Abstract
The consumption of fiber-rich foods such as cereal bran is highly recommended due to its beneficial health effects. Pre-fermentation of bran with lactic acid bacteria can be used to improve the otherwise impaired flavor and textural qualities of bran-rich products. These positive effects are attributed to enzymatic modification of bran components and the production of functional metabolites like organic acids and exopolysaccharides such as dextrans. The aim of this study was to investigate dextran production in wheat and rye bran by fermentation with two Weissella confusa strains. Bran raw materials were analyzed for their chemical compositions and mineral content. Microbial growth and acidification kinetics were determined from the fermentations. Both strains produced more dextran in rye bran in which the fermentation-induced acidification was slower and the acidification lag phase longer than in wheat bran. Higher dextran production in rye bran is expected to be due to the longer period of optimal pH for dextran synthesis during fermentation. The starch content of wheat bran was higher, which may promote isomaltooligosaccharide formation at the expense of dextran production. W. confusa Cab3 produced slightly higher amounts of dextran than W. confusa VTT E-90392 in all raw materials. Fermentation with W. confusa Cab3 also resulted in lower residual fructose content which has technological relevance. The results indicate that wheat and particularly rye bran are promising matrices for producing technologically significant amounts of dextran, which facilitates the use of nutritionally valuable raw bran in food applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The current consumer demand for healthy and high-quality food products has evoked an increasing interest in the utilization of nutritionally rich cereal ingredients such as cereal bran or whole grain products in human nutrition. In the recent years, especially wheat and rye bran have been in the focus due to their nutritional properties (dietary fiber, good quality proteins, source of minerals, vitamins, and other phytochemicals) and also due to technological challenges in the utilization of bran (negative influences on dough rheology, texture, and sensory quality) (Prinsen et al. 2014; Bakke and Vickers 2007; Poutanen et al. 2009). Wheat bran is the major side stream generated in the milling of wheat grains with an estimated annual global production of 90 million tons (Reisinger et al. 2014). Currently, wheat bran has been used as a low-value ingredient for both human consumption and animal feed, but interest in its use in human diet is increasing (Coda et al. 2015; Katina et al. 2012; Swennen et al. 2006). Rye is often used as whole grain flour in cereal products. Nonetheless, rye bran is also a byproduct of conventional milling and is therefore commercially available in large quantities and can be used as a nutritional value-enhancing ingredient (Nordlund et al. 2012). Despite the increasing evidence on healthiness of whole grain and fiber-rich cereal products, consumers tend to prefer cereal products which are made from refined white flours. This is mainly due to the favorable textural and flavor properties of refined cereal products compared to the whole grain or bran-enriched alternatives (Bakke and Vickers 2007). In bread baking, the negative technological effects of bran supplementation are derived from its influence on the starch-gluten matrix. The bran decreases gluten hydration and subsequently disrupts the gluten network formation, which negatively affects the structure of the wheat dough (Laurikainen et al. 1998; Rosell et al. 2006).
Sourdough utilization improves bread texture and prevents staling and has therefore been a prominent part of traditional whole grain baking. These positive effects are attributed to enzymatic modification of the dough components during fermentation and the production of functional metabolites such as organic acids and exopolysaccharides (EPS) (Arendt et al. 2007). Together with other sourdough lactic acid bacteria (LAB) species, food-derived Weissella spp. have gained attention especially due to their ability to produce technologically significant amounts of EPS, particularly dextran, which act as a hydrocolloid in the cereal systems. They also acidify sourdoughs to a lesser extent than conventional sourdough LAB species providing wider usability than conventional strongly acidifying LAB (Di Cagno et al. 2006; Katina et al. 2009; Wolter et al. 2014; Shukla et al. 2014). Dextran-producing LAB secrete dextransucrases (DSRs), which catalyze dextran synthesis from sucrose and are classified as a part of the glycoside hydrolase family 70 (EC 2.4.1.5) (Leemhuis et al. 2013; Patel et al. 2011). DSRs are also capable of transferring glucose residues from sucrose to other acceptors, such as oligosaccharides or other hydroxylated molecules resulting in oligosaccharides or glucoconjugates that may have physiological functionality (Monsan et al. 2001; Korakli and Vogel 2006; Monsan et al. 2010). Weissella spp. produce dextran comprising of mainly α-(1 → 6) linkages with a few α-(1 → 3) branch linkages (ca. 3 %) which has been found suitable for cereal applications (Fusco et al. 2015). Recently, we demonstrated dextran production in wheat bran with heterologously produced Weissella confusa DSR and that the obtained bioprocessed wheat bran significantly improved the quality of high-fiber wheat bread (Kajala et al. 2015).
The relationship between specific metabolic activities of sourdough cultures and product quality has been well-described in traditional wheat and rye sourdoughs. However, using the bran fraction of kernel as a substrate for fermentation, and especially for dextran production during in situ fermentation, has been less studied (Galle et al. 2010). In general, EPS formation and DSR activity in growth media or in sourdough have been reported to be stimulated by several factors, such as initial sucrose and glucose content, various mono- and divalent metal ions, initial pH and dough yield (DY) (Shukla and Goyal 2011; Shukla and Goyal 2012; Rühmkorf et al. 2012; Rühmkorf et al. 2013; Kaditzky et al. 2008). but few studies have been performed with bran matrix (Kaditzky and Vogel 2008; Kajala et al. 2015). Kaditzky and Vogel (2008) indicated that fermentation with Lactobacillus reuteri in rye bran with 10 % added sucrose (flour basis) is a promising method to obtain high glucan formation within 8 h of fermentation.
The aim of this work was to compare dextran production in wheat and rye bran during fermentation with two W. confusa strains isolated from two different raw materials and continents. The role of chemical composition of the bran and the growth and acidification kinetics of the starters in dextran production were established by using multivariate statistical methods, partial least squares (PLS) analysis.
Materials and methods
Microbial strains and growth media
Two dextran-producing LAB strains, W. confusa VTT E-90392 (E392, DSM 20194) and Cab3, isolated from carrot mash and fermented cabbage, were obtained from VTT Culture Collection and the Indian Institute of Technology Guwahati (Prof. Arun Goyal), respectively. The strains were routinely cultivated anaerobically in de Man-Rogosa-Sharpe (MRS) growth media (Oxoid, Basingstoke, UK) or MRS-S (MRS supplemented with 20 g/l sucrose) broth at 30 °C.
Raw materials
Brans were obtained from Fazer Group (Lahti, Finland). The particle size of wheat bran was 750 μm. Starch in the rye bran was reduced from 30 to 12 % by air classification as described by Nordlund et al. (2012) to obtain comparable dietary fiber and starch content for both brans. The chemical composition including total dietary fiber, insoluble and soluble fiber, total and soluble arabinoxylans, starch, fructans, and ash content of brans were measured as described by Nordlund et al. (2012).
Bran fermentations
Prior to bran fermentations, the strains were passaged twice in MRS-S. Cells were recovered from an overnight culture by centrifugation (3900×g, 20 min, 4 °C) and re-suspended in sterile tap water. Cell suspensions were used to inoculate bran fermentations at the density of 6.0 log colony-forming unit (cfu)/g.
Bran fermentations were carried out in a total weight of 600 g with or without sucrose supplementation. The fermented samples contained 480 g (80 % w/w) sterile tap water including the cell suspension and 120 g (20 % w/w) wheat bran (WB) or rye bran (RB). To study dextran production, 12 g of wheat bran and 6 g of rye bran in their respective samples were replaced with sucrose (WB-S and RB-S). Lower level of sucrose supplementation was selected for rye bran because the raw material was known from previous studies to contain more intrinsic sucrose. The exact sucrose amounts in the samples were analyzed and are shown in Table 4. The cell suspension, water, and sucrose were first mixed together and then the mixture was combined with the bran in a large beaker. The beaker was then covered with aluminum foil and incubated at 25 °C for 20 h. Samples for total titratable acidity (TTA), lactic and acetic acid, dextran, and sugar quantification were collected at 20 h, flash-frozen in liquid nitrogen, and stored at −20 °C until analyzed. Samples for dextran and sugar analysis were freeze-dried before analysis. All fermentations were performed in duplicate.
Microbiological analysis and kinetics of growth and acidification
Microbiological analysis of the samples was carried out at the beginning and at the end of fermentation. In detail, bran samples (10 g) were homogenized with 90 ml of sterile saline in a Stomacher 400 lab blender (Seward Medical, London). Serial dilutions were made and the enumeration of LAB was carried out by plating on MRS agar after incubation for 48 h at 30 ° C. Aerobic heterotrophic bacteria were determined from plate count agar (PCA, Difco Laboratories, Detroit, MI, USA). To prevent fungal growth, 0.001 % cycloheximide was added to the PCA plates. The growth of yeasts and molds was determined on yeast mold (YM) agar (Difco Laboratories). Chloramphenicol and chlortetracycline were added at 0.01 % to YM agar to prevent bacterial growth, and 0.02 % of Triton-X 100 was used to limit the spreading of fungal colonies. The plates were incubated as follows: MRS and MRS-S anaerobically at 30 °C for 2–3 days, and PCA and YM plates aerobically at 25 °C for 3–5 days.
To assess the kinetics of growth and acidification for the LAB, samples from fermenting bran were collected for viable count analysis and pH measurement every 2 h throughout the fermentation process. The kinetics were determined and modeled in agreement with the Gompertz equation as modified by Zwietering et al. (1990):
where y is the growth expressed as log cfu per gram per hour or the acidification rate expressed as ΔpH/Δt (units of pH/h) at the time t; k is the initial level of the dependent variable to be modeled (log cfu/g or pH units); A is the cell density or pH (units) variation (between inoculation and the stationary phase); μ max or v max is the maximum growth rate expressed as Δlog colony-forming unit per gram per hour or the maximum acidification rate expressed as ΔpH/h, respectively; λ is the length of the lag phase measured in hours. The experimental data were modeled by the non-linear regression procedure of the Statistica 8.0 software (Statsoft, Tulsa, USA).
The values of pH from acidification kinetics were determined on-line by a pH meter (Model HI 99161, Hanna Instruments, Woonsocket, RI, USA) with a food penetration probe. Final pH and TTA was determined with automatic titrator (EasyPlus Titrator, Mettler-Toledo, Schwerzenbach, Switzerland). A suspension of 10 g of fermented bran in 100 ml of distilled water was titrated with 0.1 M NaOH to a final pH of 8.5. TTA was expressed as the amount of NaOH used (ml). Lactic and acetic acids were determined from fermented bran with commercial enzymatic assay (Megazyme, Wicklow, Ireland). All samples were analyzed in duplicate.
Total and initial soluble mineral content
Bran total mineral contents were determined by inductively coupled plasma optical emission spectrometry (ICP-OES; Thermo Scientific iCAP 6000 Series ICP Spectrometer, MA, USA) method using a microwave system (CEM MARSXpress 5. NC, USA) to digest the samples.
Dry samples (0.5 g) were weighed into a digestion tube; after which, 5 ml concentrated HNO3 (Romil, SpA, Super Purity Acid, Cambridge, Great Britain) and 2 ml 30 % hydrogen peroxide (Merck, Empurre Iso, Darmstadt, Germany) were added. The samples were allowed to stand for 30 min at room temperature and digested in a microwave system at 1600 W power. The temperature was gradually increased to 170 °C and then kept at this temperature for 40 min. The tubes were then cooled overnight and the samples were transferred into the 50-ml volumetric flasks using fresh ultraclean water (Q-Pod Element, Millipore Corporation, MA, USA).
The mineral elements were analyzed by inductively coupled plasma optical emission spectrometer using two different wavelengths (Table S1). The operating conditions were set according to Zand et al. (2011). All measurements were performed in triplicate.
For quantitation, a multielement standard mixture was used to obtain a five-point external standard curve (0.5 –5 mg/l). The multielement standard was prepared from single-element ICP grade standards 10,000 mg/l (Romil PrimAg-plus cert. ref. material, Cambridge, Great Britain [Ca, Cu, Fe, P, Zn]; AccuStandard USA [K, Mg, Mn, S]), 10 % HNO3 and ultraclean fresh water. Phosphorous and sulfur standards were prepared separately from the metals. The accuracy and precision of the mineral element determination were tested using NBS 1567a wheat flour reference material and one in-house wheat flour reference material in every analysis.
To determine the initial mineral solubilization and availability from raw materials, the wheat and rye bran samples (20 g) were mixed with tap water (180 g) and vortexed. The bran-water suspensions were then centrifuged (10,000 rpm for 10 min, Sorvall RC-5C, Rotor SLA-1500) and supernatants collected and filtered through 0.45-μm hydrophilic PTFE Millipore Millex-LCR filter (Merck). From each sample, 15 ml of filtrate was collected and used for mineral content determination as described above except that dry sample (0.5 g) was replaced with 15 ml of liquid sample. Milli-Q water (Merck) was used as a negative control. From each bran sample, the mineral content was determined in triplicates. The solubility of the minerals was calculated with the following equation:
Enzyme-assisted method for dextran analysis
Dextran was measured from the duplicate bran fermentations as described by Katina et al. (2009) with minor changes. The glucose formed after enzyme hydrolysis was quantified with high-performance anion-exchange chromatography coupled with pulsed amperometric detector (HPAEC-PAD). The amount of dextran was estimated as the sum of anhydroglucose, corrected with a factor of 1.5 to take into account the recovery of dextran in bran matrices. The recovery factor (1.5) was determined with preliminary experiments (data not shown) as described by Katina et al. (2009). using purified W. confusa E392 dextran and the bran raw materials used in this study.
Dextran yields (DY) were calculated according to the following equation,
All amounts are given as percentages of dry weight (% d.w.). IS is the initial sucrose (sum of the added sucrose and the endogenous sucrose in the raw material), FS is the final sucrose after fermentation, and FD is the final dextran. The amount of anhydroglucose in sucrose is calculated by multiplying with the molar mass ratio of anhydroglucose to sucrose (0.4737).
HPAEC-PAD analysis of sugars
For the analysis of sugars (glucose, fructose, sucrose, maltose), 100 mg of bran or freeze-dried fermented samples were weighed into 10-ml centrifuge tubes, and 5 ml of 0.05 M sodium citrate buffer, pH 5.5, was added to extract the sugars. Then samples were vortexed and centrifuged (10,000 rpm for 15 min, Sorvall RC-5C, Rotor SLA-1500), and the supernatants were collected and placed in the boiling water bath for 10 min. From each boiled sample, 500 μl was transferred into Amicon Ultra 0.5 ml, 10 K (Merck, MA, USA) centrifugal filter units and centrifuged (13,000 rpm for 20 min). Four hundred microliters of filtrate was collected and Milli-Q water was added to dilute the sample to a final volume of 1 ml. Samples were further diluted 1:2 with Milli-Q water before analysis. A five-point standard curve was prepared for the quantification of glucose, fructose, sucrose, and maltose (Sigma-Aldrich, St. Louis, MO, USA). For both standards and samples, deoxygalactose (Sigma-Aldrich) was used as an internal standard. The standard and samples were measured by HPAEC-PAD according to (Juvonen et al. 2015). All samples were analyzed in triplicates.
Statistical analysis and modeling
Statistical analysis was performed with IBM SPSS Statistics 22.0 (Armonk, NY, USA) by using one-way ANOVA and Tukey’s test. A multivariate data analysis was performed with partial least squares regression analysis (PLS, Simca 13.5, MKS Umetrics AB, Malmö, Sweden). All raw material properties, fermentation final products (incl. pH and TTA), and results from the growth and acidification kinetics were chosen as X-variables, and final dextran amount (%) and dextran yield were chosen as Y-variables.
Results
Chemical composition of raw materials
The chemical compositions of the rye and wheat brans are shown in Table 1. The total dietary fiber and soluble fiber contents were higher in rye bran (56.10 and 5.01 %, respectively) than in wheat bran (48.01 and 3.11 %, respectively). The contents of both total and soluble pentosans were higher in rye bran, 30.87 and 1.31 %, respectively, than in wheat bran (20.00 and 0.41 %, respectively). Another distinct raw material property was the content of starch, which was 15.40 % in wheat bran and 11.72 % in rye bran.
The mineral compositions of wheat and rye bran also varied (Table 1). In rye bran, the total mineral content was slightly higher compared to that of wheat bran, 2667.62 mg/100 g bran and 2473.44 mg/100 g bran, respectively. In wheat bran, copper (Cu), iron (Fe), magnesium (Mg), manganese (Mn), and zinc (Zn) contents were higher than in rye bran while calcium (Ca), potassium (K), phosphor (P), and sulfur (S) contents were higher in rye bran than in wheat bran. Soluble minerals were predominantly the same when solubilization percentages (Eq. 2) were taken into account (Table 1). Liberation percentages in wheat bran varied from 0.46 to 2.69 %, being the lowest for Mg and the highest for K. In rye bran, the lowest mineral liberation percentage obtained was for Zn (0.51 %) and the highest for K (2.99 %). The most significant difference in mineral liberation occurred with Mg (0.46 and 0.86 % from wheat and rye bran, respectively).
Microbiological analyses of bran fermentation
Before fermentation, heterotrophic aerobic bacteria cell density varied from 4.3 to 5.0 log cfu/g in wheat bran and less than 4.0 log cfu/g in rye bran, while cell density of yeasts and molds was less than 2.90 log cfu/g in both brans. After fermentation, cell densities of heterotrophic bacteria or fungi were less than 3.0 log cfu/g in all the samples. Only uniform colonies of dextran producing LAB were observed on MRS-S agar before and after fermentation, which indicates the domination of the starter culture.
After 20 h of fermentation at 25 °C, the cell density of W. confusa E392 and W. confusa Cab3 varied from 9.4 to 9.9 log cfu/g in both bran types. The increase in cell density during fermentation was in the range of 3.4–3.7 log cycles (Table 2), except for W. confusa E392 in RB (2.8 log cycles). With W. confusa E392, higher growth rates (μ max) and longer lag phases (λ) were observed for wheat bran fermentations in relation to rye bran fermentations. When sucrose was added, the final cell density of the LAB was 9.9 log cfu/g in all cases, with a growth increase >3.6 log cycles. The addition of sucrose to bran increased maximum growth rates and lengthened the lag phase of W. confusa E392. The sucrose addition decreased the lag phase with W. confusa Cab3 in both brans and increased the maximum growth rate in rye bran (Table 2).
Acidification of bran fermentation
After 20 h of fermentation at 25 °C, similar pH drop was observed for both strains in all raw materials (Table 2). Differences in final pH values were not significant between different strains or raw materials (Table 3). TTA values varied from 11.32 to 12.60 ml and did not differ significantly.
More lactic and especially acetic acid was produced in wheat bran than in rye bran during fermentation. The addition of sucrose increased lactic acid formation with W. confusa Cab3 but did not affect acetic acid production in any of the samples. Thus, the ratio of lactic to acetic acid (fermentation quotient) was higher in rye bran than in wheat bran in all fermentations. When comparing the microbial strains, W. confusa Cab3 produced more lactic and acetic acid in all fermentations than W. confusa E392.
Clear differences could be seen in the acidification kinetics between the raw materials (Table 2). A much longer λ, over 7 h on average, was observed in rye bran compared to wheat bran (5 h on average). The addition of sucrose to the brans caused a decrease in λ with both strains, with the effect being more pronounced with W. confusa Cab3. The maximum acidification rates were in the range of 0.16–0.20 ΔpH/h except for RB-S fermented with W. confusa E392 (0.26 ΔpH/h).
Dextran formation and utilization of sucrose
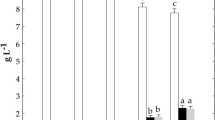
Wheat bran fermentation with W. confusa E392 without and with sucrose supplementation resulted in dextran contents of 0.11 and 1.94 % (dry weight), respectively (Fig. 1). Dextran contents after wheat bran fermentation with W. confusa Cab3 were 0.18 % without added sucrose and 2.12 % with sucrose supplementation. Dextran contents in rye bran were higher than in wheat bran. Fermentation of rye bran by W. confusa E392 produced 0.87 % dextran without added sucrose and 2.57 % when sucrose was added. With W. confusa Cab3, the dextran contents in rye bran were higher, 0.98 and 3.07 % without and with sucrose supplementation, respectively.
Dextran contents and yields of wheat and rye brans fermented by W. confusa strains E392 and Cab3. Rye bran samples had both higher dextran contents and yields than the corresponding wheat bran samples. The only significant difference between the strains was observed in rye bran supplemented with sucrose where Cab3 produced more dextran than E392. Dextran content is presented as percentage of the freeze-dried sample’s dry weight (% d.w.); E392 (dark bars) and Cab3 (light bars). Dextran yields are calculated according to Eq. (3) presented in “Materials and methods”; W. confusa E392 (triangle) and W. confusa Cab3 (square); WB wheat bran, WB-S wheat bran with sucrose supplementation, RB rye bran, RB-S rye bran with sucrose supplementation. Values with different superscript letters under x-axis (a, b, c, d) differ significantly (P < 0.05)
Dextran yields, taking into account the initially available and residual glucose from sucrose, were significantly higher in fermented rye bran with both strains. Yields in WB-S were 35 and 38 % with fermentation by W. confusa E392 and Cab3, respectively. Similarly, in RB-S, dextran yields were 54 and 66 %. Dextran yields were significantly lower in samples without added sucrose and slightly higher in samples fermented with W. confusa Cab3 than with W. confusa E392 in the respective samples.
As shown in Table 4, sucrose was almost completely utilized after 20 h in all the samples. A tendency towards slightly higher residual sucrose was observed in W. confusa Cab3-fermented brans. As expected, residual fructose amounts were higher in WB-S and RB-S samples than in samples without added sucrose. Fermentation with W. confusa E392 resulted in a higher amount of residual fructose in both brans.
Impact of chemical composition and final metabolic products on dextran production
PLS modeling showed how each individual raw material property and final metabolite product correlated with dextran production and yields (Fig. 2). The principle component (PC) 1 was mainly explaining the model (0.77), and PC 2 has very small role in the model (0.085). In general, those raw material properties which were higher in rye bran correlate positively with the final dextran amounts and yields, and those raw material properties which were dominant in wheat bran correlated negatively with resulted dextran amounts and yields. Other emerging features were variables related to acidification and growth, which were either highly positively or negatively correlated with the final dextran amounts and yields. Acidification (diamonds)-related variables and growth (triangles)-related variables correlated negatively with dextran amount and yield.
PLS biplot for different bran samples with varying chemical compositions, acidification kinetics, and dextran production. The abbreviations of sample and attribute codes are (circle) i DF insoluble dietary fiber, Pro protein, Sta starch, Glc1 initial glucose, DF dietary fiber, SDF soluble DF, Fru1 initial fructose, Frn fructan, Tpe total pentoses, s Pe sol. pentoses, TMi total mineral, s Mi sol. minerals, S/TM sol./tot. minerals, Fru2 final fructose, Suc1 initial sucrose, Suc2 final sucrose; (diamonds) Ace acetic acid, Lac lactic acid, v max maximal acedification rate, LpH λ pH (length of the aceidification lag phase), FpH final pH, Lac/Ace lac to ace ratio, ΔpH pH change during fermentation; (triangle) cfu/g: final LAB count, μ max maximal growth rate, Lgr λgrowth (length of the growth lag phase), Δlog10 logarithmic growth during fermentation; (square) Dex final dextran, DexY dextran yield; (hexagon) WB wheat bran, RB rye bran, 1 non sucrose, 2 sucrose supplementation, E fermented with E392, C fermented with Cab3 ssoluble
Discussion
In this study, wheat and rye bran fermented with two strains of W. confusa (E392 and Cab3) were compared as a medium for dextran production with and without sucrose supplementation. Rye bran was found to enhance dextran production compared to wheat bran. EPS formation has been extensively investigated in wheat and rye sourdoughs (Katina et al. 2009; Tieking et al. 2005; Di Cagno et al. 2006). and only one study reports the use of rye bran as a medium for dextran production (Kaditzky and Vogel 2008). We have previously demonstrated enzymatic production of dextran in autoclaved wheat bran (Kajala et al. 2015). but this is the first study to use non-heated, enzymatically active wheat bran as dextran production matrix.
Chemical composition
The comparison of bran chemical compositions showed that rye bran contained less starch but had higher concentrations of fructans and soluble pentosans, which contributes to its higher soluble dietary fiber content. The rye bran contained more intrinsic sucrose (5.42 % d.w.) than wheat bran (2.06 %). The total mineral content of the brans was similar which corresponded also to similar ash content. On the whole, the total mineral content was in agreement with the values reported by Kamal-Eldin et al. (2009). With respect to single minerals, rye bran had higher concentrations of Ca, K, P, and S and lower concentrations of Cu, Fe, Mg, Mn, and Zn. Initial soluble mineral contents showed a trend similar to the total mineral content, rye bran having slightly higher overall solubility and soluble mineral content. The initial mineral solubility was low for both types of bran (0.3–3 %), possibly due to the presence of phytic acid and dietary fibers. Lioger et al. (2007) reported the initial solubility of 15 and 5 % for wheat bran Mg and Ca, respectively, which is higher than found in this study.
Starter culture growth
The LAB starters dominated in both bran matrices after 20 h of fermentation, reaching final cell density above 9 log cfu/g, even with a relatively low initial inoculum. Similar final pH was observed in both fermented brans, although some differences in the interaction between the raw material and metabolic activity of the strains could be observed. The strains had a higher μ max in wheat bran than in rye bran. As the native brans had limited amounts of fermentable sugars (Table 1), it can be hypothesized that the effect on microbial growth could be due to a different release of fermentable sugars by the endogenous cereal enzymes. Both rye and wheat grains are known to have α-amylases, as well as xylanolytic and proteolytic enzymes which are known to affect the fermentation kinetics (Gys et al. 2004; Cleemput et al. 1997; Hansen et al. 2002; Gänzle 2014). Higher growth rates in wheat bran could indicate that nutrients are more efficiently released by intrinsic wheat bran enzymes. The maximum growth rates were mainly enhanced by sucrose addition.
Acidification of the bran matrix
The interaction between the enzymatic activities of the bran and the metabolism of the two strains were also reflected in the acidification kinetics. Whereas the growth lag phase was typically shorter in rye bran, the acidification was delayed in rye bran in all cases compared to wheat bran. The addition of sucrose mostly affected the lag phase but showed little or no effect on ΔpH or TTA.
Final lactic acid content did not differ between wheat or rye bran fermentations which was also reflected in similar pH and TTA values of both raw materials. Acidity levels were comparable to those of wheat flour fermented with W. confusa E392 (Katina et al. 2009). but significantly higher compared to EPS-producing fermentations earlier reported for rye bran (Kaditzky and Vogel 2008) or for sorghum and quinoa sourdoughs (Wolter et al. 2014). The amount of acetic acid was low throughout the study but was significantly higher in wheat than in rye bran. It was shown that some Weissella strains produce very little acetic acid when grown on glucose but produce significantly higher amounts when grown on xylose or xylooligosaccharides (Patel et al. 2013). Therefore, a different activity of the endogenous non-starch polysaccharide-hydrolyzing enzymes releasing pentoses or short fermentable pentosans from wheat and rye (Hansen et al. 2002) can be hypothesized. W. confusa Cab3 produced 19–38 % more organic acids than W. confusa E392 in all the fermentations but this was not reflected in pH and TTA values.
Utilization of sucrose
Essentially, all sucrose was depleted during the fermentations. However, there were significant differences in residual fructose levels between fermented bran samples. In wheat bran, residual fructose was rather well in line with the theoretical amount of fructose originating from native bran and the amount released from added sucrose, assuming it was completely consumed for dextran synthesis during fermentation, thus liberating fructose. In rye bran, residual fructose levels were lower than the theoretical maximal amounts of liberated fructose in all the cases. Fermentation with W. confusa Cab3 resulted in lower residual fructose amounts than fermentation with W. confusa E392. A study by Galle et al. (2010) showed that Weissella strains ferment fructose but do not reduce it to mannitol in the presence of maltose and glucose, which contributes to low acetic acid production of these strains. In this study, sucrose addition did not affect acetic acid production in any of the samples which means that the W. confusa strains fermented fructose to lactic acid through glycolysis, if they utilized it all. An increase in lactic acid was observed upon sucrose addition in bran fermented with W. confusa Cab3, which indicates fermentation of fructose liberated from dextransucrase reaction. It is possible that carbon sources more preferable to the strains than fructose become available during wheat bran fermentation and fructose remains largely untouched, but in rye bran, fructose is more essential for the energy metabolism of the cells and is partly consumed. Sucrose supplementation had a different effect on growth kinetics of the strains; in particular, in both brans, an increase in growth lag phase for W. confusa E392 fermentation was observed, whereas a decrease was shown for W. confusa Cab3. This could indicate either more efficient use of sucrose or fructose, liberated from sucrose by DSR, for energy metabolism by W. confusa Cab3 compared to W. confusa E392.
The effect of bran matrix and starter growth characteristics on dextran production
Rye bran was found to be a more suitable matrix for dextran production with both strains. In the native raw materials, very little dextran was detected after 20 h in wheat bran whereas 1 % (d.w.) of dextran was produced in rye bran. With sucrose supplementation, up to 2–3 % (20–30 g/kg) of dextran was produced in rye bran by fermentation with W. confusa E392 and Cab3. This is the highest reported amount of dextran produced in sourdough fermentations (Galle and Arendt 2014) advocating bran, and especially rye bran, suitability for EPS production. Dextrans as hydrocolloids in bread are effective dough improvers when used at a concentration of 0.3 % (Armero and Collar 1998). The formation of dextran in situ at concentration of 2–3 d.w. %, as reported in this study, is a promising technology for substituting the addition of hydrocolloids.
To depict the efficiency of the conversion of sucrose to dextran in the different fermentations, dextran yield was calculated from initial and final sucrose concentrations and final dextran content. Dextran yield describes the amount of sucrose converted to polymeric dextran during fermentation. Dextran yields were significantly (P < 0.05) higher in rye bran with and without sucrose supplementation and with both the strains.
The main reason for superior dextran production in rye bran appears to be due to the differences in raw material composition and overall acidification rate in two matrixes. Overall, PLS model indicates that individual raw material properties had an impact in dextran production. Raw material properties of rye bran, such as higher dietary fiber content, higher content of soluble fibers, lower content of starch, and differences in mineral composition correlated positively with high dextran production and yield, while those of wheat bran correlate negatively. Rye bran had significantly higher content of calcium and phosphorus, which correlated positively with dextran production. On the other hand, rye bran had lower content of Fe, Cu, Zn, and Mg in comparison to wheat bran. Especially Ca is known to affect DSR activity (Miller and Robyt 1986). even though contrasting results have been reported for W. confusa DSRs (Amari et al. 2013; Shukla et al. 2014). Ca was present in both brans but the initial solubility was very low and, at least in wheat bran, the solubility may remain low during fermentation (Lioger et al. 2007). Other ions have also been studied for their effect on DSR activity. For example, K and Mg have a positive impact on dextran production (Rühmkorf et al. 2013). and it is noteworthy that these ions were present in higher level in soluble form in rye bran. On the other hand Cu and Fe, present in higher levels in wheat bran, have been reported to have negative impact (Rühmkorf et al. 2013; Shukla et al. 2014; Miller and Robyt 1986). It should be noted, though, that when enzyme activities are studied, the enzymes are usually produced in rich growth medium from where the essential ions might already be incorporated to the enzymes, which is not the case here.
Both W. confusa strains grew relatively well in the two studied matrices, but the growth-related variables negatively correlated with produced dextran and dextran yields. This seems to indicate that a faster growth and high final cell density in wheat bran do not support higher dextran production, because in wheat bran, bacterial strains produce more acids in shorter time during their growth affecting the acidification and thus the performance of the DSR. Acidification-related variables were evenly distributed in the PLS model. Higher pH change and amount of acetic acid negatively correlated with produced dextran, while v max, λ pH, and the ratio of lactic/acetic acid appeared to have a positive influence, and especially the last two were different in wheat and rye and bran. In rye bran fermentations, λ pH is longer and the pH value after fermentation is higher compared to wheat bran, thus providing conditions close to optimal for the DSR activity for a longer time. In previous studies, the DSR enzymes of the strains used here were characterized using a recombinant enzyme in the case of W. confusa E392 (Kajala et al. 2015) and the crude extract and purified native enzyme for W. confusa Cab3 (Shukla and Goyal 2011; Shukla et al. 2014). The optimum pH for both the enzymes is 5.4, which corresponds to the pH observed during the fermentation in this study. The positive effects of pH maintenance at its optimum on EPS production during dough fermentation were also observed in previous works, in pH controlled experiments (Kaditzky and Vogel 2008; Kaditzky et al. 2008). In rye bran, the delay in acidification as expressed by a long lag phase might be beneficial for dextran production as the bacteria reach higher cell numbers and thus may produce more DSR before the optimum pH is reached. Thus, higher dextran yields could be explained by higher total DSR activity in rye bran.
Other possible explanation for higher dextran yields in rye bran could be lower concentrations of maltose and maltooligosaccharides, which act as acceptors for DSR and would thus result in the formation of isomaltooligosaccharides at the expense of dextran production (Katina et al. 2009). Maltose could not be quantified from the samples after 20 h of fermentation, but in some replicates, trace amounts were detected (data not shown). Maltose is, however, released from the bran matrix during fermentation, which was confirmed by incubating wheat and rye bran in antibiotic solution for 20 h with two-step pH adjustment at 10 and 15 h imitating pH drop during fermentation (Figure S1). Maltose released from raw material is expected as starch degradation is the predominant source of fermentable carbohydrates during sourdough fermentation (Gänzle 2014). As no residual maltose was quantified in the fermentations, it appears to be released from the matrix slower than it is consumed by the bacteria or is used as substrate for isomaltooligosaccharide formation. If more maltose was to be released from wheat than rye bran, due to, for instance, higher α-amylase activity and or its higher starch content, it would contribute to higher maximum growth rates observed in WB than RB as well as lower dextran yields in wheat bran. Higher isomaltooligosaccharide production in wheat bran than in rye bran is also supported by higher residual fructose contents and lower dextran yieds in wheat bran. As dextran yield depicts the conversion efficiency of sucrose to dextran, free fructose in wheat bran samples suggests the presence of isomaltooligosaccharide reaction rather than sucrose transport inside the cell for fermentation.
This study demonstrated rye bran to be a superior substrate for in situ dextran production in comparison to wheat bran. Endogenous differences in chemical composition of bran induced different acidification behavior of the two W. confusa strains in which resulted in more advantageous conditions for dextran production in rye bran compared to wheat bran. Further studies are in progress to establish technological functionality of dextran-enriched rye bran in food applications.
References
Amari M, Arango L, Gabriel V, Robert H, Morel S, Moulis C, Gabriel B, Remaud-Siméon M, Fontagné-Faucher C (2013) Characterization of a novel dextransucrase from Weissella confusa isolated from sourdough. Appl Microbiol Biotechnol 97:5413–5422
Arendt EK, Ryan LAM, Dal Bello F (2007) Impact of sourdough on the texture of bread. Food Microbiol 24:165–174
Armero E, Collar C (1998) Crumb firming kinetics of wheat breads with anti-staling additives. J Cereal Sci 28:165–174
Bakke A, Vickers Z (2007) Consumer liking of refined and whole wheat breads. J Food Sci 72:S473–S480
Cleemput G, Hessing M, Van Oort M, Deconynck M, Delcour JA (1997) Purification and characterization of a [beta]-D-xylosidase and an endo-xylanase from wheat flour. Plant Physiol 113:377–386
Coda R, Rizzello CG, Katina K (2015) Bran bioprocessing for enhanced functional properties. Curr Opin Food Sci 1:50–55
Di Cagno R, De Angelis M, Limitone A, Minervini F, Carnevali P, Corsetti A, Gaenzle M, Ciati R, Gobbetti M (2006) Glucan and fructan production by sourdough Weissella cibaria and Lactobacillus plantarum. J Agric Food Chem 54:9873–9881
Fusco V, Quero GM, Cho GS, Kabisch J, Meske D, Neve H, Bockelmann W, Franz CM (2015) The genus Weissella: taxonomy, ecology and biotechnological potential. Front Microbiol 6:155
Galle S, Arendt EK (2014) Exopolysaccharides from sourdough lactic acid bacteria. Crit Rev Food Sci Nutr 54:891–901
Galle S, Schwab C, Arendt E, Gänzle M (2010) Exopolysaccharide-forming Weissella strains as starter cultures for sorghum and wheat sourdoughs. J Agric Food Chem 58:5834–5841
Gänzle MG (2014) Enzymatic and bacterial conversions during sourdough fermentation. Food Microbiol 37:2–10
Gys W, Gebruers K, Sørensen J, Courtin C, Delcour J (2004) Debranning of wheat prior to milling reduces xylanase but not xylanase inhibitor activities in wholemeal and flour. J Cereal Sci 39:363–369
Hansen HB, Andreasen M, Nielsen M, Larsen L, Knudsen BK, Meyer A, Christensen L, Hansen Å (2002) Changes in dietary fibre, phenolic acids and activity of endogenous enzymes during rye bread-making. Eur Food Res Technol 214:33–42
Juvonen R, Honkapää K, Maina NH, Shi Q, Viljanen K, Maaheimo H, Virkki L, Tenkanen M, Lantto R (2015) The impact of fermentation with exopolysaccharide producing lactic acid bacteria on rheological, chemical and sensory properties of pureed carrots (Daucus carota L.). Int J Food Microbiol 207:109–118
Kaditzky S, Vogel RF (2008) Optimization of exopolysaccharide yields in sourdoughs fermented by lactobacilli. Eur Food Res Technol 228:291–299
Kaditzky SB, Behr J, Stocker A, Kaden P, Gänzle MG, Vogel RF (2008) Influence of pH on the formation of glucan by Lactobacillus reuteri TMW 1.106 exerting a protective function against extreme pH values. Food Biotechnol 22:398–418
Kajala I, Shi Q, Nyyssola A, Maina NH, Hou Y, Katina K, Tenkanen M, Juvonen R (2015) Cloning and characterization of a Weissella confusa dextransucrase and its application in high fibre baking. PLoS One. doi:10.1371/journal.pone.0116418
Kamal-Eldin A, Laerke HN, Knudsen KE, Lampi AM, Piironen V, Adlercreutz H, Katina K, Poutanen K, Man P (2009) Physical, microscopic and chemical characterisation of industrial rye and wheat brans from the Nordic countries. Food Nutr Res 53:8349–8356
Katina K, Maina NH, Juvonen R, Flander L, Johansson L, Virkki L, Tenkanen M, Laitila A (2009) In situ production and analysis of Weissella confusa dextran in wheat sourdough. Food Microbiol 26:734–743
Katina K, Juvonen R, Laitila A, Flander L, Nordlund E, Kariluoto S, Piironen V, Poutanen K (2012) Fermented wheat bran as a functional ingredient in baking. Cereal Chem 89:126–134
Korakli M, Vogel RF (2006) Structure/function relationship of homopolysaccharide producing glycansucrases and therapeutic potential of their synthesised glycans. Appl Microbiol Biotechnol 71:790–803
Laurikainen T, Härkönen H, Autio K, Poutanen K (1998) Effects of enzymes in fibre‐enriched baking. J Sci Food Agric 76:239–249
Leemhuis H, Pijning T, Dobruchowska JM, van Leeuwen SS, Kralj S, Dijkstra BW, Dijkhuizen L (2013) Glucansucrases: three-dimensional structures, reactions, mechanism, α-glucan analysis and their implications in biotechnology and food applications. J Biotechnol 163:250–272
Lioger D, Leenhardt F, Demigne C, Remesy C (2007) Sourdough fermentation of wheat fractions rich in fibres before their use in processed food. J Sci Food Agric 87:1368–1373
Miller AW, Robyt JF (1986) Activation and inhibition of dextransucrase by calcium. Biochim Biophys Acta 880:32–39
Monsan P, Bozonnet S, Albenne C, Joucla G, Willemot R, Remaud-Siméon M (2001) Homopolysaccharides from lactic acid bacteria. Int Dairy J 11:675–685
Monsan P, Remaud-Siméon M, André I (2010) Transglucosidases as efficient tools for oligosaccharide and glucoconjugate synthesis. Curr Opin Microbiol 13:293–300
Nordlund E, Aura A, Mattila I, Kössö T, Rouau X, Poutanen K (2012) Formation of phenolic microbial metabolites and short-chain fatty acids from rye, wheat, and oat bran and their fractions in the metabolical in vitro colon model. J Agric Food Chem 60:8134–8145
Patel S, Kothari D, Shukla R, Das D, Goyal A (2011) Scale up of dextran production from a mutant of Pediococcus pentosaceus (SPAm) using optimized medium in a bioreactor. Braz Arch Biol Technol 54:1125–1134
Patel A, Falck P, Shah N, Immerzeel P, Adlercreutz P, Stålbrand H, Prajapati JB, Holst O, Karlsson EN (2013) Evidence for xylooligosaccharide utilization in Weissella strains isolated from Indian fermented foods and vegetables. FEMS Microbiol Lett 346:20–28
Poutanen K, Flander L, Katina K (2009) Sourdough and cereal fermentation in a nutritional perspective. Food Microbiol 26:693–699
Prinsen P, Gutiérrez A, Faulds CB, del Río JC (2014) Comprehensive study of valuable lipophilic phytochemicals in wheat bran. J Agric Food Chem 62:1664–1673
Reisinger M, Tirpanalan Ö, Huber F, Kneifel W, Novalin S (2014) Investigations on a wheat bran biorefinery involving organosolv fractionation and enzymatic treatment. Bioresour Technol 170:53–61
Rosell CM, Santos E, Collar C (2006) Mixing properties of fibre-enriched wheat bread doughs: a response surface methodology study. Eur Food Res Technol 223:333–340
Rühmkorf C, Jungkunz S, Wagner M, Vogel RF (2012) Optimization of homoexopolysaccharide formation by lactobacilli in gluten-free sourdoughs. Food Microbiol 32:286–294
Rühmkorf C, Bork C, Mischnick P, Rübsam H, Becker T, Vogel RF (2013) Identification of Lactobacillus curvatus TMW 1.624 dextransucrase and comparative characterization with Lactobacillus reuteri TMW 1.106 and Lactobacillus animalis TMW 1.971 dextransucrases. Food Microbiol 34:52–61
Shukla S, Goyal A (2011) Optimization of fermentation medium for enhanced glucansucrase and glucan production from Weissella confusa. Braz Arch Biol Technol 54:1117–1124
Shukla R, Goyal A (2012) Optimization and scale-up of fermentation of glucansucrase and branched glucan by Pediococcus pentosaceus CRAG3 using Taguchi methodology in bioreactor. J Biosci Biotechnol 1:73–82
Shukla S, Shi Q, Maina NH, Juvonen M, Tenkanen M, Goyal A (2014) Weissella confusa Cab3 dextransucrase: properties and in vitro synthesis of dextran and glucooligosaccharides. Carbohydr Polym 101:554–564
Swennen K, Courtin CM, Lindemans GC, Delcour JA (2006) Large‐scale production and characterisation of wheat bran arabinoxylooligosaccharides. J Sci Food Agric 86:1722–1731
Tieking M, Ehrmann MA, Vogel RF, Gänzle MG (2005) Molecular and functional characterization of a levansucrase from the sourdough isolate Lactobacillus sanfranciscensis TMW 1.392. Appl Microbiol Biotechnol 66:655–663
Wolter A, Hager A, Zannini E, Galle S, Gänzle M, Waters D, Arendt E (2014) Evaluation of exopolysaccharide producing Weissella cibaria MG1 strain for the production of sourdough from various flours. Food Microbiol 37:44–50
Zand N, Chowdhry BZ, Zotor FB, Wray DS, Amuna P, Pullen FS (2011) Essential and trace elements content of commercial infant foods in the UK. Food Chem 128:123–128
Zwietering MH, Jongenburger I, Rombouts FM, van ’t Riet K (1990) Modeling of the bacterial growth curve. Appl Environ Microbiol 56:1875–1881
Acknowledgments
This study was supported by the Academy of Finland (contract number 255755) and the Department of Biotechnology, Ministry of Science and Technology, New Delhi, India, to AG via the joint WISEDextran project, and the ABS Graduate School (QS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was funded by a grant from Academy of Finland (contract number 255755) and by ABS graduate school (QS). All co-authors have consented to submitting this manuscript. The authors state that they have no conflict of interest.
Additional information
Ilkka Kajala and Jari Mäkelä contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 286 kb)
Rights and permissions
About this article
Cite this article
Kajala, I., Mäkelä, J., Coda, R. et al. Rye bran as fermentation matrix boosts in situ dextran production by Weissella confusa compared to wheat bran. Appl Microbiol Biotechnol 100, 3499–3510 (2016). https://doi.org/10.1007/s00253-015-7189-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-7189-6