Abstract
Although serological detection is a practical strategy for early detection and diagnosis of tuberculosis (TB), inconsistent and imprecise estimates of sensitivity and specificity block its development and application for clinic. New or alternative serological antigens with improved accuracy are urgently needed. A phage-displayed random peptide library was employed to screen for immunoactive peptides using specific immunoglobulin G (IgG) of TB patients as target molecules. With two screening strategies, 20 single phages displaying different sequences were obtained and no sequence homology was found among these phages. From the results of phage-ELISA, H12, TB6, TB15, and TB18 phages showed higher affinity to IgGs from TB patients(S/N ≥2.1) and were identified as the positive clones. Significant differences in the detection values of sera from 47 TB patients and 37 healthy individuals were found for these four phage clones. According to the reactivity of 284 human sera to synthetic H12, TB6, TB15, and TB18 peptides as determined by ELISA, TB15 showed significantly higher areas under the curve (AUC) and sensitivity than other peptides, providing a lead molecule for the development of new serology diagnostic strategies for TB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuberculosis (TB) remains as one of the world’s deadliest infectious diseases. In 2013, an estimated 9.0 million people developed TB and 1.5 million died from the disease (WHO 2014). China has the second highest TB burden with a 0.9–1.1 million active TB incidence accounting for 10 % of global cases in 2013 (WHO 2014). From China’s fifth national TB epidemiology survey in 2010, the prevalence of active TB was 459/100,000 among the population over 15 years old (technical guidance group of the fifth national TB epidemiological survey 2012). Early diagnosis of TB is especially important for providing early treatment and curtailing the spread of infection. In most of the high TB burden countries, however, clinical diagnosis of tuberculosis is still based primarily on acid-fast bacillus smears and mycobacterial culture.
Serological detection is one of the three major means of achieving TB diagnosis. Compared with traditional bacterial culturing, which takes 6 to 8 weeks to obtain results, serological detection (predominantly by antibody detection), is simple, rapid, and can be applied to the bedside of patients, and has therefore become the ideal choice for early detection and diagnosis of TB (Bekmurzayeva et al. 2013). Many different mycobacterial-specific antigens have been evaluated and applied to develop serological assays (Bahk et al. 2004). However, from the policy statement regarding commercial serodiagnostic tests for diagnosis of TB published by WHO in 2011 (WHO 2011), based on a bivariate meta-analysis of commercially available tests from 1990 to 2010 (Steingart et al. 2011), currently commercially available serological tests for TB diagnosis continue to produce inconsistent and imprecise estimates of sensitivity and specificity. WHO warned against the use of current serological tests for the immunodiagnosis of TB and strongly recommended that Mycobacterium tuberculosis (Mtb) antibody tests should not be used for the diagnosis of pulmonary and extrapulmonary Mtb infections. The WHO expert group strongly encouraged further research to identify new/alternative serological tests with improved accuracy (WHO 2011), placing higher requirements for research into serological detection of TB.
High-throughput proteome microarray technology has been used to assess antibody responses to the Mtb proteome and revealed that during active tuberculosis, antibody responses focused on only approximately 0.5 % of the proteome. Thirteen of these TB-associated proteins were found to have a significant association with active TB (Kunnath-Velayudhan et al. 2010). Recently, a functional Mtb proteome microarray covering most of the proteome and an ORFome library used for serum biomarker discovery has been reported. Fourteen Mtb proteins together were able to differentiate between patients with active TB disease and recovered individuals. The diagnostic accuracy of the 14 candidate biomarkers reached 78.4 %, with a sensitivity of 80.2 % and specificity of 79.0 %, indicating the potential that this set of biomarkers has as an index for monitoring treatment outcome (Deng et al. 2014). All of these studies provide directions for designing assays for antibody detection, but to the best of our knowledge, did not demonstrate diagnostic potential (Zhang et al. 2013; Li et al. 2014). A major difficulty that is encountered involves identifying reagents that are more sensitive and specific for active disease or that can distinguish Mtb exposure from infection. The low specificity of the current antigens can be attributed to cross-reactivity with other mycobacteria, which becomes even more problematic in countries such as China that routinely provide Mycobacterium bovis bacillus Calmette-Guerin (BCG) vaccinations (Kunnath-Velayudhan et al. 2012).
From the proteome study, many Mtb proteins can be detected by serum antibodies but the reactions are not necessarily of diagnostic quality (Schubert et al. 2013). The key requirement for diagnostic antigens is to contain immunodominant epitopes whose antibodies are abundant in each infected patients (Gonzalez et al. 2014). Therefore, immunodominant epitopes of the Mtb antigens should be present for adequate diagnosis of TB. In this context, the high-throughput screening of combinatorial phage displaying random peptide libraries has emerged as a straightforward methodology for the discovery of novel antigens and immunogens (Finlay et al. 2011). With specific antibodies as the target molecule, peptides mimicking the structure and chemical characteristics of the pathogen antigen can be obtained with strong specificity and high affinity after only several rounds of screening (Yang et al. 2015). Furthermore, synthetic peptides can be produced cheaply, are well-defined, highly reproducible, and could potentially avoid cross-reactivity (Ulises et al. 2009). Phage display random peptide library has been used successfully for the selection of mimotopes of important Mtb antigens such as lipoarabinomanan (LAM), Hsp16.3, and neutral polysaccharides (NPS) (Gevorkian et al. 2005; Saha et al. 2005; Sharma et al. 2006; Barenholz et al. 2007), providing good substitutes for serological diagnosis of TB. We previously obtained simulation antigen epitope peptides of the Mtb protein 64 (mycobacterium protein 64, tuberculosis MPT64) and CFP10/ESAT-6 (CE) protein, and our preliminary results indicated that the epitope peptide could completely replace the corresponding antigen for serological detection (Yang et al. 2011, 2013). These studies effectively established a more comprehensive serological detection method based on the immunoactive peptide. However, due to different TB antibody spectrum, the sensitivity was still not able to meet the requirements; only testing for single antigen mimotopes (Khan et al. 2008).
Recently, peptide ligands for antibodies from leprosy patients were selected from phage-displayed peptide libraries and data support the use of phage-displayed peptides as promising biotechnological tools for the design of diagnostic serological assays (Alban et al. 2013, 2014).Accordingly, this study screened a phage-display random peptide library using antibody immunoglobulin G (IgG) directly purified from TB patients as target molecules to identify new peptide mimotopes which may improve the sensitivity and be effectively used in diagnostic assays for TB.
Material and methods
Serum samples
Venous blood samples were collected from individuals attending the Shanghai Pulmonary Hospital. According to the Chinese Laboratory Science Procedure of Diagnostic Bacteriology in Tuberculosis (Chinese Antituberculosis Association 1995), TB patients (n = 130) were diagnosed with active pulmonary TB by sputum culture. No patients presented with systemic or extra-pulmonary TB as assessed by x-ray test. All patients had a history of BCG vaccination and were PPD positive (indurations of >10 mm). Blood was collected before the initiation of treatment. Seventy patients were male, with average age 42 ± 18 years; 60 patients were female, with average age 35 ± 15 years. Blood samples were also collected from 20 non-tuberculosis patients with respiratory infections disease, and 134 healthy individuals, all of which had been vaccinated with BCG during childhood and were PPD positive. All study recruits were negative for human immunodeficiency and hepatitis B and C viruses. Sera were prepared by centrifugation and stored in aliquots at −70 °C until analysis.
Purification of serum IgG
Sera from 47 TB patients and 37 healthy controls were selected and screened with CE and MPT64 antigen epitope peptide. Twelve TB patients with negative results for these tests were used as the screening target samples and 12 healthy controls similarly negative for these tests were used as the reverse screening samples. IgG antibodies were purified by protein A/G plus agarose according to the manufacturer’s instructions (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Relative molecular mass of the antibodies were identified by SDS-PAGE and concentrations were measured by the Bradford method.
Phage display and peptide sequencing
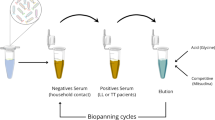
Phage display libraries Ph.D.-7 was purchased from New England Biolabs (Beverly, MA, USA). According to the manufacturer’s recommendations (http://www.neb.com/nebecomm/products/productE8100.asp), the library was screened with IgG antibodies from TB patients and healthy controls by two strategies: (1) The first screening strategy: purified IgG from TB patients diluted with 0.1 M NaHCO3, pH 8.6 was coated on the 96-well ELISA plate overnight. After blocking, the library (2 × 1011 phage for a library with 2 × 109 clones) was added onto coated plate and unbound phage was discarded. Bound phage was eluted with free purified IgG from TB patients to compete the bound phage away from the immobilized antibodies on the plate. From the second round of screening, after the unbound phage was discarded, free purified IgG from health control was added first to get the healthy IgG elution phage, followed by adding free purified IgG from TB patients to get the second generation of TB patients IgG specific binding elution phage. A third round of panning was conducted in a similar manner. (2) The second screening strategy: purified IgGs from TB patients and health control were coated on the 96-well ELISA plate, separately. The library was added onto the healthy IgG coated plate first, and the unbound phage supernatant was added to TB patients IgG package plate and interacted like before. Free healthy IgG was added to the healthy IgG-coated plate to elute the healthy IgG binding phage, same as the TB IgG coated plate. The same strategy with the first round was used for the 2–4 rounds of screening. After the panning with two strategies were finished, phage plaques were taken from TB IgG and healthy IgG eluted plates at random, and amplified in Escherichia coli ER2738 followed with DNA extraction and sequencing with −96III primer performed by the Sangon Biotech Co. Ltd. (Shanghai, People’s Republic of China). The DNA and amino acids sequences of peptides were analyzed and aligned by computer with DNAStar software (www.dnastar.com). Individual phage clones with different sequences were amplified according to the manual. The peptide sequences from positive clones were analyzed for sequence similarity using protein BLAST program on the sequence data of Mtb reference strain H37Rv (NCBI Reference Sequence: NC_000962.3).
Reverse phage ELISA
To evaluate the binding abilities of IgG from either TB patients or healthy controls to the selected phage displayed peptides, 96-well ELISA plates were coated with 100 μg/ml purified TB and health IgG in 100 μl 0.1 mol/l NaHCO3 buffer overnight at 4 °C, individually. Both coated plates and another without coating (to detect non-specific binding of BSA to the selected phage-displayed peptides), were incubated with blocking buffer. After washing with 0.5 % Tween-20 in TBS, phage particles were added to the wells with 109 plaque-forming units. After incubation with gentle shaking at room temperature, the plates were washed six times. Subsequently, capture of the phages by different IgGs was detected with anti-M13 mAb conjugated to horseradish peroxidase (HRP) (GE Healthcare, Piscataway, NJ) using tetramethylbenzidine (TMB) solution as a substrate. The reaction was read at 460 and 620 nm using an ELISA reader (MK3, Thermo). All samples were tested in triplicate.
Peptides synthesis
Synthetic peptides carrying amino acid sequence of four mimotope H12 (HVQLLIFGGGS), TB6 (FHFPPVYGGGS), TB15 (WHLPLSLGGGS), and TB18 (LPPPPPFSGGGS) were synthesized, purified and identified by Shanghai Bootech Bioscience & Technology, Co. Ltd. (Shanghai, China). A structurally flexible linker (GGGS) was added to the representative consensus sequences at the C-terminal ends to obtain the effective conformations of peptides.
Indirect ELISA
Antibodies IgG binding by the immunoactive mimotopes were detected by indirect ELISA, as described previously (Yang et al. 2011). In brief, 1010 phages ml−1 or 4 μg peptides ml−1 were coated on the 96-well ELISA plates overnight at 4 °C with 100 μl per well of coating solutions (17.5 mmol/l Na2CO3, 32.5 mmol/l NaHCO3, 15 mmol/l MgCl2•6 H2O, pH 9.6). Plates were washed thrice with 0.02 % Tween 20 in PBS and blocked with 1.5 % nonfat milk powder in PBS at 1 h at 37 °C. After washing thrice with PBST, serum dissolved in PBST (100 μl per well) was then added and incubated for 1 h at 37 °C. After the plates were washed thrice, HRP-labeled goat anti-human Ig was added 100 μl per well for 1 h at 37 °C, followed by the addition of TMB substrate and measurement with ELISA reader. All samples were tested in triplicate.
Statistical analysis
Graphpad Prism 5 statistical software was used for data processing. Test results were presented as mean ± standard deviation (SD). The two different groups were compared using the two-tailed, unpaired Student’s t test. One-way analysis of variance (ANOVA) and Turkey’s multiple comparison tests were used for data of more than two groups. P values of <0.05 were considered to be significant. The receiver operating characteristic (ROC) curve was used to evaluate the performance of the ELISA tests with two categories (TB patients and controls) with the MedCalc statistical software. The difference between the areas under the curve (AUC) was compared using the pair-wise comparison of ROC curves, P values of <0.05 were considered to be significant.
Results
Selection of phage clones and analysis of peptides sequences
In order to identify immunoactive peptides specifically recognized by antibodies in the serum of TB patients, phage display peptide libraries were selected by affinity using TB-specific antibodies. IgG antibodies were purified by the protein A/G Sepharose column chromatography from pools of sera from either TB patients or healthy controls and used for the direct isolation of TB-specific immunoactive mimotopes. After panning using two strategies, the peptide-coding regions of 34 randomly selected phage clones were sequenced. Twelve unique sequences were obtained using strategy one (Table 1). Twelve of the 34 phage clones had the same NTWHNSR sequence while 9 clones shared the same WHKEQFW sequence. Seven clones had unique sequences, while clones T6 and T18, T1 and H15, and T14 and H1, contained the same sequences, respectively. No analogs were found among the 12 identified amino acids sequences. Eight sequences were received using screening strategy two (Table 2), with 19 of the 34 phage clones having the same TTAGIRQ sequence. The second highest frequency was observed for the SNAPQKL sequence, which was returned for eight clones. Two clones shared the FHFPPVY sequence while the other clones had unique sequences. Sequence homology was also not found among these 8 sequences, even for 20 sequences obtained from two strategies.
Binding affinity of selected phage clones
To confirm the binding affinity of selected phage clone, clones displaying different peptide sequences (12 identified by screening strategy one and 8 identified by strategy two) were amplified and tested by phage ELISA. Surprisingly, from strategy one, only 1 phage clone (H12) showed higher affinity to IgG from TB patients and the ratio was more than five times that of the negative control clone (TF1), which should be a positive clone. The affinity of H12 to IgG from healthy donors was significantly lower than that of the TB patients IgG (Fig. 1a). Other clones from strategy one, even for the clone with highest frequency, did not show any affinity to the two kinds of IgG. For strategy two, phage clones TB6, TB15, and TB18 showed more than four times higher affinity to IgG from TB patients, which identified as positive clones, and the rest of the single phages were not detected with the affinity to both IgGs (Fig. 1b).
ELISA immunoreactivity of selected phage clones (a strategy one; b strategy two) against IgG from TB patients and health control. Phage clones with different peptide sequences were used for the phage ELISA analysis. Phage clones were added to the plates coated with either IgG from TB patients or health control. Capture of the phages by antibodies was detected with anti-M13 mAb conjugated to horseradish peroxidase using tetramethylbenzidine as a substrate. The reaction was read at 460 and 620 nm. The data represent the mean and standard deviation of triplicate wells. The first eluted phage library (TF1, TBF1) was used as negative control
Sequence alignment against Mtb H37Rv proteins
To reveal likely protein sources of the reactive peptide sequences, we conducted a bioinformatics alignment against the amino acid sequence of all proteins from Mtb strain H37Rv. Alignment of the peptide sequences displayed by four positive clones, indicated that the 1st–5th amino acid (HVQLL) of H12 was conserved as the 80th–84th amino acid of Rv0323c, a conserved hypothetical protein of Mtb. The sequence of TB6 demonstrated significant alignment with Rv3176c, probable the epoxide hydrolase MesT. The 1st–6th amino acid of TB15 was identical to the 142nd–147th amino acid of Rv2515c, also a conserved hypothetical protein, and the 2nd–7th amino acid corresponded with the 284th–289th amino acid of Rv1184c, a possible exported protein of Mtb. The core amino acid (PPPPP) of TB18 was conserved in most of the membrane or transmembrane proteins, PE family proteins in Mtb, suggesting the important role of PE family proteins as targets of the humoral response after Mtb infection.
Positive single phage detection by serum antibody test
To evaluate the binding of phage displayed peptides to antibodies in the sera of TB patients, four positive phage clones (H12, TB6, TB15, and TB18) were used for detection by ELISA (Fig. 2). Using two independent sample t test analysis, stronger reactivities were observed for all of the four single phages among sera from TB patients than sera from healthy controls (H12: t = 2.912, P = 0.0046; TB6: t = 4.275, P < 0.0001; TB15: t = 3.883, P = 0.0002; TB18: t = 4.565, P < 0.0001).
Scatter dot plot representation of the distribution of IgG levels to the four-phage clone in TB patients and health control. (Error bars 95 % confidence interval for mean) The plates were coated with 1010 phages ml−1 per well, as described in “Material and methods.” Sera from TB patients (n = 47) and healthy individuals (n = 37) were tested. The results are shown as the mean OD value of each triplicate sample read 20 min after the addition of substrate. Significance was determined against the negative control group of four-phage clone
TB-specific reactivity of immunoactive peptide mimotopes
The reactivities of the four peptides encoded by the selected clones (H12, TB6, TB15, and TB18) were evaluated by indirect ELISA. To determine the sensitivity and specificity of reactions, 130 sera from confirmed TB patients and 154 samples from control individuals (20 from non-tuberculosis patients with respiratory infections disease and 134 from healthy individuals), were examined (Fig. 3). Using the one-way ANOVA analysis, significant differences were detected for all of the four peptides between the three groups and all P values were <0.0001. Using t test analysis, two between the three groups of samples were compared, respectively, and the statistical outcomes are shown in Table 3. The areas under the receiver operating characteristic (ROC) curve (AUC) of four peptides were evaluated using the TB and control sera panels (Fig. 4). The statistical results for AUC, cutoff value, sensitivities, and specificities are shown in Table 4. According to the pair-wise comparison, there was no significant difference between the AUC of H12 and TB6 (z = 0.207, P = 0.836); H12 and TB18 (z = 1.359, P = 0.174); and TB6 and TB18 (z = 1.621, P = 0.105). But the AUC of TB15 was significantly higher than the other three peptides (H12 and TB15: z = 2.444, P = 0.015; TB6 and TB15: z = 2.818, P = 0.005; TB18 and TB15: z = 3.389, P = 0.001). Together, these data suggest that among the clones investigated, the TB15 peptide has the greatest capacity for the serological diagnosis of TB.
Scatter dot plot representation of the distribution of IgG levels to the four IgG binding peptides (a H12; b TB6; c TB15; d TB18) in TB patients, disease control, and health control. (Error bars 95 % confidence interval for mean). The plates were coated with 4 μg peptides per well, as described in “Material and methods.” Sera from TB patients (n = 130), disease control (n = 20), and healthy individuals (n = 134) were tested. The results are shown as the mean OD value of each triplicate sample read 20 min after the addition of substrate
The receiver operating characteristic (ROC) curve of four IgG binding peptides evaluated with the panel of TB and controls serum samples. According to the distribution of levels of antibodies (IgG) to the four peptides in TB patients and negative controls, the ROC curve was produced with the Medcalc software (http://www.medcalc.be/). According to the pair-wise comparison results of ROC curves, there was no significant difference between the AUC of H12 and TB6 (z = 0.207, P = 0.836); H12 and TB18 (z = 1.359, P = 0.174); and TB6 and TB18 (z = 1.621, P = 0.105). But the AUC of TB15 was significantly higher than the other three peptides (H12 and TB15: z = 2.444, P = 0.015; TB6 and TB15: z = 2.818, P = 0.005; and TB18 and TB15: z = 3.389, P = 0.001)
Discussion
We previously used a phage display random peptide library to identify the B-cell epitopes of MPT64 and CE protein in Mtb with useful attributes for serological diagnosis of TB, but our data also revealed that the sensitivities were still not sufficiently high enough to discriminate all the TB patients from health control (Yang et al. 2011, 2013). One reason for this limitation may be the complexity of antigen-specific antibodies that arise during Mtb infection. Such that mimotopes of these two antigens do not represent all of the antigenic targets and cannot therefore react with serum from the patients that lack these antibodies. Not including the carbohydrate and lipid antigens, antibody responses against more than ten protein antigens have, to date, been indicated to have significant association with active TB (Kunnath-Velayudhan et al. 2010). It is time-consuming, and potentially meaningless given that the complete antigen may still not meet the requirements for stringent detection of Mtb infection to screen all of the peptide mimotopes of these antigens. Theoretically, since Mtb-specific antibodies are induced during infection, the serum IgG of TB patients can be used as the direct target for the screening of immunoactive peptides and this can be used to rapidly and effectively discover more sensitive peptides. In this study, we therefore evaluated the serum antibody responses against peptides that were selected from random peptide phage-display libraries with IgG purified from the sera of TB patients that could not be distinguished by the epitope peptides of MPT64 and CE protein (M2 and E5). This strategy was designed to identify peptides that could effectively supplement M2 and E5 to enhance the sensitive serological detection of TB.
A major difficulty when directly using serum IgG as a molecular target for screening is excluding the impact of numerous non-specific IgG that are also present in the sample. We therefore adopted two screening strategies of adding the reverse screening IgG from healthy control sera to exclude/reduce the number of non-specific hits obtained during screening. For the first strategy, we adopted a subtraction method to obtain the phages binding to all the IgG in TB patient sera, before then removing the non-specific phages by eluting with IgG purified from healthy control sera as a means to effectively select positive clones. For the second strategy, to obtain specific rather than non-specific phage clones with high affinity, the phage displayed library was first interacted with IgG from health individuals and then only those phage clones that did not bind were further screened against IgG from TB patients. As expected, these screening strategies identified different phage clones with variant affinities and potential for diagnosis. Under the first, less stringent screening strategy, 12 kinds of sequence were obtained; with the exception of one clone (H12) that showed high affinity to TB IgG, however, none reacted with any of the screening molecules. These data indicate the complexity and diversity of the IgG response of TB patients, resulting in many non-specific clones and reduced screening efficiency. On the contrary, for the second strategy, we obtained eight kinds of sequences and three clones displayed effective binding affinities to the target molecules, indicating that the screening efficiency had been improved. We also found that the most frequently recurring clones from strategies one and two did not display significant binding efficiencies to the screening molecules by phage ELISA assay, which could be the ineffective mimotopes of unknown target, and this frequency may have risen as a result of non-specific biopanning, a major disadvantage of the immobilized screening.
With these two screening strategies, we identified four positive clones based on the phage-ELISA evaluation and part of sera sample detection, which all displayed higher reactivity to the TB patient samples than the health control samples. The search for similarity of peptide sequences in H37Rv database showed that the selected peptides are likely derived from some previously unidentified proteins in Mtb. Furthermore, mimotopes could also be isolated from antibodies against the carbohydrate and lipid antigens, which would not be detected by FASTA approach. Further studies of the selected mimotopes and their probable native antigen against these serum samples could reveal if those peptides represented mimotopes of the same antigen or of different antigens. Such data would also promote the better understanding of molecular mechanisms that participate in the immune response and allow the informed design of peptides for diagnostic purposes (Christiansen et al. 2015). To further estimate the value of these mimotopes for serological detection of Mtb, we synthesized four peptides based on the phage displayed amino acid sequence and assessed reactivity in ELISA against a large panel of about 300 serum samples from TB patients and controls. Similar results were obtained for four peptides, each demonstrating greater than 90 % specificity and greater than 45 % sensitivity. For TB15, the sensitivity reached 54 %, similar to that attained with the M2 and E5 peptides in our previous work (Yang et al. 2011, 2013). Pukazhvanthen et al. (2014) reported sensitivities for 38 kDa, MPT64, Adk, and BfrB ranging from 20 to 52.5 %, respectively, for TB serological diagnosis, with related specificities of around 95 % (2014). It was also recently reported that using PGL - Tb1 and ESAT - 6/CFP10 recombinant antigen, the sensitivities were 72.3 and 72.3 % respectively, with specificities about 91 % for TB HIV coinfection (Simonney et al. 2008; Lagrange et al. 2014). Thus, the diagnostic capability of the TB15 immunoactive peptide identified here is comparable to that of other mimotopes and antigens that have been reported, supporting the potential for broader application in the serological detection of TB. Furthermore, analyses of additional samples from cured patients and patients with relapse TB are required to evaluate the application value of TB15.
In conclusion, by using phage display random peptide library, we successfully obtained four immunoactive peptides that specifically bound IgG from the sera of TB patients. Since these peptides were identified in selective screening using samples that had not reacted with CE and MPT64 antigen epitope peptides we previously identified, it is our belief that integration and combined use of these three peptides will yield much higher sensitivity and specificity for the serological diagnosis of TB.
References
Alban SM, de Moura JF, Minozzo JC, Mira MT, Soccol VT (2013) Identification of mimotopes of Mycobacterium leprae as potential diagnostic reagents. BMC Infect Dis 13:42. doi:10.1186/1471-2334-13-42
Alban SM, de Moura JF, Thomaz-Soccol V, Bührer Sékula S, Alvarenga LM, Mira MT, Olortegui CC, Minozzo JC (2014) Phage display and synthetic peptides as promising biotechnological tools for the serological diagnosis of leprosy. PLoS ONE 9(8):e106222. doi:10.1371/journal.pone.0106222
Bahk YY, Kim SA, Kim JS, Euh HJ, Bai GH, Cho SN, Kim YS (2004) Antigens secreted from Mycobacterium tuberculosis: identification by proteomics approach and test for diagnostic marker. Proteomics 4(11):3299–3307
Barenholz A, Hovav AH, Fishman Y, Rahav G, Gershoni JM, Bercovier H (2007) A peptide mimetic of the mycobacterial mannosylatedlipoarabinomannan: characterization and potential applications. J Med Microbiol 56(Pt 5):579–586
Bekmurzayeva A, Sypabekova M, Kanayeva D (2013) Tuberculosis diagnosis using immunodominant, secreted antigens of Mycobacterium tuberculosis. Tuberc (Edinb) 0.93(4):381–388. doi:10.1016/j.tube.2013.03.003
Chinese Antituberculosis Association (1995) Chinese laboratory science procedure of diagnostic bacteriology in tuberculosis. Beijing, pp. 9–21
Christiansen A, Kringelum JV, Hansen CS, Bøgh KL, Sullivan E, Patel J, Rigby NM, Eiwegger T, Szépfalusi Z, de Masi F, Nielsen M, Lund O, Dufva M (2015) High-throughput sequencing enhanced phage display enables the identification of patient-specific epitope motifs in serum. Sci Rep. 5:12913. doi:10.1038/srep12913
Deng J, Bi L, Zhou L, Guo SJ, Fleming J, Jiang HW, Zhou Y, Gu J, Zhong Q, Wang ZX, Liu Z, Deng RP, Gao J, Chen T, Li W, Wang JF, Wang X, Li H, Ge F, Zhu G, Zhang HN, Gu J, Wu FL, Zhang Z, Wang D, Hang H, Li Y, Cheng L, He X, Tao SC, Zhang XE (2014) Mycobacterium tuberculosis proteome microarray for global studies of protein function and immunogenicity. Cell Rep 9(6):2317–2329. doi:10.1016/j.celrep.2014.11.023
Finlay WJ, Bloom L, Cunningham O (2011) Phage display: a powerful technology for the generation of high specificity affinity reagents from alternative immune sources. Methods Mol Biol 681:87–101. doi:10.1007/978-1-60761-913-0_6
Gevorkian G, Segura E, Acero G, Palma JP, Espitia C, Manoutcharian K, López-Marín LM (2005) Mimotopes of Mycobacterium tuberculosis carbohydrate immunodeterminants. Bichem J 387(Pt 2):411–417
Gonzalez JM, Francis B, Burda S, Hess K, Behera D, Gupta D, Agarwal AN, Verma I, Verma A, Myneedu VP, Niedbala S, Laal S (2014) Development of a POC test for TB based on multiple immunodominant epitopes of M. tuberculosis specific cell-wall proteins. PLoS ONE 9(9):e106279. doi:10.1371/journal.pone.0106279
Khan IH, Ravindran R, Yee J, Ziman M, Lewinsohn DM, Gennaro ML, Flynn JL, Goulding CW, DeRiemer K, Lerche NW, Luciw PA (2008) Profiling antibodies to Mycobacterium tuberculosis (M.tb.) by multiplex microbead suspension arrays for serodiagnosis of TB. Clin Vaccine Immunol 15(3):433–438
Kunnath-Velayudhan S, Salamon H, Wang HY, Davidow AL, Molina DM, Huynh VT, Cirillo DM, Michel G, Talbot EA, Perkins MD, Felgner PL, Liang X, Gennaro ML (2010) Dynamic antibody responses to the Mycobacterium tuberculosis proteome. ProcNatlAcadSci U S A 107(33):14703–14708. doi:10.1073/pnas.1009080107
Kunnath-Velayudhan S, Davidow AL, Wang HY, Molina DM, Huynh VT, Salamon H, Pine R, Michel G, Perkins MD, Xiaowu L, Felgner PL, Flynn JL, Catanzaro A, Gennaro ML (2012) Proteome-scale antibody responses and outcome of Mycobacterium tuberculosis infection in nonhuman primates and in tuberculosis patients. J Infect Dis 206(5):697–705. doi:10.1093/infdis/jis421
Lagrange PH, Thangaraj SK, Dayal R, Deshpande A, Ganguly NK, Girardi E, Joshi B, Katoch K, Katoch VM, Kumar M, Lakshmi V, Leportier M, Longuet C, Malladi SV, Mukerjee D, Nair D, Raja A, Raman B, Rodrigues C, Sharma P, Singh A, Singh S, Sodha A, Kabeer BS, Vernet G, Goletti D (2014) A toolbox for tuberculosis (TB) diagnosis: an Indian multi-centric study (2006–2008); evaluation of serological assays based on PGL-Tb1 and ESAT-6/CFP10 antigens for TB diagnosis. PLoS ONE 9(5):e96367. doi:10.1371/journal.pone.0096367
Li D, Zhang C, Lu N, Mu L, He Y, Xu L, Yang J, Fan Y, Kang Y, Yang C (2014) Cloning and characterization of Clp protease proteolytic subunit 2 and its implication in clinical diagnosis of tuberculosis. Int J Clin Exp Pathol 7(9):5674–5682 eCollection 2014
Pukazhvanthen P, Anbarasu D, Basirudeen SA, Raja A, Singh M (2014) Assessing humoral immune response of 4 recombinant antigens for serodiagnosis of tuberculosis. Tuberculosis (Edinb) 94(6):622–633
Saha A, Sharma A, Dhar A, Bhattacharyya B, Roy S, Das Gupta SK (2005) Antagonists of Hsp16.3, a low-molecular-weight mycobacterial chaperone and virulence factor, derived from phage-displayed peptide libraries. Appl Environ Microbiol 71(11):7334–7344
Schubert OT, Mouritsen J, Ludwig C, Röst HL, Rosenberger G, Arthur PK, Claassen M, Campbell DS, Sun Z, Farrah T, Gengenbacher M, Maiolica A, Kaufmann SH, Moritz RL, Aebersold R (2013) The Mtb proteome library: a resource of assays to quantify the complete proteome of Mycobacterium tuberculosis. Cell Host Microbe 13(5):602–612. doi:10.1016/j.chom.2013.04.008
Sharma A, Saha A, Bhattacharjee S, Majumdar S, Das Gupta SK (2006) Specific and randomly derived immunoactive peptide mimotopes of mycobacterial antigens. Clin Vaccine Immunol 13(10):1143–1154
Simonney N, Dewulf G, Herrmann JL, Gutierrez MC, Vicaut E, Boutron C, Leportier M, Lafaurie M, Abgrall S, Sereni D, Autran B, Carcelain G, Bourgarit A, Lagrange PH (2008) Anti-PGL-Tb1 responses as an indicator of the immune restoration syndrome in HIV-TB patients. Tuberc (Edinb). 88(5):453–461. doi:10.1016/j.tube.2008.01.006
Steingart KR, Flores LL, Dendukuri N, Schiller I, Laal S, Ramsay A, Hopewell PC, Pai M (2011) Commercial serological tests for the diagnosis of active pulmonary and extrapulmonary tuberculosis: an updated systematic review and meta-analysis. PLoS Med 8(8):e1001062. doi:10.1371/journal.pmed.1001062
Technical guidance group of the fifth national TB epidemiological survey, the office of the fifth national TB epidemiological survey (2012) The fifth national tuberculosis epidemiological survey in 2010. Chin J Antituberc. 34(8):485–508
Ulises HC, Tatiana G, Karlen G, Guillermo MH, Juan XC, Carlos E (2009) Peptide sequences identified by phage display are immunodominant functional motifs of pet and pic serine proteases secreted by Escherichia coli and Shigella flexneri. Peptides 30(12):2127–2135. doi:10.1016/j.peptides.2009.09.019
World Health Organization (2011) Commercial Serodiagnostic Tests for Diagnosis of Tuberculosis. Policy Statement. Geneva, Switzerland. WHO/HTM/TB/2011.5
World Health Organization (2014) Global tuberculosis report 2014.Geneva, Switzerland. WHO/HTM/TB/2014.08
Yang H, Liu ZH, Zhang LT, Wang J, Yang HS, Qin LH, Jin RL, Feng YH, Cui ZL, Zheng RJ, Hu ZY (2011) Selection and application of peptide mimotopes of MPT64 protein in Mycobacterium tuberculosis. J Med Microbiol 60(Pt 1):69–74. doi:10.1099/jmm.0.025098-0
Yang H, Chen H, Liu Z, Ma H, Qin L, Jin R, Zheng R, Feng Y, Cui Z, Wang J, Liu J, Hu Z (2013) A Novel B-Cell Epitope Identified with Mycobacterium tuberculosis CFP10/ESAT-6 Protein. PLOS ONE 8(1):e52848. doi:10.1371/journal.pone.0052848
Yang Y, Cao MJ, Alcocer M, Liu QM, Fei DX, Mao HY, Liu GM (2015) Mapping and characterization of antigenic epitopes of arginine kinase of Scylla paramamosain. Mol Immunol 65(2):310–320. doi:10.1016/j.molimm.2015.02.010
Zhang X, Su Z, Zhang X, Hu C, Yu J, Gao Q, Wang H (2013) Generation of Mycobacterium tuberculosis-specific recombinant antigens and evaluation of the clinical value of antibody detection for serological diagnosis of pulmonary tuberculosis. Int J Mol Med 31(3):751–757. doi:10.3892/ijmm.2013.1254
Acknowledgments
This work was supported by the grant from the National Key Project for Infectious Disease (no. 2013ZX10003009-001-010), the National Natural Science Foundation of China (no. 81301391), and the Science and Technology Commission of Shanghai Municipality, Shanghai, P.R. China (no. 124119a1500).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics statement
All of the experimental procedures and human blood sample collections were performed in accordance with the institutional guidelines. All of the individuals provided written informed consent prior to venipuncture. Experiments involving human subjects were approved by the Tongji University Ethics Committee (permit number: 2011-fk-03).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Hua Yang and Wei Sha contributed equally to this paper
Rights and permissions
About this article
Cite this article
Yang, H., Sha, W., Song, P. et al. Screening and identification of immunoactive peptide mimotopes for the enhanced serodiagnosis of tuberculosis. Appl Microbiol Biotechnol 100, 2279–2287 (2016). https://doi.org/10.1007/s00253-015-7122-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-7122-z