Abstract
The Arthrobacter sp. strain AK-YN10 is an s-triazine pesticide degrading bacterium isolated from a sugarcane field in Central India with history of repeated atrazine use. AK-YN10 was shown to degrade 99 % of atrazine in 30 h from media supplemented with 1000 mg L−1 of the herbicide. Draft genome sequencing revealed similarity to pAO1, TC1, and TC2 catabolic plasmids of the Arthrobacter taxon. Plasmid profiling analyses revealed the presence of four catabolic plasmids. The trzN, atzB, and atzC atrazine-degrading genes were located on a plasmid of approximately 113 kb.
The flagellar operon found in the AK-YN10 draft genome suggests motility, an interesting trait for a bioremediation agent, and was homologous to that of Arthrobacter chlorophenolicus. The multiple s-triazines degradation property of this isolate makes it a good candidate for bioremediation of soils contaminated by s-triazine pesticides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Each year, approximately 5.6 billion pounds of pesticides are used worldwide in agriculture amongst which herbicides and insecticides have wide applications (Michael and Alavanja 2009). Their presence in soil and surface water is known to create serious health hazards (Hayes et al. 2010).
Although banned in Europe, atrazine remains a very popular herbicide in different parts of the world including India and the rest of Asia and North and South America, where it is used for pre- and post-control of grassy weeds in corn and sugarcane fields. It is an endocrine disruptor reported to cause sex reversal in frogs (Hayes et al. 2002, 2010). Furthermore, s-triazine compounds and their metabolites have been reported to induce mammary gland tumors in Sprague–Dawley female rats (Sene et al. 2010). Hence, removal of these toxic compounds from the environment is important for the ecological balance.
Bioremediation has the potential to offer an environmental friendly solution to restore contaminated biotopes inexpensively yet effectively. A large number of bacteria have been isolated demonstrating the degradation of atrazine. Amongst them, Actinobacteria are known for degradation of many s-triazine pesticides (Krutz et al. 2010). Rapid and broad spectrum activity of actinobacteria for s-triazine degradation makes it a suitable candidate for field bioremediation. But bioremediation remains challenging due to the lack of detailed knowledge of soil bacteria, their degradative pathways, efficiencies in low nutrient conditions, and survival capabilities under environmental stress.
Some difficulties in understanding the degradative pathways are due to the nature and specificity of the enzymes involved. All pesticide degrading-enzymes reported to date are members of different protein superfamilies, most of them having multiple functions that are different from the target enzyme. Besides, biodegradative functions can arise independently based on the environmental conditions and hence genes with completely different sequences may catalyze the same reaction (Fenner et al. 2013). Understanding these complicate systems thus requires new tools like next generation sequencing and bioinformatic analyses that offer unexplored insights in understanding physiologies of s-triazine degrading microorganisms relevant for bioremediation (Lovley 2003).
Bacteria from the genus Arthrobacter possess various catabolic pathways for the detoxification of xenobiotic compounds, most of them being plasmid-encoded (Supplementary Table S1) (Igloi and Brandsch 2003; Jerke et al. 2008). Members of this genus are ubiquitous due to their property of being nutritionally versatile and tolerant to environmental stresses (Niewerth et al. 2013). Seven complete and 35 partial genome sequences of Arthrobacter are available in NCBI database.
In this study, we report the isolation and the characterization of a bacterial strain, AK-YN10, which has great potential to be used in bioaugmentation for decontamination of s-triazine-polluted soils. This bacterium was isolated from an agricultural field in India, repeatedly treated with atrazine for weed control in sugarcane cultivation. It was identified as Arthrobacter sp. by analyzing its 16S rRNA gene sequence. Multi substrate degradation capacity was demonstrated using microbiological and analytical chemistry analyses. The isolate was characterized by analyzing its draft genome, and its potential for bioremediation was demonstrated by microcosm experiments using pesticide-contaminated soil.
Materials and methods
Chemicals and strains
Atrazine, simazine, terbuthylazine, ametryn, prometryn, atratone, and prometon of 99 % analytical purity were purchased from Sigma Aldrich, USA.
Two bacterial strains used in this study, Arthrobacter sp. strain AK-YN10 (laboratory isolate) and Arthrobacter aurescens TC1, ATCC-BAA-1386 (control). In the manuscript, Arthrobacter sp. strain AK-YN10 is abbreviated as AK-YN10. This strain is deposited in the collection of microorganisms of interest for agriculture and environment (MIAE) of INRA of Dijon (France) under the registration no. MIAE01508.
Isolation and identification of the Arthrobacter isolate
Isolation of bacteria was carried out from soil samples collected from agricultural field (Yadavmala, Maharashtra, India) cultivating sugarcane and reporting the use of atrazine for the last 3 years (Sagarkar et al. 2013). Soil samples of 100 g were enriched on 250 mL Basal media (BS media) (Cai et al. 2003) supplemented with 300 mg L−1 atrazine. Every 15 days, the enrichment was subjected to centrifugation (7000×g, 10 min) and the pellet was transferred into 250 ml of fresh BS media. The process was repeated for eight such transfers after which atrazine degrading bacteria were selected on the basis of zone of atrazine clearance obtained on BS agar plates with 300 mg L−1 atrazine.
The 16S rRNA gene from AK-YN10 was amplified and cloned in pCRTM 2.1 vector (TA cloning kit, Invitrogen, USA). Colonies obtained after transformation were screened and a 1.5 kb fragment of the 16S rRNA was sequenced.
Atrazine degradation
Atrazine degradation capacity of the lab isolate was tested in BS media where 1000 mg L−1 atrazine was used as both carbon and nitrogen source. The seed culture was prepared by growing the strain in 0.1× Luria Broth (LB) (HiMedia, India) containing 100 mg L−1 atrazine. Growth was measured as OD600 and cell quantity of 5 OD600 was harvested by centrifugation. The pellet was washed in sterile BS media, without any carbon source and resuspended in the same so as to yield 0.1 OD600 mL−1. Atrazine was added and flasks were incubated at 30 °C at 120 rpm. Growth and atrazine degradation was monitored for 70 h. All experiments were performed in triplicates.
Atrazine mineralization assay
A mineralization assay was performed as reported by Udiković-Kolić et al. (2008). AK-YN10 was grown in MS medium with 30 mg L−1 atrazine until the exponential phase of growth at 30 °C. Bacterial cell quantities of 0.3OD600 were introduced into sterile respirometers with 10 mL Knapp buffer and 30 mg L−1 atrazine containing 147.7 Bq mL−1 of 14C ring-labeled atrazine (14C-ATZ-Ring, 24.6 mCi mmol−1, Sigma) in one flask and 56.4 Bq mL−1 of 14C ethyl-chain-labeled atrazine (14C-ATZ-Chain, 1776 Mbq mmol−1, Isotophim) in another flask at 30 °C and 100 rpm. The mineralization assay was performed in triplicates.
The mineralization of atrazine was monitored by counting the 14CO2, trapped in 5 mL of 0.2 M NaOH by liquid scintillation counting (Packard) using 10 mL of ACSII scintillation fluid (Amersham) and the kinetics were calculated as previously reported (Udiković-Kolić et al. 2008).
Analysis of multi-substrate degrading property
Multi-substrate degrading properties of the AK-YN10 isolate were assessed by analyzing degradation of the following s-triazines; simazine, terbuthylazine, atratone, prometon, ametryn, and prometryn. The seed culture was prepared as mentioned above and a bacterial suspension of 0.1 OD600 per mL−1 was inoculated in M9 media (Kapley et al. 2001) with 100 mg L−1 of substrate. The s-triazine pesticides were used either (i) as sole source of carbon and nitrogen, or (ii) as sole source of carbon, supplemented with 1 g L−1 ammonium chloride as the nitrogen source, or (iii) as sole source of nitrogen supplemented with 3 g L−1 citrate as carbon source. Un-inoculated controls were also set up. Degradation was carried out at 30 °C for 8 days and was monitored by HPLC as described below.
Analyzing the bioremediation potential of isolate AK-YN10 by microcosm experiments
The bioremediation potential of the lab-isolate AK-YN10 was studied with atrazine contaminated soil in microcosm experiments. Utilization of atrazine under bioaugmentation conditions was compared against the degradative capacity under the condition of natural attenuation. The two conditions were analyzed in duplicate microcosms set up using soil collected from the NEERI garden premises. The microcosms were set up as follows: Composite soil was prepared according to ISO 10381–6 recommendations (soil quality–sampling–Part 6: Guidance on the collection, handling, and storage of soil for the assessment of aerobic microbial processes in the laboratory). After sieving (2-mm mesh), soil samples were thoroughly mixed and atrazine was applied at 100 mg kg−1 of soil (dry weight equivalent). Four aliquots of 250 g were then transferred into wide mouth 1 L jars to generate four microcosms. Two were used to study the effect of bioaugmentation with AK-YN10 isolate and two were used to analyze the capacity of natural attenuation. The isolate was grown in 0.1 × LB for 18 h at 30 °C. Cells were harvested by centrifugation and the cell pellet was washed with buffer and resuspended in 5 mL of 20 × BS media (Sagarkar et al. 2014b). AK-YN10 was inoculated at 2.5 × 107 CFU per 250 g soil (dry weight equivalent) into two of the four experimental microcosms (bioaugmentation), while the remaining two other replicates were not inoculated (control). Before harvesting the cells, the presence of all genes was confirmed by PCR to exclude the possibility of plasmid abortion. Microcosms were kept at room temperature (30 ± 1 °C). The soil moisture content was monitored once per week and was maintained at 40 % until the end of the experiment by addition of sterile distilled water. Degradation of atrazine was monitored by HPLC on day 0,3,6,9, and 12.
Molecular biology methods
Atrazine-degrading genetic potential characterization
AK-YN10 was grown until its exponential phase of growth and harvested for total DNA extraction done with the Fast DNA™ Spin Kit from MP Biomedicals. DNA aliquots of 50 μL were stored at −20 °C until required. The atrazine-degrading genetic potential was determined by PCR using different primer pairs listed in Table 1 targeting atzA, B, C, D, and trzN,D sequences.
The total genomic DNA of AK-YN10 was digested either with NruI or SmaI restriction enzymes. Digested DNAs were separated by electrophoresis on a 1 % agarose gel, vacuum-transferred onto a Biodyne Plus membrane (Gelman Sciences, Merck Eurolab, France), and analyzed by southern hybridization under high stringency conditions using digoxigenin labeled probes targeting either atzA, B, C, D, E, and trzD,N genes (Devers et al. 2007).
Plasmid profiling and hybridization
Eckhardt’s method as modified by Wheatcroft (Eckhardt 1978; Wheatcroft et al. 1990) was applied to obtain plasmid DNA profiles. Briefly, bacterial cultures were grown in BS medium containing 100 mg L−1 atrazine at 30 °C and 120 rpm. After their separation and transfer to a membrane, plasmids were hybridized with digoxigenin probes of atzA, B, C, D, E, and trzD, N sequences as described above. The size of the plasmid was estimated according to Udiković-Kolić et al. (2008).
Genome sequencing and annotation
The genomic DNA of AK-YN10 was sequenced two times. In the first version, total DNA of AK-YN10 was prepared using the Fast DNA™ Spin Kit for bacterial DNA isolation from MP Biomedicals (France). In the second version, DNA isolation was carried out by the plasmid enrichment protocol (Anderson and Mckay 1983). DNA purity was analyzed by a NanoDrop 1000 Spectrophotometer (Thermo Scientific). The genome was sequenced using the Illumina MiSeq20 sequencing platform (Sagarkar et al. 2014a). The paired-end sequencing libraries were prepared using Illumina Nextera DNA Library Preparation Kit. Paired-End sequencing allows the template fragments to be sequenced in both the forward and reverse directions. The amplified library was analyzed in Bioanalyzer 2100 (Agilent Technologies) using high sensitivity (HS) DNA chip as per manufacturer’s instructions. The raw data were filtered using trimmomatica with a minimum read length of 50 and a minimum quality of 20. Sequencing reads were assembled by using a GS assembler version 2.6. Version one was assembled using Arthrobacter reference and yielded 36× sequence coverage, while version two of the draft genome was assembled de novo. The genome was annotated using the NCBI Prokaryotic Genomes Automatic Annotation Pipeline (PGAAP) and was independently analyzed on the RAST server (Aziz et al. 2008). A circular genome map was generated using CGView server V 1.0 (2008), http://stothard.afns.ualberta.ca/cgview_server/) to compare the draft genome of the lab isolate, AK-YN10 (AVPD00000000.1, AVPD00000000.2) with the complete genome of its nearest neighbor, A. aurescens TC1 (CP000474.1) using default parameters.
The assembled scaffolds were used to predict CDS. The predicted CDS were annotated using BLASTX and BLASTN search against NCBI non-redundant database. We considered the top hit species distribution of annotated CDS from BLASTN for identification of plasmids. In order to identify plasmid regions, contigs were submitted to similarity searches carried out by using Microbial nucleotide BLAST with default parameters against the Arthrobacter taxon 1663; with complete genome (6) and complete plasmids (15). A plasmid dataset extracted from the genome of AK-YN10 was aligned with the corresponding sequences of plasmids of Arthrobacter nicotinovorans (pAO1 plasmid) and A. aurescens (TC1 plasmid) using Mauve 2.1 with default parameters.
Complete TrzN protein sequences were retrieved from the NCBI database. They were aligned with the AK-YN10 TrzN protein using multiple sequence alignment functions in ClustalW in Mega 5.2 and viewed using Jal view version 2.8 (Sagarkar et al. 2013).
Motility assay
Motility test medium was prepared as given in the ASM microbial library protocol. For motility assay, a test medium tube was stabbed with A. aurescens TC1 and AK-YN10 and was incubated at 30 °C for 72 h or until growth was evident.
HPLC analysis
A 1 mL sample was harvested from growth medium, mixed with an equal volume of methanol, and centrifuged at 10,000 rpm for 5 min. The supernatant was collected and filtered through a 0.22 μm filter (Millipore) and analysed by HPLC.
A Licrosphere RP18 column was used in a HPLC system (Waters e2695), equipped with a PDA detector and a quaternary phasic auto injector. The conditions reported by Farre et al. (2005) were used for detection of triazine herbicides.
Nucleotide sequence accession number
The 16S rRNA, atzB, atzC, and trzN gene sequences of AK-YN10 were deposited in GenBank under the accession numbers HE716859, HE716866, HE716867, and HE716868. The whole genome shotgun sequence of AK-YN10 was deposited under the accession numbers NZ_AVPD00000000 version AVPD01000000, AVPD02000000.
Results
Isolation and characterization of the s-triazine degrading bacterial strain
A gram positive, yellow colored, smooth margined bacterial isolate, producing a zone of clearance on BS plates containing atrazine was isolated from enrichments. The isolate, referred to AK-YN10, was identified as Arthrobacter genus by sequence analysis of its 16S rRNA gene. The 16S rRNA phylogeny demonstrated that A. aurescens TC1 and Arthrobacter sp. Rue61a were the closest relatives of AK-YN10 with 98 % of sequence similarity (Supplementary Fig. S1). A circular map comparing an Arthrobacter sp AK-YN10 version 2 (AVPD00000000.2) and complete genome of A. aurescens TC1 (CP000474.1) can be viewed in Supplementary Fig S2.
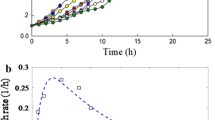
The capacity of the laboratory strain, AK-YN10, to degrade atrazine was demonstrated by monitoring of atrazine dissipation (Fig. 1a) and atrazine mineralization (Fig. 1b). Modeling of dissipation kinetics allowed estimating an atrazine degradation rate of 35.58 ± 0.36 mg L−1 h−1, an atrazine half-life of 9.45 ± 0.07 h, and a growth rate of AK-YN10 of 0.24 ± 0.06 OD mL−1 day−1 (Table 2). The potential of AK-YN10 to mineralize atrazine was analyzed by radiorespirometry using both 14C-ATZ-Ring and 14C-ATZ-chain. Mineralization kinetics showed that AK-YN10 was only able to mineralize 14C-ATZ-chain (Fig. 1b). 14CO2 was rapidly formed reaching a maximum mineralization of 61.83 ± 4.4 % of initially added 14C-atrazine after 5 days of incubation. Modeling of the mineralization kinetics with the modified Gompertz model allowed estimating a mineralization rate of 34.40 ± 5.5 h−1 and a lag time (K) of 0.054 ± 0.01 h.
Utilization of atrazine under different conditions by bacterial isolate AK-YN10. a Atrazine dissipation and growth kinetics of Arthrobacter sp. AK-YN10 when 1000 mg L−1 atrazine was used as sole nitrogen source. Vertical bars indicate standard deviation and b atrazine mineralization kinetics of strain AK-YN10 obtained with 14C-chain- or 14C-ring-labeled atrazine. Median values of cumulated 14CO2 evolved from 14C-atrazine are shown. Vertical bars indicate standard deviation. c Demonstration of bioremediation potential of lab isolate AK-YN10 in microcosm experiments with 100 mg kg−1 atrazine. The vertical bars indicate the percentage of atrazine remaining in the bioaugmented microcosm and the line graph indicates the percentage of atrazine remaining in the control microcosms (natural attenuation)
AK-YN10 also demonstrated the capacity to degrade other s-triazine pesticides, utilizing them as a source of nitrogen. Growth kinetic parameters were estimated and are shown in Table 2. No growth was observed when the s-triazine pesticides were used as sole carbon source (data not shown). AK-YN10 was able to rapidly degrade simazine, ametron, and prometron with high rate of degradation superior to 70 % day−1, while ametryn, prometryn, and terbuthylazine demonstrated relatively low degradation rates (i.e., inferior to 50 % degradation day−1). A maximum growth rate of 0.28 ± 0.05 OD day−1 was observed with ametron.
Figure 1c demonstrates the efficiency of the lab isolate in enhancing bioremediation of contaminated soil. In contrast to weak atrazine dissipation in control soil microcosms, atrazine was completely dissipated within 12 days in the soil microcosms bioaugmented with AK-YN10.
Localization of atrazine degradation genes in total DNA
Amplification results demonstrated the atrazine-degrading genetic potential of AK-YN10 comprised of trzN, atzB, and atzC genes. All amplicons were cloned, sequenced, and compared to known sequences confirming their sequence similarity (Table 1).
Figure 2 demonstrates the localization of atrazine degradation genes as analyzed by Southern blot hybridization. Total DNA (genomic + plasmid) (Fig. 2a) and plasmid DNA (Fig. 2b) were hybridized with trzN, atzB, and atzC probes. Before electrophoresis, total DNA was digested with (i) NruI and (ii) SmaI restriction enzymes. Results indicate that all three probes hybridized to the plasmid DNA as well as total DNA digested with SmaI. The absence of a hybridization signal with trzN probe on the total DNA digested by NruI may be attributed to the presence of this gene on the NruI-restriction fragment inferior to 4 kb. As it can be seen in Fig. 2b, the isolate shows the presence of at least four plasmids. All probes hybridized to only one of those, indicating that the three atrazine degradation genes are located on a plasmid of approximately 113 kb.
Confirmation of genes from the atrazine degradation pathway in isolate Arthrobacter sp. AK-YN10 by Southern Hybridization. a Southern blot hybridization of atrazine degrading genes with total DNA of AK-YN10 digested with NruI and SmaI restriction enzymes. Lanes labeled as “M” indicate the molecular size marker where size in bp are shown on the left hand side. Lanes 1 and 2 demonstrate the digestion profile of total DNA digested with restriction enzymes NruI and SmaI, respectively. Lanes 3–5 show the results of hybrization of trzN, atzB, and atzC probes, respectively, on NruI digested DNA. Lanes 6–8 show the results of hybrization of trzN, atzB, and atzC probes, respectively, on SmaI digested DNA. b Plasmid profiling and southern blot hybridization of atrazine degrading genes with plasmid DNA. Sizes of plasmid markers (in Kbp) are shown on the left hand side. Lane 1 indicates the presence of four plasmids in the lab isolate. Lanes 2–4 show the hybridization results with trzN, atzB, and atzC probes, respectively
General features on Arthrobacter sp. AK-YN10 draft genome and genes involved in atrazine metabolism
Two versions of the draft genome (V1 and V2) have been submitted to NCBI (accession no. AVPD01000000, AVPD02000000). According to the first version sequencing, the genome size of about 4.84 Mb is distributed in 107 contigs with an average GC content of 63.3 %, containing 4578 putative coding sequences (CDSs). Distribution of 107 contigs into the bacterial plasmids and chromosome is presented in Supplementary Fig. S3. About 4.2 Mb of the AK-YN10 genome, representing approximately 87 % of the sequence data, demonstrated 80–90 % similarity to the complete genome of A. aurescens TC1 (accession no. NC_008711). The remaining 13 % data demonstrates homology to different plasmids as follows; (i) the ∼94 kb region shows >90 % similarity to A. nicotinovorans pAO1 plasmid pAO1 (NC_021229), (ii) the 31.427 kb region shows >90 % similarity to the A. aurescens TC1 plasmid TC1 (NC_008712), (iii) the 248.260 kb region of contig 7 and contig 26 demonstrates >85 % similarity but <20 % query coverage with A. aurescens TC1 plasmid TC2 (NC_008713), and (iv) the 195 kb region contains mainly hypothetical proteins. A list of whole genome sequences of different Arthrobacter species available in the NCBI database is listed in the Supplementary Table S1.
Microbial nucleotide BLAST carried out against plasmid dataset demonstrated >90 % identity with A. aurescens TC1 plasmid TC2 and A. nicotinovorans pAO1 plasmid (Supplementary Table S2). Since our results suggested the presence of multiple plasmids, mauve alignment was used to analyze homology with plasmids showing high similarity. Ten contigs showed similarity to A. nicotinovorans pAO1 plasmid (Supplementary Fig. S4, panel I), wherein six contigs were highly similar (>90 % similarity) and four were poorly similar (<70 % similarity). All together, a ∼108 kb fragment was reconstituted from the sequencing data where annotation revealed the presence of a 6-hydroxy-d-nicotine oxidase gene for nicotine degradation, a complete set of ORFs for enzymes essential for the biosynthesis of the molybdenum dinucleotide cofactor, as well as ORFs related to uptake and utilization of carbohydrates, sarcosine, and amino acids.
RAST annotation revealed that the atzB and atzC genes coding for atrazine-degrading enzymes were respectively located on contigs 63 (M707_23105) and 35 (Fig. 3a). The trzN gene sequence was not found in the first version of the draft genome of AK-YN10 even though its presence was shown by amplification and Southern hybridization done on total DNA. Alignment with the TC1 plasmid revealed 12 contigs out of which ten contigs were 99 % identical to the TC1 plasmid (Supplementary Fig. S4, panel II) and two V2 contigs numbers 12 and 35 had 90 % similarity but <45 % query coverage. V1 and V2 indicate the two different versions of the draft genome of isolate AK-YN10.
Organization of genes involved in atrazine degradation and organism motility. Panel a depicts the organization and comparison of catabolic genes involved in atrazine degradation in Arthrobacter sp. AK-YN10 and A. aurescens TC1 plasmid TC1. Analyses were performed using RAST. The trzN gene was found in version 2 of the draft genome on contig 12, while atzB and atzC were found in version 1 of the draft genome and are hence shown separately. Hypothetical proteins are indicated in gray color. Panel b depicts the organization and comparison of functional flagellar genes of A. chlorophenolicus (CP001341) with Arthrobacter sp. AK-YN10 contig 22
The second version of the draft genome, V2, yielded 4.4 Mb data distributed in 464 contigs with 4020 CDS, with 1437 of them being hypothetical proteins. The trzN gene was located on a 30.951 kb contig 12, at 28,925–27,555 nucleotides (RAST annotation). This contig demonstrated 99 % similarity in the region from 26,119–29,103 to region 100,512–103,496 of A. aurescens TC1 plasmid (Fig. 3a).
Amino acid sequence comparison of the AK-YN10 triazine hydrolase to complete s-triazine hydrolases from the NCBI database indicated that AK-YN10 has distinct Pro(299) residue instead of Ala(299). Whereas Tyr(68) and Glu(117) residues were present instead of Phe(68) and Gln(117) like in Pseudomonas sp. AD39 (FJ161692). At position 305, Met to Leu sequence variation was observed like in Nocardioides sp. C190 (AF416746) (Supplementary Fig. S5). The amino acid sequence of AtzB and AtzC was 100 % identical to the TC1 plasmid.
Motility associated genes
Mobility is an interesting phenotype for microbial candidates for bioremediation. Hence, we carried out soft agar motility assay and looked for flagellar genes in the draft genome. As can be seen in Fig. 3b, the complete operon for flagellar biosynthesis genes was present on contig 22 with 60–90 % sequence similarity to the flagellar cassette of Arthrobacter chlorophenolicus (CP001341). The functional aspect is demonstrated in Supplementary Fig S6 by soft agar motility assay.
Discussion
In this study, we report the isolation and characterization of Arthrobacter sp. AK-YN10 with the ability to degrade 1000 mg L−1 atrazine in 24 h under laboratory conditions. This result is in accordance with previous studies reporting the isolation of several others Gram-positive bacteria able to transform atrazine. Nocardioides sp. C190 was the first Gram-positive atrazine degrading bacterium isolated from a Canadian agricultural soil frequently exposed to this herbicide (Topp et al. 2000). Subsequently, other Gram-positive atrazine degraders have been characterized, belonging to Nocardioides (Piutti et al. 2003; Yamazaki et al. 2008) or the Arthrobacter genus (Strong et al. 2002; Devers et al. 2007; Arbeli and Fuentes 2010). Most of these strains harbor a trzN–atzBC gene combination allowing the transformation of atrazine to cyanuric acid (Sajjaphan et al. 2004; Udiković-Kolić et al. 2012). Several strains harbor a more complete gene combination of trzN-atzCDEF (Arbeli and Fuentes 2010) or trzN-atzBC-trzD (Vaishampayan et al. 2007) allowing the complete mineralization of atrazine to simple compounds naturally present in the environment. Likewise, our isolate has the trzN–atzBC gene combination responsible for the transformation of atrazine to cyanuric acid. Accordingly, the AK-YN10 atrazine degradation pathway was found to start by de-halogenation of atrazine to hydoxyatrazine, catalysed by TrzN. Hydroxyatrazine is then transformed to cyanuric acid by AtzB and AtzC enzymes. In addition, AK-YN10 was shown to degrade several other s-triazines including simazine, ametron, prometron, ametryn, prometryn, and terbuthylazine. This is in line with several other studies reporting that strains harboring the trzN–atzBC gene combination have a wide s-triazine substrate range. A. aurescens TC1 is reported to use 23 different s-triazines as substrate (Strong et al. 2002) and Udiković-Kolić et al. 2008 reported that a bacterial community, harboring Gram-positive isolates, enriched from waste water from an herbicide factory, was able to degrade a range of substituted s-triazines.
Southern blot analysis confirmed localization of these genes to a single plasmid. trzN and atzBC genes of AK-YN10 were 99 % homologous to that found on the ∼300 kb TC1 plasmid of A. aurescens TC1 (Mongodin et al. 2006).
The trzN gene was located on a 30.951 kb contig, with only a 2984 bp region containing the trzN gene being 99 % identical to the TC1 plasmid. The rest of the contig does not have similarity with anything in the database. It is noteworthy that a sharp increase in G + C content was observed in the region flanking the trzN gene (Supplementary Fig. S7) which might be the signature of a genetic rearrangement mediated by an IS element with a lower G + C content. This is in contrast with the TC1 plasmid showing no segmentation in the G + C profile in the ∼34 kb fragment containing the trzN gene.
The atzB gene was present on contig 63, and the atzC gene was present at the end of contig 35. Southern blot analysis indicated that atzB and atzC genes were located on a single fragment of 7.3 kb. To explain this discrepancy, one could hypothesize that contigs 63 and 35 might be separated by a transposon similar to that of the atzBC gene arrangement in the TC1 plasmid not allowing their assembly following full sequencing (Fig. 3a) highlighting the difficulty of genome assembly in the presence of insertion sequences rich in repeated sequences.
The atzC gene was located on the 37.732 kb contig which has 42 % query coverage and 92 % similarity to the TC1 plasmid, i.e., half of the contig did not match to sequences in the database suggesting horizontal gene transfer of atzBC gene fragment.
The trzN–atzBC gene combination along with IS elements is widely reported over the years and in different strains isolated worldwide mainly present amongst actinobacteria (Vaishampayan et al. 2007; Devers et al. 2007; Arbeli and Fuentes 2010). As these genes were reported to be flanked by IS elements, genetic rearrangement mediated by them cannot be excluded.
In AK-YN10, besides the plasmid harboring atrazine degrading genes, three other plasmids were observed. Total genome sequencing revealed contigs with >90 % similarity to the plasmid pAO1 but its functional role has not been explored in this study. Even though the isolate was grown in minimal medium with atrazine, the pAO1-like plasmid was maintained. Hence, future work can be targeted to study the effect of co-existence of these plasmids implied in atrazine degradation.
Mongodin et al. 2006 reported that genes involved in plasmid partitioning in TC1 and pAO1 share a common ancestor. But in case of AK-YN10, ParA proteins were different from TC1 and pAO1 but ParB and NrdH proteins on contig 50 had 50 and 66 % amino acid identity to the plasmid TC1. This suggests that TC1 and one of the plasmids out of four have a common ancestor but the ancestors of the remaining three plasmids are different.
A broad s-triazine substrate range has been reported for the triazine hydrolase of actinobacteria like A. aurescens TC1 and Nocardiodies sp. C190 (Yamazaki et al. 2008). The broad substrate specificity and high enzyme activity of TrzN provides an evolutionary advantage over AtzA making it dominant amongst Gram-positive soil bacteria (Arbeli and Fuentes 2010). AK-YN10 had four amino acid sequence mutations in triazine hydrolases. Residues 76 and 78 are active sites of triazine hydrolases. Variation at residue phe(68) to tyr(68) changes polar amino acids to non-polar compounds. This variation may affect enzyme activity as these residues are near the active site.
Arthrobacter are robust soil dwelling bacteria. The draft genome of AK-YN10 indicates the presence of genetic potential towards environmental stress tolerance capacity by the presence of a number of stress response genes (data not shown). Furthermore, it is resistant to many heavy metals (cobalt, copper, zinc, arsenic, and mercury) and antibiotics (Gentamycin (10 μg/disc), rifampicin (15 μg/disc), penicillin G (10 μnits/disc), ampicillin (25 μg/disc), polymyxin (50 μg/disc) like Arthrobacter phenanthrenivorans type strain (Sphe3) (Kallimanis et al. 2011) and Arthrobacter sp. Rue61a (Niewerth et al. 2013). Apart from all these features, flagellar motility is an interesting feature of this strain rarely observed in the Arthrobacter genus. Among the six full genome sequences, only A. chlorophenolicus A6 has the motility phenotype (Nordin et al. 2005). In silico analysis demonstrated the flagellar gene cassette of AK-YN10 is similar to A. chlorophenolicus while the rest of the chromosome was similar to A. aurescens suggesting its acquisition by horizontal gene transfer.
The bioremediation assay carried out with AK-YN10 in soil microcosms incubated under laboratory conditions showed that its degrading capability along with its environmental stress tolerance and motility makes it a suitable candidate for bioremediation of s-triazine contaminated soils. As the degrading genes are plasmid born and surrounded by insertion sequences (IS), following its inoculation to soil, AK-YN10 may spread its degrading-genetic potential to related microbial populations by horizontal gene transfer and further genetic rearrangement mediated by IS elements has already been shown under controlled conditions (Devers et al. 2007).
This study reported the isolation and characterization of a multiple s-triazines degrading bacterium that could be a good candidate for bioremediation. Draft genome analysis enabled us to understand the s-triazine degrading characteristics of this isolate and provided knowledge that would be helpful to assess the efficiency of this isolate and monitor its survival once released into contaminated environments that are to be restored.
References
Anderson DG, Mckay LL (1983) Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Microbiol Biotechnol 46:549–552
Arbeli Z, Fuentes C (2010) Prevalence of the gene trzN and biogeographic patterns among atrazine-degrading bacteria isolated from 13 Colombian agricultural soils. FEMS Microbiol Ecol 73:611–623
Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, Meyer F, Olsen GJ, Olson R, Osterman AL, Overbeek RA, McNeil LK, Paarmann D, Paczian T, Parrello B, Pusch GD, Reich C, Stevens R, Vassieva O, Vonstein V, Wilke A, Zagnitko O (2008) The RAST server: rapid annotations using subsystems technology. BMC Genomics 9:75
Cai B, Han Y, Liu B, Ren Y, Jiang S (2003) Isolation and characterization of an atrazine-degrading bacterium from industrial waste water in China. Lett Appl Microbiol 36:272–276
De Souza ML, Wackett LP, Sadowski MJ (1998) The atzABC genes encoding atrazine catabolism are located on a self transmissible plasmid in Pseudomonas sp. strain ADP. Appl Environ Microbiol 64:2323–2326
Devers M, Soulas G, Martin-Laurent F (2004) Real-time reverse transcription PCR analysis of expression of atrazine catabolism genes in two bacterial strains isolated from soil. J Microbiol Methods 56:1–3
Devers M, Azhari NE, Kolic NU, Martin-Laurent F (2007) Detection and organization of atrazine-degrading genetic potential of seventeen bacterial isolates belonging to divergent taxa indicate a recent common origin of their catabolic functions. FEMS Microbiol Lett 273:78–86
Eckhardt T (1978) A rapid method for identification of plasmid deoxyribonucleic acid in bacteria. Plasmid 1:584–588
Farre MJ, Franch MI, Malato S, Ayllon JA, Peral J, Domenech X (2005) Degradation of some biorecalcitrant pesticides by homogeneous and heterogeneous photocatalytic ozonation. Chemosphere 58:1127–1133
Fenner K, Canonica S, Wackett LP, Elsner M (2013) Evaluating pesticide degradation in the environment: blind spots and emerging opportunities. Science 341:752–758
Fruchey I, Shapir N, Sadowsky MJ, Wackett LP (2003) On the origins of cyanuric acid hydrolase: purification, substrates, and prevalence of AtzD from Pseudomonas sp. strain ADP. Appl Environ Microbiol 69:3653–3657
Grant JR, Stothard P (2008) The CGView Server: a comparative genomics tool for circular genomes. Nucleic Acids Res 36:181–184
Hayes TB, Haston K, Tsui M, Hoang A, Haeffele C, Vonk A (2002) Feminization of male frogs in the wild. Nature 419:895–896
Hayes TB, Khoury V, Narayan A, Nazir M, Park A, Brown T, Adame L, Chan E, Buchholz D, Stueve T, Gallipeau S (2010) Atrazine induces complete feminization and chemical castration in male African clawed frogs (Xenopus laevis). Proc Natl Acad Sci U S A 107:4612–4617
Igloi GL, Brandsch R (2003) Sequence of the 165-kilobase catabolic plasmid pAO1 from Arthrobacter nicotinovorans and identification of a pAO1-dependent nicotine uptake system. J Bacteriol 185:1976–1986
Jerke K, Nakatsu CH, Beasley F, Konopka A (2008) Comparative analysis of eight Arthrobacter plasmids. Plasmid 59:73–85
Kallimanis A, Labutti KM, Lapidus A, Clum A, Lykidis A, Mavromatis K, Pagani I, Liolios K, Ivanova N, Goodwin L, Pitluck S, Chen A, Palaniappan K, Markowitz V, Bristow J, Velentzas AD, Perisynakis A, Ouzounis CC, Kyrpides NC, Koukkou AI, Drainas C (2011) Complete genome sequence of Arthrobacter phenanthrenivorans type strain (Sphe3). Stand Genomic Sci 29:123–130
Kapley A, Tolamare A, Purohit HJ (2001) Role of oxygen in partial utilization of phenol in continuous culture. World J Microbiol Biotechnol 17:801–804
Krutz LJ, Shaner DL, Weaver MA, Webb RM, Zablotowicz RM, Reddy KN, Huang Y, Thomson SJ (2010) Agronomic and environmental implications of enhanced s-triazine degradation. Pest Manag Sci 66:461–481
Lovley DR (2003) Cleaning up with genomics: Applying molecular biology to Bioremediation. Nat Rev Microbiol 1:35–44
Michael CR, Alavanja PH (2009) Pesticides use and exposure extensive worldwide. Rev Environ Health 24:303–309
Mongodin EF, Shapir N, Daugherty SC, Deboy RT, Emerson JB, Shvartzbeyn A, Radune D, Vamathevan J, Riggs F, Grinberg V, Khouri H, Wackett LP, Nelson KE, Sadowsky MJ (2006) Secrets of soil survival revealed by the genome sequence of Arthrobacter aurescens TC1. Plos Genet 2:2094–2106
Mulbry WW, Zhu H, Nour SM, Topp E (2002) The triazine hydrolase gene trzN from Nocardioides sp. strain C190: Cloning and construction of gene-specific primers. FEMS Microbiol Lett 206:75–79
Niewerth H, Schuldes J, Parschat K, Kiefer P, Vorholt JA, Daniel R, Fetzner S (2013) Complete genome sequence and metabolic potential of the quinaldine-degrading bacterium Arthrobacter sp. Rue61a. BMC Genomics 13:534
Nordin K, Unell M, Jansson JK (2005) Novel 4-chlorophenol degradation gene cluster and degradation route via hydroxyquinol in Arthrobacter chlorophenolicus A6. Appl Environ Microbiol 71:6538–6544
Piutti S, Semon E, Landry D, Hartmann A, Dousset S, LichtfouseE TE, Soulas G, Martin-Laurent F (2003) Isolation and characterization of Nocardioides sp. SP12, an atrazine degrading bacterial strain possessing the gene trzN frombulk- and maize rhizosphere soil. FEMS Microbiol Lett 221:111–117
Sagarkar S, Mukherjee S, Nousiainen A, Björklöf K, Purohit HJ, Jørgensen KS, Kapley A (2013) Monitoring bioremediation of atrazine in soil microcosms using molecular tools. Environ Pollut 172:108–115
Sagarkar S, Bhardwaj P, Yadav T, Qureshi A, Khardenavis A, Purohit HJ, Kapley A (2014a) Draft genome of atrazine-utilizing bacteria isolated from Indian agricultural soil. Genome Announc 2:e01149–13
Sagarkar S, Nousiainen A, Shaligram S, Björklöf K, Lindström K, Jørgensen KS, Kapley A (2014b) Soil mesocosm studies on atrazine bioremediation. J Environ Manage 139:208–216
Sajjaphan K, Shapir N, Wackett LP, Palmer M, Blackmon B, Tomkins J, Sadowsky MJ (2004) Arthrobacter aurescens TC1 atrazine catabolism genes trzN, atzB, and atzC are linked on a 160-kilobase region and are functional in Escherichia coli. Appl Environ Microbiol 70:4402–4407
Sene L, Converti A, Secchi GAR, Simão RDCG (2010) New aspects on atrazine biodegradation. Braz Arch Biol Technol 53:487–496
Strong LC, Rosendahl C, Johnson G, Sadowsky MJ, Wackett LP (2002) Arthrobacter aurescens TC1 metabolizes diverse s-triazine ring compounds. Appl Environ Microbiol 68:5973–5980
Topp E, Mulbry WM, Zhu H, Nour SM, Cuppels D (2000) Characterization of s-triazine herbicide metabolism by a Nocardioides sp. isolated from agricultural soils. Appl Environ Microbiol 66:3134–3141
Udiković-Kolić N, Martin-Laurent F, Devers M, Petrić I, Kolar AB, Hršak D (2008) Genetic potential, diversity and activity of an atrazine degrading community enriched from a herbicide factory effluent. J Appl Microbiol 105:1334–1343
Udiković-Kolić N, Scott C, Martin-Laurent F (2012) Evolution of atrazine-degrading capabilities in the environment. Appl Microbiol Biotechnol 96:1175–1189
Vaishampayan PA, Kanekar PP, Dhakephalkar PK (2007) Isolation and characterization of Arthrobacter sp. strain MCM B-436, an atrazine-degrading bacterium, from rhizospheric soil. Int Biodeterior Biodegrad 60:273–278
Wheatcroft R, McRae DG, Miller RW (1990) Changes in the Rhizobium meliloti genome and the ability to detect supercoiled plasmids during bacteroid development. Mol Plant-Microbe Interact 3:9–17
Yamazaki K, Fujii K, Iwasaki A, Takagi K, Satsuma K, Harada N, Uchimura T (2008) Different substrate specificities of two triazine hydrolases (TrzNs) from Nocardioides species. FEMS Microbiol Lett 286:171–177
Acknowledgments
Funds from the Department of Biotechnology, New Delhi are gratefully acknowledged. Author Sneha Sagarkar acknowledges the Raman Charpak funding to work in the laboratory of Agroecology at INRA of Dijon (France). Pooja Bhardwaj is grateful to CSIR/UGC (India) for a senior research fellowship award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Compliance with Ethical Standards
ᅟ
Conflict of Interest
All authors declare that they have no competing interests.
Ethical approval
No ethical approval is required since this study did not involve animals/human samples.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1072 kb)
Rights and permissions
About this article
Cite this article
Sagarkar, S., Bhardwaj, P., Storck, V. et al. s-triazine degrading bacterial isolate Arthrobacter sp. AK-YN10, a candidate for bioaugmentation of atrazine contaminated soil. Appl Microbiol Biotechnol 100, 903–913 (2016). https://doi.org/10.1007/s00253-015-6975-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6975-5