, Xia YL, Xiao GH, Xiong CH, Hu X, Zhang SW, Zheng HJ, Huang Y, Zhou Y (2011) Genome sequence of the insect pathogenic fungus Cordyceps militaris, a valued traditional Chinese medicine. Genome Biol 12(11):287–302
Zhou X, Gong Z, Su Y, Lin J, Tang K (2009) Cordyceps fungi: natural products, pharmacological functions and developmental products. J Pharm Pharmacol 61:279–291
Acknowledgments
This work was financially supported by the National Spark Key Program of China (2015GA610001), the Foundation of Tianjin University of Science and Technology (Nos. 20120106), the International Science and Technology Cooperation Program of China (2013DFA31160), and the Foundation of Tianjin Educational Committee (20090604).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Zhu, ZY., Liu, XC., Dong, FY. et al. Influence of fermentation conditions on polysaccharide production and the activities of enzymes involved in the polysaccharide synthesis of Cordyceps militaris . Appl Microbiol Biotechnol 100, 3909–3921 (2016). https://doi.org/10.1007/s00253-015-7235-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-7235-4