Abstract
Fluoroaromatics are widely and—in recent years—increasingly used as agrochemicals, starting materials for chemical syntheses and especially pharmaceuticals. This originates from the special properties the carbon-fluorine bond is imposing on organic molecules. Hence, fluoro-substituted compounds more and more are considered to be important potential environmental contaminants. On the other hand, the microbial potentials for their transformation and mineralization have received less attention in comparison to other haloaromatics. Due to the high electronegativity of the fluorine atom, its small size, and the extraordinary strength of the C-F bond, enzymes and mechanisms known to facilitate the degradation of chloro- or bromoarenes are not necessarily equally active with fluoroaromatics. Here, we review the literature on the microbial degradation of ring and side-chain fluorinated aromatic compounds under aerobic and anaerobic conditions, with particular emphasis being placed on the mechanisms of defluorination reactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unlike chlorinated, brominated and iodinated organic compounds, organofluorines are rarely found in nature. While some of these compounds, including fluoroaromatics, are formed abiotically by volcanic activity (Gribble 2002), only about 20 compounds are known to be synthesized biogenically to date (Walker and Chang 2014). In contrast, a wide variety of fluorinated chemicals is produced and processed industrially.
Fluoroaromatics are an important group of organofluorines used as pesticides, pharmaceuticals, or starting materials for synthesis thereof. As such, they are released into the environment deliberately, as metabolites of drug detoxification or components of industrial waste waters. While substitution of fluorine for hydrogen has only minor impact on steric demand, the extreme electronegativity of the fluorine atom (EN according to Pauling: F, 4.0; Cl, 3.0; Br, 2.8) and strength of the carbon-fluorine bond (bond energies: CH3-F, 116 kcal/mol; CH3-H, 99 kcal/mol; CH3-Cl, 81 kcal/mol; CH3-Br, 68 kcal/mol) can alter bioavailability and metabolic stability drastically (Hiyama 2000; Purser et al. 2008). Substance properties like biological and chemical inertness are often considered beneficial in product design but affect behavior and degradability in the environment as well.
The biodegradation of halogenated aromatic hydrocarbons in general has been subject to extensive study. However, the main focus has been placed on chlorinated and brominated derivatives (see, for example, Reineke and Knackmuss 1988; Engesser and Fischer 1991; Schlömann 1994; Häggblom et al. 2000; Pieper et al. 2010) with less attention being paid to fluoroaromatics (Neilson and Allard 2002; Natarajan et al. 2005; Murphy et al. 2009; Murphy 2010; Zhang et al. 2012).
Due to the above-mentioned physical and chemical differences among the halogens, insights gained for one type of halo substituent are not necessarily valid for the others as well. Here, we review the literature on the microbial degradation of fluorinated mainly monocarbocyclic aromatic compounds under aerobic and anaerobic conditions with special regard to the dehalogenation mechanisms involved. The examples are grouped according to aromatic ring or side chain fluorination. Within these two major classes, mono- and polyfluorinated compounds are distinguished and subdivided according to their functional groups. The latter classification comprises carboxylic acids, phenols, arenes (meaning fluorinated benzenes and biphenyls), anilines, quinolones, and a miscellaneous class (including complex molecules used as herbicides and insecticides).
Within all these cases, the transformation of the substrates into fluorinated or initially defluorinated central metabolites of the aromatic degradation pathways will be shown first. A detailed discussion of these catecholic metabolites, highlighting historical findings and recent insights regarding their metabolic fate and degradability, will be given in a later section.
The degradation of fluorinated aromatic heterocyclic compounds as well as the plant or animal metabolism of fluoroaromatics is not within the scope of this review paper.
Definitions and general considerations
The term degradation is usually used to describe a variety of very different processes: for example, the substrate in question may either be mineralized completely or it may undergo partial transformation before a non-utilizable metabolite is excreted and accumulates in the medium. In some cases, this metabolite is immobilized by bonding to matrix components like humic acids.
While the original starting compound is eliminated in all three alternatives, the latter two may produce metabolites that are eventually even more problematic if purification of waste air or water or remediation of a polluted environmental compartment is desired. Biodegradation sensu strictu, meaning complete mineralization to CO2, H2O, and inorganic F−, therefore has to be distinguished from the more general use of this term describing mere biotransformation yielding organic products which may not be further metabolized (dead-end metabolites).
As a consequence, biodegradability cannot be deduced from disappearance of the substrate alone. Several approaches have been developed for the comprehensive assessment of the fate of chemicals in the environment or under laboratory conditions. Transformation can be followed by isotopic labeling of specific carbon or hydrogen atoms. For this purpose, radioactive (14C) as well as stable (13C, 2H) isotopes may be applied. Isotope ratios may also be used to distinguish biological from chemical or physical processes if a mixture of 12C and 13C labeled molecules is present: Enzymes preferably convert those molecules containing the 12C isotope, causing a difference in the 12C/13C ratios of the substrate and the product (see, for example, Meckenstock et al. 2004).
In the case of fluorinated substrates, a stoichiometric release of the fluoride ion into the medium provides nearly conclusive evidence for complete degradability, since dehalogenation is the most critical step in the biodegradation of haloorganics. A partial release of fluoride, however, may also occur by chemical transformation of halogenated dead-end metabolites (Wunderwald et al. 2000).
One particularly important tool for biodegradation studies with fluorinated compounds is the 19 F NMR technique due to several favorable properties of the 19 F isotope: its high intrinsic sensitivity and the absence of 19 F NMR relevant endogenous compounds in biological systems which facilitate the detection of fluorinated metabolites even at low concentrations and with an excellent signal-to-noise ratio. Furthermore, the 19 F nucleus has a broader chemical shift range than other nuclei (19 F, about 500 ppm; 1H, 15 ppm; 13C, 250 ppm). Alterations of its molecular surroundings cause severe and drastic changes in its chemical shift patterns simultaneously reducing chance of peak overlap (Boersma et al. 1998).
Growth with a substrate yielding an increase in biomass concentration can easily be detected under laboratory conditions. However, it may stem from partial degradation only and is difficult to monitor under environmental conditions. Growth does not fulfill the above definition of biodegradation in the strictest sense due to the possibly partial use only of carbon for biomass production instead of complete mineralization. Since most biomass is readily degradable, this process is classified as biodegradation nonetheless—as opposed to humification processes in which a biochemically modified metabolite is retained in the matrix over long periods of time.
Often, microorganisms are unable to use a specific substrate as sole source of carbon and energy, but may convert it partially or completely if a cosubstrate is supplied. This cosubstrate may be a structurally related compound that, unlike the substrate itself, induces enzymes suitable for transformation of both compounds (structure-driven cometabolism, e.g., the cometabolic degradation of fluorobenzoates in the presence of benzoate). In other cases, it is sufficient to provide energy by feeding an easily degradable cosubstrate without any structural similarity (energy-driven cometabolism, e.g., the cometabolic degradation of fluorobenzoates in the presence of or after preincubation with glucose).
Research with pure strains under laboratory conditions allows the detailed description of biodegradation processes and the catabolic pathways involved. It may, however, not assist in predicting the fate of a certain contaminant in the “real world” environment, which is influenced by the much greater biodiversity found in nature and additional physical and chemical factors that cannot precisely be simulated in vitro.
Ring-fluorinated aromatic hydrocarbons
In haloaromatic compounds, the p-orbitals of the halogen substituents overlap with the π-electrons of the aromatic ring, thereby increasing electron density in the system (positive mesomeric effect). This is especially true for fluorine and less important for the other halogens as has been shown for the relative rates for 4-halocatechol turnover. Once cationoid transition states are reached during enzymatic reactions, a fluoro substituent in comparison to other halogens may even accelerate the overall reaction (Engesser 1982). At the same time, the halo-substituents withdraw electrons from the ring due to their high electronegativity (negative inductive effect). The latter effect frequently predominates, in particular if an extremely electronegative substituent like fluorine substituent is present. The aromatic ring is deactivated and the electrophilic attack of molecular oxygen—the first step in most aerobic aromatic degradation pathways—generally is impaired (Guzik et al. 2013). The CF3-group (perfluoromethyl group), however, does show a negative inductive influence only, with no positive mesomeric effect being possible (Kieltsch 2008).
Elimination of fluorine as fluoride is a critical step in the degradation of core-fluorinated aromatic compounds in order to facilitate substrate breakdown and to avoid formation of dead-end or toxic metabolites. It may occur during initial oxidation of the aromatic core or after cleavage of the aromatic ring, spontaneously or by enzymatic activity.
Monofluorinated compounds
Carboxylic acids
The degradation of 2-fluorobenzoate has been a subject of research for several decades. The compound is utilized as sole source of energy and carbon by various bacterial strains (Goldman et al. 1967; Engesser et al. 1980, 1990a; Hickey and Focht 1990; Higson and Focht 1990; Kozlovsky et al. 1993; Emanuelsson et al. 2009; Duque et al. 2012). In other cases, a cosubstrate is needed to achieve substantial degradation (Fewson et al. 1968; Clarke et al. 1975; Schreiber et al. 1980).
The aerobic degradation of 2-fluorobenzoate is initiated by attack of a dioxygenase in 1,2- or 1,6-position, introducing molecular oxygen into the aromatic core (Milne et al. 1968) and yielding non-aromatic cyclohexadienediols (Fig. 1).
Aerobic bacterial metabolism of benzoate and the isomeric monofluorobenzoic acids. Reactions of the upper (benzoic acids > catechols) and lower (catechols > TCA cycle) degradative pathway are shown as an example of a complete pathway for the catabolism of fluorinated aromatic compounds. Consumption or recovery of a reductive equivalent is indicated by [2H] as a reaction substrate or product, respectively. The degradative pathways for fluorinated and non-fluorinated compounds converge at the stage of 3-oxoadipate. B12O, benzoate 1,2-dioxygenase; DHBD, dihydrodihydroxybenzoate dehydrogenase; C12O, catechol 1,2-dioxygenase; CC12O, chlorocatechol 1,2-dioxygenase; MCI, muconate cycloisomerase; CMCI, chloromuconate cycloisomerase; MLI, muconolactone isomerase; DLH, dienelactone hydrolase; ELH, enol-lactone isomerase; MAR, maleylacetate reductase
Early mechanistic suggestions for further transformation of the product of 1,2-dioxygenation, 2-fluoro-3,5-cyclohexadiene-1,2-diol-1-carboxylic acid, involved an analogous NAD+-dependent oxidation with a fluoride transfer to NAD+ rather than hydride transfer (Clarke et al. 1975). This, however, seems to be chemically impossible. Moreover, in mutants lacking dienediol dehydrogenase activity, the dienediol was shown to be chemically unstable, rearomatizing spontaneously under formation of carbon dioxide, fluoride and catechol (Engesser et al. 1980; Fetzner et al. 1992), the latter being a common intermediate of aromatic hydrocarbon degradative pathways. It is further metabolized by intradiol (“ortho”) or extradiol (“meta”) cleavage of the aromatic ring. Both pathways have been well studied (see “Degradation of central metabolites”). Initial defluorination therefore facilitates further degradation of the xenobiotic compound along these widely spread pathways without need for specialized enzymes.
Dioxygenation in 1,6-position, however, does not induce spontaneous dehalogenation during rearomatization. Instead, highly reactive and toxic 3-fluorocatechol is formed by enzymatic decarboxylation and dehydrogenation. Catechol 1,2-dioxygenases may, albeit very slowly, be able to cleave 3-fluorocatechol in an ortho cleavage fashion. The thereby derived product, 2-fluoromuconate, however, will in general not be processed further by the enzymes of the ortho pathway in essentially all microorganisms investigated (Schreiber et al. 1980; Schmidt and Knackmuss 1984; Engesser et al. 1990a; Boersma et al. 2004).
As a consequence of the problems arising from 1,6-dioxygenation, 1,2-dioxygenation is the predominant pathway in strains that grow with 2-fluorobenzoate (Goldman et al. 1967; Engesser and Schulte 1989; Boersma et al. 2004). Prolonged adaptation to the substrate was shown to effectively select for change of regioselectivity patterns in favor of dioxygenation at the 1,2-position rather than 1,6-position. This shift was indicated by an increase in fluoride elimination efficiency, evolving from <80 to 97 % of the stoichiometric amount in Pseudomonas sp. B13 (Engesser et al. 1980) and 42 to 83 % in another Pseudomonas species (Vora et al. 1988), respectively. This change in regioselectivity of the initial dioxygenation is only one of several thinkable strategies to avoid poisoning by 3-fluorocatechol: others are the inactivation of the dehydrogenase catalyzing rearomatization of the 1,6-dienediol (Alcaligenes eutrophus B9, Engesser et al. 1980), or the fast and quantitative cleavage of 3-fluorocatechol to 2-fluoromuconic acid (Schmidt and Knackmuss 1984). The dead-end metabolites accumulating in the latter two cases cannot be utilized for generation of biomass and energy, although not being toxic to the bacteria either. Thus, the lesion of the dienediol dehydrogenase gene in Alcaligenes eutrophus B9 is an example of a genotypic loss being expressed as a phenotypic gain under specific circumstances.
Anaerobic degradation of 2-fluorobenzoate has been observed under denitrifying (Taylor et al. 1979; Vargas et al. 2000; Mechichi et al. 2002), sulfate-reducing (Drzyzga et al. 1994) and methanogenic conditions (Mouttaki et al. 2009). Some denitrifiers are able to aerobically degrade the substrate as well (Song et al. 2000, 2001). By contrast, Schennen and coworkers observed growth with 2-fluorobenzoate in several Pseudomonas strains under denitrifying conditions only. Ligation of CoA to the carboxyl group was determined to be the first step in benzoate and 2-fluorobenzoate degradation. Substrate specificity analysis revealed that the ATP dependent benzoyl-CoA synthetase was unable to discriminate between benzoate and the sterically similar monofluorobenzoates. Other 2-halobenzoates were not degraded and were transformed at much slower rates by benzoyl-CoA synthetase in crude extracts (Schennen et al. 1985). A similar influence of substituent size on the rate of substrate turnover by benzoate 1,2-dioxygenase has been shown under aerobic conditions (Reineke and Knackmuss 1978).
Azoarcus evansii KB740 (formerly assigned to the Pseudomonas genus) utilized 2-fluorobenzoate as a sole substrate under denitrifying conditions, too (Ziegler et al. 1987, 1989; Anders et al. 1995). Anaerobic growth with 2-aminobenzoate induced two 2-aminobenzoate-CoA ligases and with benzoate as a substrate, a benzoate CoA ligase was induced. All three enzymes showed a high activity with 2-fluorobenzoate (Altenschmidt et al. 1991). The anaerobically induced benzoate-CoA ligase was not related to the isoenzyme from aerobically grown cells (Altenschmidt et al. 1993).
Due to the stability of the haloarene bond, a direct dehalogenation reaction in native aromatic systems does not seem to be possible by definition. In anaerobic environments, no molecular oxygen is available which could very effectively destroy the haloarene system by activation to a non-aromatic system. As seen with chlorinated substrates (Löffler et al. 1995), a direct dehalogenation of halogenated aromatics under conditions of hydride transfer seems to be chemically impossible. Therefore, haloarenes have to be activated, for example, by addition of CoA to the carboxyl group. After derivatization, a mesomerically active system is generated, allowing attack of hydride (see Fig. 2).
Possible mechanism for anaerobic degradation of fluorobenzoic acids. By addition of CoA, the resistance of the aromatic mesomeric system against a nucleophilic attack of hydride ion is bypassed, facilitating fluoride elimination. The actual attacking species (e.g., sulfhydryl) is unknown. According to the resonance structures shown in the upper row, only fluoro substituents in o- and p-position may be eliminated. This mechanistic hypothesis is supported by the observation that many fluorobenzoic acid degraders metabolize only the 2- and 4-fluorinated isomers under anaerobic conditions
3-Fluorobenzoate may serve as a single growth substrate as well (Engesser et al. 1990a; Zaitsev et al. 1995; Boersma et al. 2004; Emanuelsson et al. 2009; Duque et al. 2012) or be cooxidized with structurally related or nonrelated sources of carbon and energy (Fewson et al. 1968; Clarke et al. 1975). It is initially dioxygenated in 1,2- or 1,6-position under aerobic conditions (Fig. 1). The resulting, still fluorinated cyclohexadienediols are decarboxylated by a dehydrogenase, forming fluorinated catechols.
In the case of initial 1,2-dioxygenation, the dehydrogenation product is 3-fluorocatechol which, as mentioned above, tends to accumulate in the medium and damage the culture. Otherwise, it is transformed into 2-fluoromuconate by ortho cleavage of the aromatic ring. Dioxygenation in 1,6-position produces 4-fluorocatechol which—at least theoretically—can be used productively by many bacterial strains. Via ortho cleavage, 3-fluoromuconic acid is formed, which, in comparison to the 2-fluorinated derivative, is readily degradable.
Like in 2-fluorobenzoate degradation, regioselectivity of the initial dioxygenation determines the degree of 3-fluorobenzoate degradation that can be achieved by non-specialized benzoate degraders. While dioxygenation in 1,2-position is favorable in 2-fluorbenzoate degradation, only 1,6-dioxygenation of 3-fluorobenzoate leads to a readily degradable catecholic metabolite. Consequently, dioxygenation in 1,6-position was identified as the major pathway in bacteria growing with 3-fluorobenzoate (Engesser et al. 1990a; Boersma et al. 2004). Interestingly, these conflicting strategies seem to impair a simultaneous evolution toward improved degradation of both 2- and 3-fluorobenzoate. “Normal” benzoate dioxygenases are sterically comparatively “closed” (i.e., possess a narrow “pocket”) in 2-position but “open” in 3-position (Reineke and Knackmuss 1978). In the present case, it should shift sterical pattern to narrow space in 3-position and specifically open in 5-position. These speculations, however, have never been proven by spatial analysis of the oxygenase proteins.
Under denitrifying conditions, enrichment of monofluorobenzoate-degrading strains by Song and coworkers yielded a variety of 2- or 4-fluorobenzoate degraders, but none that could use 3-fluorobenzoate as sole source of carbon and energy. However, some of the strains obtained grew with 3-fluorobenzoate under aerobic conditions (Song et al. 2000, 2001). This exceptional behavior may be explained by analysis of Fig. 2, as fluoride liberation from an activated, reduced, non-aromatic system seems not to be facilitated in case of 3-fluorobenzoate due to mesomeric constraints. Instead, the system would tend to rearomatize without halide removal.
Crude extracts of denitrifying A. evansii KB740 grown with benzoate converted 3-fluorobenzoate to 3-fluorobenzoyl-CoA (Schennen et al. 1985). The two 2-aminobenzoate-CoA ligases and the benzoate-CoA ligase from strain KB740 were shown to catalyze this reaction, albeit at a lower rate compared to the respective reactions with 2- or 3-fluorobenzoate (Ziegler et al. 1989; Altenschmidt et al. 1991).
CoA thioester synthesis was also observed during incubation of 3-fluorobenzoate with cell extract from 3-chloro- or 3-bromobenzoate-grown denitrifying Thauera chlorobenzoica. While 3-chloro- and 3-bromobenzoyl-CoA were further transformed to benzoyl-CoA, the extract dehalogenated the 3-fluoro derivative only to a minor extent. Instead, a fluorinated cyclic dienoyl-CoA compound was produced and fluorinated dead-end metabolites accumulated (Kuntze et al. 2011). The same pattern was also observed when 3-fluorobenzoyl-CoA was incubated with benzoyl-CoA-reductase from Thauera aromatica K172 (Kuntze et al. 2011). The 3-hydroxybenzoate CoA ligase from 3-hydroxybenzoate-grown K172 transformed 3-fluorobenzoate at only 4 % of the rate obtained with the growth substrate (Laempe et al. 2001).
A methanogenic diculture of Syntrophus aciditrophicus and Methanospirillum hungatei was able to transform 3-fluorobenzoate cometabolically with crotonate as a cosubstrate (Mouttaki et al. 2009). Of the 3-fluorobenzoate supplied, 26 % was transformed, and stoichiometric amounts of benzoate and fluoride were detected. Furthermore, a 1-carboxy-3-fluorocyclohexadiene with unspecified positions of the double bonds was found as an intermediate.
Growth with 4-fluorobenzoate has been reported for numerous bacterial strains (Vora et al. 1988; Zaitsev et al. 1995; Carvalho et al. 2005; Emanuelsson et al. 2009; Misiak et al. 2011; Hoskeri et al. 2011; Duque et al. 2012; Hatta et al. 2012). Others were shown to cometabolize it employing aromatic or non-aromatic cosubstrates for growth (Ali et al. 1962; Fewson et al. 1968; Clarke et al. 1975; Mc Cullar et al. 2002).
Like the other monofluorobenzoates, 4-fluorobenzoate is initially attacked by a dioxygenase at the carbon carrying the carboxy group (C-1) and an adjacent carbon atom, forming 4-fluoro-3,5-cyclohexadiene-1,2-diol-1-carboxylic acid (Schreiber et al. 1980; Boersma et al. 2004, see Fig. 1). Via dehydrogenation and decarboxylation, 4-fluorocatechol is produced and further degraded by ortho cleavage and formation of 3-fluoromuconic acid (Harper and Blakley 1971b; Engesser et al. 1990a; Schlömann et al. 1990a; Perez-Pantoja et al. 2009; Hasan et al. 2011). The risk of formation of toxic 3-fluorocatechol and non-degradable 2-fluoromuconic acid present in 2- or 3-fluorobenzoate degradation naturally does not play a role in 4-fluorobenzoate degradation.
As an alternative to defluorination after ring cleavage, fluorine may also be eliminated initially during the first steps of degradation. Oltmanns and coworkers isolated several Pseudomonas and Alcaligenes sp. strains degrading 4-fluorobenzoate via 4-fluorocatechol as described above. Aureobacterium sp. strain RHO25, however, produced 4-hydroxybenzoate as the first intermediate, which was then hydroxylated by a 4-hydroxybenzoate-3-hydroxylase to form protocatechuate. By this initial defluorination reaction, this strain obviously prevents any toxic effects caused by possible accumulation of 4-fluorocatechol (Oltmanns et al. 1989). Energetically however, this strategy seems unfavorable compared to initial dioxygenation leading to 4-fluorocatechol. Here, the reducing equivalent required for dioxygenation is recovered in the following dehydrogenation step (see Fig. 1). A fluorocatechol-avoiding pathway would make more sense in degradation of 3-fluorobenzoate since an initial dioxygenation reaction may lead to toxic or dead-end metabolites in this case.
Degradation of 4-fluorobenzoate has been observed in a number of denitrifying bacteria. Song and coworkers isolated various strains growing anaerobically on 4-fluorobenzoate, some of whom were able to utilize the substrate under aerobic conditions as well (Song et al. 2000, 2001). Complete defluorination of and growth with 4-fluorobenzoate was reported to occur in a denitrifying mixed culture (Vargas et al. 2000). Pseudomonas PN-1 could not utilize the substrate alone, but resting cells defluorinated 2- and 4-fluorobenzoate after anoxic growth with 4-hydroxybenzoate. While 2-fluorobenzoate was attacked immediately, 4-fluorobenzoate transformation happened only after a lag phase and was inhibited by chloramphenicol, indicating that at least one additional enzyme was needed for conversion of the latter substrate (Taylor et al. 1979). A. evansii strain KB740 did not grow with 4-fluorobenzoate either. However, 4-fluorobenzoyl-CoA was produced by the crude extract of benzoate-grown KB740 and by its purified benzoate- and 2-aminobenzoate-CoA ligases (Schennen et al. 1985; Ziegler et al. 1987, 1989; Altenschmidt et al. 1991). The same reaction was catalyzed by 4-chlorobenzoate-CoA ligase from Arthrobacter sp. TM-1 grown aerobically on 4-chlorobenzoate (Zhou et al. 2004). The 3- and 4-hydroxybenzoate-CoA ligases from T. aromatica K172 exhibited a very low activity with 4-fluorobenzoate compared to the respective hydroxybenzoates (Biegert et al. 1993; Laempe et al. 2001).
Under sulfate-reducing conditions, 4-fluorobenzoate was used as sole source of carbon and energy by a diculture of strains belonging to the Desulfotomaculum and Desulfovibrio genera (Drzyzga et al. 1994).
Comparably few strains which degrade or transform carboxylic acids other than the monofluorobenzoates have been described in literature.
The degradation of 4-fluorocinnamic acid, a common reagent in the synthesis of pharmaceuticals, was investigated by New and coworkers. The substrate was completely converted by activated sludge, and 4-fluoroacetophenone as well as 4-fluorobenzoic acid were detected as metabolites. However, only 14.2 % of the fluoride expected to accumulate in the case of complete mineralization was recovered from the medium (New et al. 2000). The same intermediates, but no defluorination were found by Freitas dos Santos et al. (2001) whereas Creaser et al. (2002) observed a fluoride release of approximately 80 %.
Several pathways have been proposed for 4-fluorocinnamic acid degradation: one possibility is initial monooxygenation of the double bond with formation of an epoxide. After decarboxylation, it isomerizes to form 4-fluoroacetophenone which is then transformed into 4-fluorobenzoic acid (Creaser et al. 2002). Another proposal suggests initial decarboxylation, oxidation of the double bond to form a diol and, after a loss of H2O, an epoxide which is further processed as described above (New et al. 2000). Arthrobacter sp. strain G1 utilizes 4-fluorocinnamic acid as sole source of carbon and energy. However, only the C1 and C2 of the side chain are used for growth and 4-fluorobenzoate is excreted, which can be degraded by other strains along the above-discussed pathway via 4-fluorocatechol (Hasan et al. 2011). In strain G1, degradation of 4-fluorocinnamic acid starts with formation of a CoA adduct by 4-fluorocinnamoyl-CoA ligase. This intermediate is hydrated and then dehydrogenated, forming 4-fluorophenyl-β-keto-propionyl-CoA. By thiolase activity, 4-fluorobenzoyl-CoA is formed, which is then converted to 4-fluorobenzoic acid. In this pathway, 4-fluoroacetophenone arises only as a dead-end side product.
Recently, complete mineralization of 4-fluorocinnamic acid by a single bacterial strain was observed. Rhodococcus sp. strain S2 was reported to produce 4-fluorobenzoic acid and trans,trans-muconic acid during growth with this substrate (Amorim et al. 2014a). The degradation of 4-fluorocinnamic was therefore suggested to occur by transformation of 4-fluorobenzoic acid to 4-hydroxybenzoic acid and protocatechuate as observed by Oltmanns et al. (1989). Via ortho cleavage, 3-carboxymuconate might be formed and then converted to trans,trans-muconic acid, a compound observed for the first time as an intermediate in aromatic hydrocarbon degradation (Amorim et al. 2014a). However, other pathways of trans,trans-muconic acid formation could not be excluded. Since muconate cycloisomerases generally are known to transform only the cis,cis-isomer at reasonable rates (Sistrom and Stanier 1954; Schmidt et al. 1980), an alternative explanation such as chemical isomerization of a precursor cis,cis-muconate to the trans forms or the formation of trans,trans-muconic acid as a minor side product and dead-end metabolite seems much more probable than a productive utilization of this unusual stereoisomer.
Crawford and coworkers described a Bacillus brevis strain able to grow with 5-fluorosalicylate and 5-chlorosalicylate (Crawford et al. 1979). The latter substrate was initially dioxygenated in 1,2-position in a reaction analogous to the one catalyzed by gentisate 1,2-dioxygenase. The ring-fission product was postulated to undergo nonenzymatic lactonization. The following dechlorination step appeared to be enzyme mediated, since crude extracts and partially purified dioxygenase rapidly formed non-halogenated maleylpyruvate from 5-chlorosalicylate, whereas in incubations containing further purified dioxygenase enzyme only slow chemical dechlorination was observed. Salicylate 1,2-dioxygenase from Pseudaminobacter salicylatoxidans BN12 converted 5-fluorosalicylate and 5-chlorosalicylate to unstable ring-fission products as well (Hintner et al. 2004). The first intermediate that could be detected was a dehalogenated dienelactone which was then hydrolyzed to maleylpyruvate. Surprisingly, 5-fluorosalicylate was oxidized at higher rates than salicylate by resting cells of strain BN12 grown with 6-aminonaphthalene-2-sulfonate and yeast extract. Unlike the 5-chlorosalicylate 1,2-dioxygenase from B. brevis (Crawford et al. 1979), salicylate 1,2-dioxygenase from strain BN12 was also able to oxidize gentisate; and both enzymes were found to be closely related to gentisate 1,2-dioxygenases. Thus, their activities represent an alternative strategy for degradation of halogenated aromatic compounds, in which ring fission is facilitated by enzymes that usually require a para-dihydroxylated substrate.
Mineralization of 4-fluorophenylacetic acid by a Pseudomonas sp. strain was studied by Harper and Blakley (1971a, c). By means of an unknown and seemingly rather unplausible ring-fission mechanism, 3-fluorohex-3-enedioic acid was formed. Similar to muconic acids, this compound was suggested to lactonize, forming two different products, a 3-fluoro- and a 4-fluorolactone. During hydrolysis of the 4-fluorinated lactone, HF is eliminated and β-ketoadipic acid, an intermediate in the common β-ketoadipate pathway for degradation of aromatic hydrocarbons, is formed. Delactonization of the 3-fluoro derivative yields a more stable intermediate which can be oxidized to 4-fluoro-3-ketoadipic acid. In this case, fluorosuccinic acid is formed after elimination of acetyl-CoA. This xenobiotic compound is likely processed by the normal enzymes of the TCA cycle and fluorine is eliminated from 2-fluoromalate (Harper and Blakley 1971c). Intermediates of both possible pathways were isolated from culture media (Harper and Blakley 1971a).
Cells of Nocardia erythropolis strain CA4 grown with 4-nitrobenzoate were able to oxidize 2-fluoro-4-nitrobenzoic acid (Cain et al. 1968). While the nitrogen from the nitro group was completely recovered from the medium in the form of ammonia, defluorination did not occur. Instead, 2-fluoroprotocatechuate was found, which was further metabolized into fluoroacetate. In mammalian as well as bacterial cells, citrate synthase transforms fluoroacetate into fluorocitrate, a potent inhibitor of the enzyme aconitase and thus TCA cycle activity (“lethal synthesis,” Peters 1952; Cain et al. 1968).
In mutants of Pseudomonas testosteroni NH1000 defective in the phthalate degradation pathway, 3-fluorophthalic acid acted as an inducer of phthalic acid catabolic enzymes. The substrate was dioxygenated to form cis-3-fluoro-4,5-dihydro-4,5-dihydroxyphthalic acid and then dehydrogenated to 3-fluoro-4,5-dihydroxyphthalic acid. Subsequent decarboxylation yielded 2-fluoroprotocatechuic acid and 5-fluoroprotocatechuic acid. The decarboxylase exhibited no preference toward one of the carboxyl groups, indicating that the fluorine substituent is not recognized. Neither the mutants nor the wild type strain utilized 3-fluorophthalic acid as a growth substrate (Martin et al. 1987).
Phenols
In most cases, the first step in aerobic degradation of 2-fluorophenol is monooxygenation at the C-2 or C-6 position relative to the hydroxyl moiety. The former alternative represents a favorable oxidative initial defluorination accompanied by formation of unsubstituted catechol. In the latter case, no dehalogenation occurs and toxic 3-fluorocatechol is formed.
In many bacterial and fungal strains, both pathways are active, with differing regioselectivities of the respective hydroxylating enzyme: the ratio of C-2:C-6 oxygenation was 80:20 in Rhodococcus opacus strain 1G (Bondar et al. 1999), 50:50 in Gloeophyllum striatum DSM 9592 (Kramer et al. 2004), 29:71 in Trichosporon cutaneum CBS-2466 (Peelen et al. 1995), and 9:91 in Penicillium simplicissimum SK9117 (Marr et al. 1996). An absolute (0:100) preference of the unfavorable 3-fluorocatechol generating pathway was seen in Penicillium frequentans Bi 7/2 (Hofrichter et al. 1994), Bacillus thermoleovorans (Reinscheid et al. 1996), Rhodococcus sp. strain FP1 (Duque et al. 2012) as well as in Burkholderia fungorum strain FLU100 (Strunk and Engesser 2013). Moreover, further hydroxylated by-products like fluorohydroquinone or fluoropyrogallols may be formed (Finkelstein et al. 2000; Kim et al. 2010).
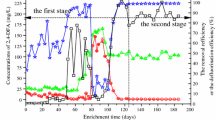
Most of these organisms required a cosubstrate for conversion of 2-fluorphenol, and the 3-fluorocatechol formed or its ortho ring-fission product, 2-fluoromuconic acid, at least transiently accumulated in the medium. Rhodococcus sp. strain FP1, however, was able to grow—albeit very slowly (1 mM within ca. 5 days)—with this substrate (Duque et al. 2012). The authors even claimed growth with 3-fluorocatechol, indicating a degradation pathway previously thought to be unproductive, and their findings were seemingly supported by the growth of B. fungorum FLU100 on 3-fluorocatechol (Strunk and Engesser 2013). Due to its toxicity and autoxidability, however, growth with 3-fluorocatechol would be expected to be difficult to measure.
Degradation of both 2-fluorophenol and 3-fluorocatechol was furthermore reported to occur in acclimated activated sludge (Chaojie et al. 2007a). The degradative pathway for 2-fluorophenol was suggested to proceed via 3-fluorocatechol and productive use of 2-fluoromuconic acid, because no unsubstituted catechol was found in the medium, 3-fluorocatechol was formed only transiently, and no accumulation of fluoromuconic acid was observed despite catechol 1,2-dioxygenase activity in the culture. Substrate concentrations of more than 50 mg/l inhibited degradation of 2-fluorophenol by this sludge as well as by a reactor bioaugmented with Rhodococcus sp. strain FP1 (Duque et al. 2011).
Apart from strain FP1, few 2-fluorophenol degraders have been reported. Duque and coworkers isolated several more 2-fluorophenol utilizing strains from the above-mentioned bioreactor belonging to the Acinetobacter, Rhodococcus, Sinorhizobium, Pimelobacter, and Microbacterium genera (Duque et al. 2014). Another Rhodococcus sp. strain (GM-14) was isolated by Zaitsev et al. (1995). The fungi P. simplicissimum SK9117 (Marr et al. 1996) and Pisolithus tinctorius (Franco et al. 2014) grew with 2-fluorophenol, but achieved only partial degradation even if phenol and glucose, respectively, were supplied as cosubstrates.
Anaerobic degradation of 2-fluorophenol was described for methanogenic cultures. Londry and Fedorak observed 97 % fluorine elimination by a phenol induced consortium (Londry and Fedorak 1993). Sharak Genthner and coworkers found evidence that, in the presence or absence of phenol, conversion of 2-fluorophenol was initiated by introduction of a carboxyl group in p-position respective to the hydroxyl group (Sharak Genthner et al. 1990). The product, 3-fluoro-4-hydroxybenzoic acid, was then transformed into 3-fluorobenzoic acid which was not further metabolized. The elimination of the hydroxyl group and introduction of the carboxyl group has been demonstrated to be the main pathway for anaerobic phenol degradation. This represents a typical example in which substitution of hydrogen by fluorine may create valuable insight into the mechanism of substrate degradation due to efficient inhibition of turnover at later steps of metabolism.
Similar to 2-fluorophenol, 3-fluorophenol is initially hydroxylated at the C-2 or C-6 position. However, concomitant dehalogenation is not possible in either case and hydroxylation yields 3-fluorocatechol (C-2, unfavorable) or 4-fluorocatechol (C-6, favorable), respectively. In most 3-fluorophenol converting organisms, a mixture of both catechols is produced. The ratio of C-2:C-6 hydroxylation was 83.5:16.5 in B. fungorum strain FLU100 (Strunk and Engesser 2013), 29:71 in T. cutaneum CBS-2466 (Peelen et al. 1995), and 0:100 in Pseudomonas putida strain F6 (Brooks et al. 2004). In the yeast-like fungus Exophiala jeanselmei CBS 658.76, 4-fluorocatechol was the main catecholic intermediate formed (Boersma et al. 1998). Chaojie and coworkers found the hydroxylation ratio in acclimated activated sludge to be pH dependent. At pH 7.5, no 4-fluorocatechol was detected. When the pH value was 7.0, the C-2/C-6 hydroxylation ratio was 7.9. Along with decreasing pH value, the ratio shifted further toward 4-fluorocatechol formation, with a C-2/C-6 hydroxylation ratio of 1.7 at pH 6.0 (Chaojie et al. 2007a, b).
R. opacus GM-14 was able to utilize 3-fluorophenol as sole source of carbon and energy (Zaitsev et al. 1995), but no indication of the pathway was given. B. fungorum FLU100 mineralized the substrate with a concomitant fluoride release of 98 % although the strain had been shown to produce mainly 3-fluorocatechol. P. tinctorius degraded 3-fluorophenol in the presence or absence of glucose as a cosubstrate, but without fluorine elimination (Franco et al. 2014). P. simplicissimum SK9117 achieved only partial defluorination when utilizing 3-fluorophenol alone. Addition of phenol as a cosubstrate increased both the rate and completeness of dehalogenation (Marr et al. 1996).
Besides the formation of 3- and 4-fluorocatechol, further hydroxylation reactions may be catalyzed by 3-fluorophenol monooxygenating enzymes. In Pseudonocardia benzenivorans, 2-fluorohydroquinone was detected as an additional product (Kim et al. 2010), and in R. opacus 1cp, 4-fluorocatechol was hydroxylated to form 5-fluoropyrogallol (Finkelstein et al. 2000). Tyrosinase from Streptomyces antibioticus, an enzyme known to catalyze the ortho-hydroxylation of monophenols and the subsequent oxidation of diphenols to quinones, converted 3-fluorophenol into 4-fluorinated 1,2-benzoquinone which then underwent nonenzymatic polymerization reactions, thereby releasing about 50 % of the fluorine contained. As an alternative, the fluoroquinone can be chemically converted to 4-fluorocatechol in the presence of reducing agents like ascorbic acid (Battaini et al. 2002).
Under anaerobic conditions, 3-fluorophenol conversion took place only when phenol was supplied as a cosubstrate. Seventy-five percent defluorination was observed in a methanogenic consortium by Londry and Fedorak (1993). Another methanogenic culture transformed 3-fluorophenol into 2-fluorobenzoate, but only at a limited extent (3 %) (Sharak Genthner et al. 1990).
Hydroxylation of 4-fluorophenol in ortho position relative to the hydroxyl group yields only one product, 4-fluorocatechol, which is then transformed into 3-fluoromuconic acid and defluorinated after lactonization. These reactions have been shown to be the first degradation steps in 4-fluorophenol mineralizing strains P. benzenivorans (Kim et al. 2010) and B. fungorum FLU100 (Strunk and Engesser 2013). During cometabolic conversion of 4-fluorophenol with phenol, the fungi E. jeanselmei and P. frequentans Bi 7/2 metabolized the substrate along the same pathway under complete defluorination (Hofrichter et al. 1994; Boersma et al. 1998). R. opacus 1cp coinduced with phenol and 4-fluorophenol excreted 4-fluorocatechol and 5-fluoropyrogallol, which was then dioxygenated to form a product tentatively identified as 2-pyrone-4-fluoro-6-carboxylate. No fluoropyrogallol was formed after induction with phenol alone, indicating that different phenol hydroxylases were induced in the presence or absence of 4-fluorophenol (Finkelstein et al. 2000). 4-Fluorocatechol was detected as a metabolite in 4-fluorophenol degrading activated sludge (Chaojie et al. 2007a), indicating mere partial transformation.
Tyrosinase from S. antibioticus converted 4-fluorophenol into 4-fluoro-1,2-benzoquinone which then polymerizes and releases about 50 % of the fluorine contained. Alternatively, it may be chemically converted to 4-fluorocatechol in the presence of a reducing agent (Battaini et al. 2002). In contrast, Brooks and coworkers suggested 4-fluorocatechol to be the first product of the oxidation of 4-fluorophenol by P. putida F6 tyrosinase, which is then transformed into 4-fluoro-1,2-benzoquinone by the same enzyme (Brooks et al. 2004). The 4-hydroxyphenylacetate 3-hydroxylase from Escherichia coli W catalyzed only the first step, yielding 4-fluorocatechol from 4-fluorophenol. For both enzymes, the rate of substrate transformation decreased with increasing size of the halogen substituent, indicating a similar effect of the halogen substituent on the activity of two mechanistically different enzymes (oxidase vs. oxygenase) (Brooks et al. 2004; Coulombel et al. 2011). Chloroperoxidase from Caldariomyces fumago catalyzed the oxidative dehalogenation of p-halophenols, although its primary biological function is the chlorination, bromination, and iodination (but not fluorination) of aliphatic substrates. A fluorophenol dimer and 1,4-benzoquinone were formed as the major and minor products, respectively (Osborne et al. 2006). In contrast, Murphy found 1,4-benzoquinone as the main product of chloroperoxidase activity and a variety of—partially defluorinated—dimers and trimers as side products (Murphy 2006).
1,4-Benzoquinone, as well, was formed through monooxygenation of 4-fluorophenol utilized as a growth substrate by Arthrobacter sp. strain IF1. By chemical or enzymatic reductive activities, hydroquinone was produced and then hydroxylated to form 1,2,4-trihydroxybenzene. The latter intermediate was suggested to be the ring-fission substrate, giving 3-hydroxymuconate after ortho cleavage, which could then be transformed into maleylacetate and finally 3-oxoadipate (Ferreira et al. 2008, 2009). Previously, the formation of hydroquinone from 4-fluorophenol had been reported to occur in 4-chlorophenol grown Arthrobacter ureafaciens CPR706 (Bae et al. 1996).
Growth with 4-fluorophenol was further observed in R. opacus GM-14 (Zaitsev et al. 1995) and Labrys portucalensis strain F11 (Carvalho et al. 2005, 2008). P. simplicissimum was able to utilize the substrate alone, but degradation rates were increased and complete defluorination was only achieved when phenol was added as a cosubstrate (Marr et al. 1996).
Arenes
Utilization of fluorobenzene as sole source of carbon and energy was first indicated for several Alcaligenes and Aureobacterium strains (Oltmanns et al. 1989) and Burkholderia kururiensis strain KP23T (Zhang et al. 2000). Its complete mineralization, however, was observed only later in a consortium of three strains (Carvalho et al. 2002). While none of these strains could grow with the substrate in the absence of the others, L. portucalensis strain F11 - isolated by the same workgroup - was able to mineralize fluorobenzene alone (Carvalho et al. 2005, 2008). Via dioxygenation, strain F11 transformed fluorobenzene into 4-fluorocatechol and catechol at a ratio of 2:1. Both were cleaved in ortho position. Degradation of fluorobenzene by strain F11 was accelerated by addition of Fe2+ ions and slowed down by addition of Cu2+ or Ag+. The latter two heavy metal ions appear to inhibit the ortho pyrocatechase of strain F11, since catechol and 4-fluorocatechol accumulated in the medium (Moreira et al. 2013).
Cometabolic conversion of fluorobenzene was reported by Gibson et al. (1968). Toluene-grown P. putida transformed the substrate into 3-fluorocatechol. No further degradation of this metabolite occurred; instead, a brown coloring of the medium indicated autooxidation. Strunk and Engesser (2013) described productive degradation of fluorobenzene via dioxygenation to 3-fluorocatechol. B. fungorum strain FLU100 mineralized the substrate after ortho fission of the aromatic ring, producing 2-fluoromuconate, an intermediate commonly thought to be a dead-end metabolite in fluoroaromatic degradation (Strunk and Engesser 2013). Fluorobenzene was almost exclusively (98.8 %) converted to 3-fluorocatechol by a FLU100 transposon mutant being defective in the catechol dioxygenase gene. When 3-fluorocatechol was offered as a substrate to the wild type in a resting cell experiment, 95 % of the stoichiometrically expected amount of fluoride could be detected in the culture medium.
Complete mineralization of fluorobiphenyls by a single strain has not been observed to date. Pseudomonas pseudoalcaligenes KF707 was able to grow with 2-fluorobiphenyl and 4-fluorobiphenyl (Murphy et al. 2008). However, the strain obtained the energy and carbon needed from the non-fluorinated aromatic ring predominantly, and 2-fluorobenzoate or 4-fluorobenzoate, respectively, accumulated in the medium. These metabolites were only partially defluorinated (35 and 25 %) and did not serve as growth substrates themselves.
Transformation of 14C labeled 4-fluorobiphenyl in soil was observed by Green et al. (1999a). Over 80 % of the fluorobiphenyl supplied were eliminated by volatilization, incorporation into the soil organic matter, or at least partial degradation as indicated by the detection of 14CO2. Addition of biphenyl as a cosubstrate increased the transformation rate. Green et al. (1999b) reported conversion 4-fluorobiphenyl by mycorrhizal fungi after growth with glucose. Tylospora fibrillosa transformed >80 % of the substrate, and 4′-hydroxy-4-fluorobiphenyl and 3′-hydroxy-4-fluorobiphenyl were identified as metabolites. Other fungi which degraded 4-fluorobiphenyl were Thelophora terrestris (>65 %), Suillus variegatus (>50 %), and Hymenoscyphus ericae (>20 %), producing a similar metabolite pattern. Likewise, Cunninghamella elegans grown with Sabouraud dextrose broth transformed 2-fluorobiphenyl and 4-fluorobiphenyl into mono- and dihydroxylated derivatives. In the case of 4-fluorobiphenyl, sulfate and β-glucuronide conjugates were detected as well (Amadio and Murphy 2010).
Under sulfate-reducing conditions, 4-fluorobiphenyl was cometabolically transformed by a biphenyl degrading mixed culture and 4-fluorobiphenyl carboxylic acid was detected as a metabolite (Selesi and Meckenstock 2009).
C. elegans ATCC 36112 growing with Sabouraud dextrose broth converted 1-fluoronaphthalene. After initial monooxygenation yielding an epoxide in the 3,4- or 5,6-positions, trans-dihydrodiols were produced by the fungal cytochrome P-450 epoxide hydrolase. Alternatively, the epoxide can rearrange non-enzymatically to give 4- or 5-hydroxy-1-fluoronaphthalene. These metabolites underwent detoxification by formation of glucoside, sulfate, and glucuronide conjugates. No initial epoxidation of the fluorinated double bond was observed (Cerniglia et al. 1984).
Anilines
Fluoroanilines can be utilized as sole sources of carbon, energy, and nitrogen by various bacterial strains. L. portucalensis F11 grew with all three monofluoroanilines; however, degradation and dehalogenation were enhanced by addition of ammonia as a nitrogen source and induction of cells with fluorobenzene. Complete conversion of the supplied 2-fluoroaniline and stoichiometric release of fluoride was observed under these conditions (Amorim et al. 2013). Ralstonia sp. strain FD-1 degraded and completely defluorinated 500 mg/l 4-fluoroaniline within 20 h without an additional nitrogen source. 3-Fluoroaniline was utilized as a growth substrate as well and 2-fluoroaniline was degraded cometabolically with 4-fluoroaniline (Song et al. 2014). Growth with 4-fluoroaniline was further reported for Moraxella sp. strain G (Zeyer et al. 1985) and a mixed culture which had been enriched using sucrose as an additional carbon source (Zhao et al. 2015).
Cometabolic transformation of 4-fluoroaniline by Pseudomonas sp. AW-2 mutant B-9 with aniline as a cosubstrate yielded 4-fluorocatechol (Kodama et al. 1997). Acinetobacter baylyi GFJ2 converted all three monofluoroanilines when succinate and yeast extract were supplied as cosubstrates. During degradation of 4-chloroaniline, 4-chlorocatechol was detected in the medium (Hongsawat and Vangnai 2011). Similarly, Moraxella sp. strain G produced 4-chlorocatechol from 4-chloroaniline (Zeyer et al. 1985). Further degradation of 4-chlorocatechol proceeded via ortho cleavage of the aromatic ring in both cases. Although meta fission of halogenated catechols may produce toxic dead-end products, meta pyrocatechase activity was found to predominate in a number of fluoroaniline-degrading cultures (Zhang et al. 2014; Zhao et al. 2015). Ralstonia sp. FD-1 produced 4-fluorocatechol from 4-fluoroaniline, which was then cleaved in meta position to form a semi-aldehyde. Based on the metabolites detected, further degradation was proposed to proceed via oxidation to 2-hydroxy-5-fluoromuconic acid, which is then decarboxylated and hydrated to form 5-fluoro-4-hydroxy-2-oxopentanoic acid. However, no defluorination mechanism could be deduced (Song et al. 2014).
Quinolones
Fluoroquinolones are inhibitors of bacterial DNA gyrase and topoisomerase IV and are thus widely used as antibiotics in human and animal medical care. Their general structure is shown in Fig. 3, with substituents R1, R2, R5, R7, and R8 controlling their activity against Gram-negative, Gram-positive, and anaerobic bacteria (Zhanel et al. 2002). R7 is an N-heterocyclic moiety, commonly a piperazine ring, that may bear alkyl substituents. C-8 is replaced by a nitrogen atom in some cases. This class of compounds, however, does not belong to the fluoroquinolones in the strictest sense and is beyond the scope of this review due to the heteroaromatic nature of the fluorinated ring.
The veterinary antibiotic enrofloxacin (see Fig. 3: R1 = cyclopropyl; R2 = R5 = R8 = H; R7 = 4-ethyl-1-piperazinyl) was among the first fluoroquinones whose biodegradability was examined. Martens and coworkers tested several basidiomycetes for their potential to degrade enrofloxacin absorbed to wheat straw or soil. Both white and brown rot fungi were able to cleave the heterocyclic ring as indicated by a release of 14CO2 from enrofloxacin labeled with 14C at the carbonyl (C-4) position. Although especially white rot fungi are known for their potential to degrade a variety of recalcitrant xenobiotic compounds, the highest degradation rates of [14C]enrofloxacin were observed in three strains of G. striatum, a brown rot fungus (Martens et al. 1996).
Various intermediates were identified during further investigation of the enrofloxacin metabolism by G. striatum. Congeners of enrofloxacin hydroxylated in 3-, 6-, or 8-position were detected, accompanied by decarboxylation in the case of C-3 hydroxylation and defluorination in the case of C-6 hydroxylation. A second hydroxylation yielded unstable dihydroxy derivatives. Cleavage of the heterocyclic ring of the fluoroquinolone core was demonstrated as well. Furthermore, the piperazinyl group was partially or completely removed from the substrate. Since neither peroxidase nor laccase activity was present in G. striatum, hydroxyl radicals formed by Fenton-type reactions were suggested to be responsible for the transformation of enrofloxacin. Chemical degradation of enrofloxacin by Fenton’s reaction produced the same monohydroxylated derivatives of enrofloxacin as found during biological conversion. Chemical degradation of the piperazinyl moiety was observed as well (Wetzstein et al. 1997).
Knowledge on the network of metabolites formed during enrofloxacine transformation by G. striatum was refined by Karl et al. (2006). Of the 87 metabolites found, 18 were identified by comparison to reference substances of known structure. The structures of the remaining 69 compounds were proposed according to their molecular mass and biochemical plausibility. Among the latter metabolites, two were suggested to be cis,cis-muconic acid-type congeners of enrofloxacin formed by cleavage of the aromatic ring. However, tetrahydroxylated constitutional isomers would provide an alternative explanation of the data presented (Wetzstein et al. 2012). At the same time, the biotransformation of enrofloxacine by seven other basidiomycetous strains known to express peroxidase or laccase activities was investigated. Of the 61 metabolites detected, 13 were also produced by G. striatum while the remaining 48 compounds had not been detected previously. No monohydroxylation of the aromatic core was detected; however, the strains produced a greater variety of metabolites with a modified piperazinyl moiety. Two strains produced defluorinated metabolites (Wetzstein et al. 2006).
A zygomycetous soil fungus, Mucor ramannianus strain R-56, transformed enrofloxacin into desethylene-enrofloxacin, enrofloxacin-N-oxide, and N-acetyl-ciprofloxacin (Parshikov et al. 2000).
Ciprofloxacin (see Fig. 3: R1 = cyclopropyl; R2 = R5 = R8 = H; R7 = 1-piperazinyl) was degraded by 16 basidiomycetous strains indigenous to agriculturally relevant sites. The pattern of metabolites formed was determined for G. striatum, showing transformation routes similar to those found in enrofloxacin degradation. Various mono- and dihydroxylated derivatives were detected, as well as metabolites with modifications to the heterocyclic ring of the fluoroquinolone core or the piperazinyl moiety. Hydroxylation of C-6 was accompanied by defluorination (Wetzstein et al. 1999). No defluorinated metabolites were produced by the fungi M. ramannianus and Pestalotiopsis guepini; instead, only modifications to or degradation of the piperazinyl moiety were observed (Parshikov et al. 1999, 2001b). Trametes versicolor additionally hydroxylated the substrate at the C-8 position (Prieto et al. 2011).
Recent investigations on the bacterial degradation of fluoroquinolones showed that ciprofloxacin is transformed by the fluoroaromatic hydrocarbon degrading strains L. portucalensis F11, Rhodococcus sp. FP1, and Rhodococcus sp. S2 in the presence of acetate as a cosubstrate (Amorim et al. 2014b; Maia et al. 2014). A mixed culture of these three strains released fluoride during successive feedings of ciprofloxacin at a steady, but lower rate compared to the elimination of the substrate itself. Of the fluorine supplied, 62 % was transformed to fluoride at the end of the test period (Maia et al. 2014). As a pure strain, L. portucalensis F11 was able to transform 0.8 μM of ciprofloxacin completely, but without defluorination to be seen. At higher concentrations, only incomplete transformation occurred, but the substrate was partly defluorinated (22 %). Two metabolites were detected, suggesting oxidative defluorination and hydroxylation at C-6, and desethylenation of the piperazinyl group (Amorim et al. 2014b).
The degradation of norfloxacin (see Fig. 3: R1 = ethyl; R2 = R5 = R8 = H; R7 = 1-piperazinyl) by fungal P. guepini and T. versicolor yielded metabolites analogous to those formed during ciprofloxacin transformation. In particular, no defluorination was observed (Parshikov et al. 2001b; Prieto et al. 2011).
Eight mycobacterial strains were able to transform norfloxacin via N-acetylation of the piperazine ring or N-nitrosation. Both metabolites were less effective antibacterial agents than the original substrate (Adjei et al. 2006). Hydroxylation and defluorination of a fluoroquinolone by a pure bacterial isolate was first reported by Kim and coworkers. Microbacterium sp. 4 N2-2 furthermore hydroxylated norfloxacin in the C-8 position and modified its piperazine ring by N-acetylation or desethylation (Kim et al. 2011). A mixed culture of L. portucalensis F11, Rhodococcus sp. FP1, and Rhodococcus sp. S2 transformed norfloxacin and liberated 60.5 % of the fluorine supplied during four successive feedings in the form of fluoride (Maia et al. 2014). L. portucalensis F11 alone completely removed up to 2 μM of norfloxacin from the medium, but achieved a defluorination of only about 38 % (Amorim et al. 2014b). The strain was able to defluorinate norfloxacin by hydroxylation at the C-6 position. The piperazine ring and the ethyl substituent in C-4 position were modified as well.
The mixed culture of L. portucalensis F11, Rhodococcus sp. FP1, and Rhodococcus sp. S2 was able to transform ofloxacin (9-Fluoro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-2,3-dihydro-7H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid) as well. After four successive feedings, 43.5 % of the fluorine supplied was released as fluoride (Maia et al. 2014). L. portucalensis F11 alone defluorinated 52 % of the substrate and was able to completely transform up to 2 μM ofloxacin. Ofloxacin was transformed via demethylation of the piperazinyl moiety, followed by two-fold hydroxylation and decarboxylation. No defluorinated metabolite was detected (Amorim et al. 2014b).
Conversion of ofloxacin by a T. versicolor strain was reported by Gros et al. (2014). No defluorinated intermediates were found; instead, transformation occurred by oxidation, hydroxylation, cleavage, and removal of the piperazine ring.
Less information is available on the degradation of other fluoroquinolone compounds. Moxifloxacin (see Fig. 3: R1 = cyclopropyl; R2 = R5 = H; R7 = [(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]; R8 = methoxy) was conversed by a mixed culture of L. portucalensis F11, Rhodococcus sp. FP1, and Rhodococcus sp. S2 at a lower extent than three other fluoroquinolones tested. Moxifloxacin has a bulkier diazabicyclo ring at the C-7 position instead of a piperazinyl moiety, which may hinder defluorination (Maia et al. 2014).
M. ramannianus formed two metabolites from sarafloxacin (see Fig. 3: R1 = 4-fluorophenyl; R2 = R5 = R8 = H; R7 = 1-piperazinyl). N-acetylsarafloxacin and desethylene-N-acetylsarafloxacin, but no defluorinated products, were found (Parshikov et al. 2001a).
Cleavage of the aromatic core part of a fluoroquinolone was observed for the first time during transformation of pradofloxacin (see Fig. 3: R1 = cyclopropyl; R2 = R5 = H; R7 = [(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]; R8 = CN) by G. striatum. A previously unknown metabolite was found, consisting of the cyclopropyl-substituted pyridone part and C atoms 7 and 8 of pradofloxacin, linked by a double bond and carrying a hydroxyl and the CN group (Wetzstein et al. 2012). The pathway leading to this metabolite was suggested to be initiated by defluorination of pradofloxacin. A catechol is then formed by further hydroxylation at the C-5 position. The amine moiety is removed from the substrate, probably yielding a pyrogallol-type intermediate whose two-fold oxidation would give a cis,cis-muconic acid-type metabolite. Two spontaneous decarboxylation reactions could then produce the new metabolite observed.
Miscellaneous compounds
Cells of Pseudomonas fluorescens strain ACB grown with 4′-hydroxyacetophenone converted 4′-fluoroacetophenone to 4-fluorophenol and 4′-fluoro-2′-hydroxyacetophenone to 4-fluorocatechol. While 4-fluorophenol was a dead-end metabolite, 4-fluorocatechol was further degraded under complete release of fluorine as fluoride. Fluorinated phenylacetic acid derivatives formed by initial monooxygenation of 2′-, 3′-, and 4′-fluoroacetophenones as well as 2′,4′-difluoroacetophenone were only observed during transformation by the strain’s purified Baeyer-Villiger monooxygenase due to fast ester hydrolysis seen in whole cell experiments. For 4′-fluoro- and 5′-fluoro-2′-hydroxyacetophenone, however, even transformation by the purified enzyme yielded no phenylacetic acids. Instead, base-catalyzed hydrolysis produced 4-fluorocatechol (Moonen et al. 2001).
The degradation of fluorinated toluenes can be initiated either by an oxygenase attack on the aromatic core or by stepwise oxidation of the side chain to a carboxy group. The hypothetical product of a monooxygenase attack on the methyl group of 4-fluorotoluene, 4-fluorobenzyl alcohol, was converted by several Pseudomonas sp. strains to fluorobenzoic acid with benzyl alcohol and succinate as cosubstrates. One strain, IE653, was able to grow on 4-fluorobenzyl alcohol alone and under complete defluorination of the substrate. The culture supernatant contained 4-fluorocatechol and products of both ortho and meta ring-fission reactions. 4-Fluorophenol was detected as well, which could arise from a hydroxylation reaction replacing the carboxyl group of the 4-fluorobenzoate formed as an intermediate (Maeda et al. 2007). Dioxygenation of 4-fluorobenzoic acid at the C-1 and C-2 positions, yielding a dienediol, could provide an alternative explanation, since dienediols easily rearomatize to phenols at acidic pH as adjusted during the metabolite extraction procedure.
Benzyl alcohol formed from toluene in the side chain oxidation pathway is further transformed to benzaldehyde and then benzoic acid. Both reactions are catalyzed by dehydrogenases. An unspecific aldehyde dehydrogenase of a Sphingomonas sp. was shown to convert the monofluorinated benzaldehydes to fluorobenzoic acids (Peng et al. 2005).
Prenafeta-Boldú and coworkers investigated the metabolism of the three monofluorotoluenes by various fungal strains, some of which were able to utilize toluene as sole source of carbon and energy. The strains that converted toluene cometabolically were found to mainly produce the respective benzoates from the fluorotoluenes by stepwise oxidation of the methyl group. The fungi able to grow with toluene transformed the fluorotoluenes considerably faster and produced a greater variety of metabolites. Besides the side chain oxidation products mentioned above, mono- and dihydroxylated benzoic acids were detected as well as 3-fluorocatechol and its ortho ring-fission product, 2-fluoromuconic acid. In particular, free fluoride ion was produced only by the toluene utilizing fungi (Prenafeta-Boldú et al. 2001).
Fluoride liberation has also been observed in strains degrading toluene via initial oxygenation of the aromatic ring. Rhodococcus sp. strain OCT 10, which attacks halotoluenes by initial oxygenation of the aromatic ring, was able to degrade 2-fluorotoluene cometabolically with 2-chlorotoluene. Of the fluorine supplied with the substrate, 48.9 % was released as fluoride (Dobslaw and Engesser 2012). Pseudomonas sp. T-12 in which the toluene-degradative pathway enzymes had been induced transformed 3-fluorotoluene to 3-methylcatechol. The reaction was suggested to proceed via dioxygenation at the C-2 and C-3 positions. The resulting dienediol should be unstable and eliminate HF, in a way analogous to the defluorination of 2-fluorobenzoic acid by 1,2-dioxygenation (see above). 3-Fluorobenzyl alcohol was a poor substrate for this reaction (Renganathan 1989). Further substrates for the toluene-degrading enzymes of Pseudomonas sp. T-12 were 3-fluoroanisole and 3-fluorobenzonitrile. While 3-fluorotoluene and 3-fluoroanisole were transformed to defluorinated catechols only, the other substrates generated catechols with and without a fluorine substituent. In general, the percentage of fluorinated catechols over non-fluorinated ones increased with an increasing steric demand of the C-1 substituent (Renganathan 1989).
From a wide range of halo-substituted fluorobenzenes and fluorophenols tested, only the 3-chloro-, 3-bromo-, and 3-iodo-1-fluorobenzenes as well as 4-chloro-2-fluorophenol served as growth substrates for R. opacus GM-14 (Zaitsev et al. 1995). This class of compounds may be dehalogenated by initial dioxygenation of the aromatic ring as well (Renganathan 1989).
Drugs and pesticides are an important source of environmental contamination by fluoroaromatics. Studies relating to their degradability have been undertaken; however, the degree of mineralization and particularly the fate of the fluorine substituent often remain unclear.
The insecticide cyfluthrin was degraded by fungal and bacterial strains, serving as a growth substrate in some cases. Diphenyl ether derivatives with a fluorine substituent in ortho position relative to the carbon carrying the ether bridge (C-1) were produced as metabolites (Saikia and Gopal 2004; Saikia et al. 2005; Chen et al. 2013). Defluorination of these intermediates has rarely been reported (Hu et al. 2014). Fluorodiphenyl ether degradation by Sphingomonas sp. strain SS3 is facilitated by attack of a dioxygenase at the C-1 position and an adjacent unsubstituted carbon. The dienediol formed is rearranged to yield phenol and a fluorocatechol, or catechol and a fluorophenol, depending on which ring was dioxygenated. The phenols are further converted by hydroxylation and catechols are cleaved by a catechol 1,2-dioxygenase. The pattern of oxygen uptake rates measured with non-halogenated, fluorinated, chlorinated, and brominated catechols or phenols as substrates suggests that only type I enzymes (Dorn and Knackmuss 1978a) are induced in strain SS3. These enzymes allow the efficient degradation of non-halogenated catechols and fluorocatechols, but not of catechols substituted with bulkier halo-substituents. With the three isomeric monofluorodiphenyl ethers as test substrates, oxygen uptake rates were highest with 4-fluorodiphenyl ether, which was also utilized as a growth substrate, and lowest with 2-fluorodiphenyl ether (Schmidt et al. 1992).
The fungus C. elegans degraded the gastroprokinetic agent mosapride by elimination of a 4-fluorobenzyl moiety (Sun et al. 2009). The herbicide pentoxazone with a 14C-label at the aromatic ring was transformed in soil. Up to 23 % of 14C were released as 14CO2 (Satsuma et al. 2000). Transformation of the pesticide dufulin in soils yielded a defluorinated metabolite probably resulting from elimination of 2-fluorobenzaldehyde (Wang et al. 2014).
Under denitrifying conditions, whole cells and cell extract of toluene-degrading T. aromatica K172 transformed 4-fluorotoluene to 4-fluorobenzoate (Biegert and Fuchs 1995; Biegert et al. 1996). The anaerobic degradation of toluene was shown to be initiated by the formation of benzylsuccinate (Biegert et al. 1996). Benzyl alcohol, which is no intermediate of this pathway, is converted to benzaldehyde by dehydrogenation. The benzyl alcohol dehydrogenase of strain K172 transformed its 2- and 4-fluoro derivatives as well (Biegert et al. 1995).
A sulfate-reducing enrichment culture transformed 4-fluorobiphenyl cometabolically with biphenyl to 4-fluorobiphenyl carboxylic acid (Selesi and Meckenstock 2009).
Various fluoronitrobenzenes were reduced to the respective anilines by a sucrose-utilizing methanogenic community. This reduction of nitro groups is rather unspecific and was frequently described. A release of fluoride ion was only observed during conversion of 3-fluoronitrobenzene (Zhao et al. 2014).
Polyfluorinated compounds
Similar to monofluorobenzoate catabolism, bacterial dioxygenases attack difluorinated benzoic acids at the carbon carrying the carboxy group and an adjacent carbon, forming cyclohexadienediols. When 3,4- or 3,5-fluorobenzoate was offered as a substrate to a P. putida JT103 mutant defective in the gene for the dienediol dehydrogenating enzyme, difluorinated dienediols accumulated. Only a small proportion of the product was further metabolized due to leakiness of the mutant (Cass et al. 1987; Rossiter et al. 1987). With 2,5-difluorobenzoate as a substrate, about 85 % of the total fluorine contained in the starting material was released as fluoride, indicating that fluorine was eliminated from both the C-2 and C-5 carbons. It was concluded that the dioxygenase had a preference for 1,2-dioxygenation, producing an unstable dienediol allowing for spontaneous elimination of CO2 and HF, and formation of 4-fluorocatechol. Thus, the genetic block of the mutant was bypassed and further degradation of the substrate facilitated (Cass et al. 1987).
2,6-Difluorobenzoic acid, which is a metabolite formed from fluorinated pesticides (Nimmo et al. 1984; Finkelstein et al. 2001), was transformed in soil as shown by a release of 14CO2 from 2,6-difluorobenzoic acid with a 14C label at the C-1 position (Nimmo et al. 1990). However, no information on the extent of defluorination was given.
Fluorinated 4-hydroxybenzoates were converted by p-hydroxybenzoate-3-hydroxylase from P. fluorescens (Husain et al. 1980). 5-Fluoroprotocatechuate was formed from both 3-fluoro-4-hydroxybenzoate and—accompanied by stoichiometric fluoride release—from 3,5-difluoro-4-hydroxybenzoate, indicating the preference of the hydroxylase for a non-fluorinated carbon atom ortho to the hydroxyl group. Fluoride elimination was also observed during conversion of tetrafluoro-p-hydroxybenzoate to 2,5,6-trifluoroprotocatechuate. The turnover of the di- and tetrafluorinated p-hydroxybenzoates required 2 mol of NADPH per mole of substrate, double the amount needed for conversion of the monofluoro derivative. An ortho-benzoquinone was suggested to be the primary product of defluorination, followed by nonenzymatic reduction to trifluoroprotocatechuate, with NADPH consumption by both reactions (Husain et al. 1980). The same phenomenon was observed by van der Bolt and coworkers during transformation of tetrafluoro-p-hydroxybenzoate by p-hydroxybenzoate hydroxylase or its active site mutant Y385F (van der Bolt et al. 1997). When ascorbate was supplied as an alternative reductant for the benzoquinone intermediate, the NADPH demand of the hydroxylation reaction was decreased to a 1:1 stoichiometry. The authors observed further defluorination to 5,6-difluoro-2,3,4-trihydroxybenzoate, 2,6-difluoro-3,4,5-trihydroxybenzoate, and 5-fluoro-2,3,4,6,-tetrafluorobenzoate. The p-hydroxybenzoate-1-hydroxylase from Candida parapsilosis decarboxylated tetrafluoro-p-hydroxybenzoate and the Y385F transformation products, giving their respective 1-hydroxy derivatives (van der Bolt et al. 1997).
No growth with polyfluorinated phenols has been reported so far. However, enzyme activity induced with phenol or chloroaromatics may catalyze their transformation.
Hydroxylation of fluorophenols is accompanied by oxidative dehalogenation if a carbon atom carrying a fluorine substituent is attacked. The phenol hydroxylases from different organisms show differing regioselectivities toward phenols fluorosubstituted in the C-2, but not C-6 positions. A variety of Rhodococcus strains showed no consistent preference pattern for C-2 or C-6 hydroxylation of 2,3-, 2,4-, and 2,5-difluorophenols, and 2,3,4-, 2,3,5-, and 2,4,5-trifluorophenols. While C-2 hydroxylation was favored by R. opacus 1G and, for most substrates, by Rhodococcus corallinus 135, Rhodococcus sp. strain 89 preferred C-6 hydroxylation. In Rhodococcus erythropolis 1CP, the regioselectivity of hydroxylation depended on the substituent pattern of the fluorophenol (Bondar et al. 1998, 1999). The conversion rates for the different substrates varied among the Rhodococcus strains as well. Difluorophenols tended to be converted at higher rates than trifluorophenols, as expected because of the ring deactivating effect of fluorine substituents. Incubation of 2,3-difluoromuconate with purified chloromuconate cycloisomerase from R. erythropolis 1CP yielded probably 5-fluoromaleylacetate (Bondar et al. 1998).
Phenol hydroxylase from T. cutaneum preferentially catalyzed hydroxylation of the di- and trifluorinated phenols in the C-6 position (Peelen et al. 1995). A similar pattern was observed in P. frequentans Bi 7/2 which produced a single catecholic product from 2,3-, 2,4-, and 2,5-difluorophenols, respectively. Both the 2- and 6-hydroxylated products, however, were formed from 3,4-difluorophenol, which lacks an ortho fluorine substituent. All substrates were partially defluorinated (Wunderwald et al. 1997). E. jeanselmei hydroxylated di- and trifluorophenols both at the C-2 and C-6 carbons. Further conversion of the resulting catechols was hindered by fluorine substituents in ortho position. High catechol 1,2-dioxygenase activity was only observed with 4-fluorocatechol and 4,5-difluorocatechol (Boersma et al. 1998).
Purified phenol hydroxylases from E. jeanselmei and T. cutaneum transformed also 2,3,5,6-tetrafluorophenol and pentafluorophenol under elimination of one fluorine substituent (Peelen et al. 1995; Boersma et al. 1998). Hydroxylation of the fluorinated ortho position was suggested to proceed via formation of a 1,2-benzoquinone intermediate which is then chemically reduced to the corresponding catechol, as proposed for tetrafluoro-p-hydroxybenzoate by Husain et al. (1980). Transformation and partial defluorination of pentafluorophenol by Pseudomonas cepacia AC1100 resting cells was reported by Karns et al. (1983). Cell extract of Mycobacterium fortuitum CG-2 transformed pentafluorophenol as well. In this strain, a halophenol para-hydroxylase produced tetrafluoro-p-hydroquinone in an oxygen dependent reaction which was blocked by cytochrome P-450 inhibitors. Further defluorination to 1,2,4-trihydroxybenzene was oxygen independent and not affected by cytochrome P-450 inhibitors (Uotila et al. 1992). Rhodococcus chlorophenolicus PCP-1 cell extract converted tetrafluoro-p-hydroquinone to 1,2,4-trihydroxybenzene, followed by formation of maleic acid. The turnover of tetrachloro-p-hydroquinone was inhibited when the air in the reaction vessel was replaced by pure oxygen. Replacement by argon or carbon monoxide did not diminish the transformation rate, indicating that the oxygen incorporated into the substrate was derived from water rather than molecular oxygen (Uotila et al. 1995).
1,3-Difluorobenzene was partially dehalogenated by Pseudomonas sp. T-12 cells after induction of the toluene-degrading enzymes. The main catecholic product formed (90 %) was 3-fluorocatechol (Renganathan 1989). 1,4-Difluorobenzene was transformed to 3,6-difluorocatechol (Renganathan and Johnston 1989). The same reaction was observed in B. fungorum FLU100, which could not grow with 1,4-difluorobenzene (Strunk and Engesser 2013). Catechols with fluorine substituents in ortho position are known to produce toxic or dead-end products after ring fission by both ortho or meta-pyrocatechases. The degradation of these intermediates is discussed in a later chapter of this review.
The utilization of 1,3-difluorobenzene as sole source of carbon an energy was reported for L. portucalensis strain F11 which could also degrade 1,4-difluorobenzene cometabolically with fluorobenzene (Moreira et al. 2012). Rhodococcus sp. strain MS11 showed slow growth with 1,4-difluorobenzene (Rapp and Gabriel-Jürgens 2003).
4,4′-Difluorobiphenyl and 2,3,4,5,6-pentafluorobiphenyl were transformed to their mono- and dihydroxy derivatives by C. elegans. Moreover, from 4,4′-difluorobiphenyl a sulfate conjugate (phase II metabolite) was formed (Amadio and Murphy 2010).
Bacterial strains P. pseudoalcaligenes KF707 and Burkholderia xenovorans LB400 utilized both compounds as growth substrates. During growth with 4,4′-difluorobiphenyl, 4-fluorobenzoate was formed by both strains. Biphenyl grown resting cells of strain KF707 produced 4-fluorocyclohexadiene-cis,cis-1,2-diol-1-carboxylate, the product of 1,2-dioxygenation of 4-fluorobenzoate, and putative 5-fluoro-4-hydroxy-2-oxovaleric acid, a metabolite of the meta degradation pathway of 4-fluorocatechol. Degradation of 4,4′-difluorobiphenyl was proposed to be initiated by dioxygenation in 2,3-position. After meta cleavage, 4-fluorobenzoate is split off and further degraded. The remaining 3-fluoro-2-hydroxypenta-2,4-dienoate is transformed to acetaldehyde and fluoropyruvate (Hughes et al. 2011). Cultures grown with 2,3,4,5,6-pentafluorobiphenyl turned yellow, indicating meta ring-fission activity. Pentafluorobenzoate was detected as a metabolite in culture supernatants of strain KF707, and biphenyl grown resting cells accumulated metabolites that were identified as 3-pentafluorophenyl-3,5-cyclohexadiene-1,2-diol and its catecholic rearomatization product (Hughes et al. 2011).
The biphenyl dioxygenase of strain LB400 attacked 2,2′-difluorobiphenyl preferentially (85-90 %) at the fluorinated side of the ortho-halogenated ring, forming 2,3-dihydroxy-2′-fluorobiphenyl. The minor product was a difluorinated dihydrodiol, probably resulting from dioxygenase attack at the non-fluorinated C-5 and C-6 (Seeger et al. 2001).
P. fluorescens 26-K degraded 3,4-difluoroaniline via 3-fluoro-4-hydroxyaniline. Addition of glucose as a cosubstrate doubled the transformation rate. Meta pyrocatechase activity, which was induced by growth with 3,4-dichloroaniline, was low in 3,4-difluoroaniline grown cells. Instead, ortho pyrocatechase activity was detected (Travkin et al. 2003). In contrast, only catechol 2,3-dioxygenase was induced in 4-fluoroaniline-degrading Ralstonia sp. FD-1. This strain converted 2,4- and 3,4-difluoroanilines and 2,3,4-trifluoroaniline cometabolically with 4-fluoroaniline (Song et al. 2014). Only meta pyrocatechase activity was found in mixed cultures enriched from activated sludge after growth with 2,4-difluoroaniline and 2,3,4-trifluoroaniline as well (Zhao et al. 2015).
4,4′-Difluorodiphenyl ether served as a growth substrate for Sphingomonas sp. strain SS33 under release of 91 % of fluorine as fluoride ion. 4-Fluorophenol and 4-fluorocatechol were produced as intermediates (Schmidt et al. 1993).
Besides 4-chlorophenylurea and 2,6-difluorobenzoic acid as major metabolites, hydrolysis of the insecticide diflubenzuron produced trace amounts of 2,6-difluorobenzamide, which readily hydrolyzes to 2,6-difluorobenzoic acid as well. After transient accumulation, 2,6-difluorobenzoic acid was further converted. Within 4 weeks, cleavage of the aromatic rings had occurred in 28 % of the diflubenzuron supplied, as indicated by a release of 14CO2 from substrate 14C-labeled at both the fluorinated and chlorinated aromatic rings. The larger part of 14CO2 formation originated from the difluorophenyl ring (Nimmo et al. 1984).
B. brevis 625, Alcaligenes sp. 1431, Acinetobacter calcoaceticus 21, and Pseudomonas sp. 10 W produced 2,6-difluorobenzamide, 2,6-difluorobenzoic acid and 2,4-difluoro-3,5-dichloroaniline from teflubenzuron in the presence or absence of acetate, succinate, glycerine, and glucose as cosubstrates. Only 30 % of the initial substrate was transformed within 12 days of incubation, however, and no evidence for further degradation of the haloaromatic metabolites by the strains was found (Finkelstein et al. 2001).
Degradation of central metabolites
To overcome the stabilizing resonance energy of the aromatic ring system, the benzene ring needs to be “activated” for further oxidative degradation (Fuchs et al. 2011). For this purpose, hydroxyl groups are introduced into the aromatic ring in the course of the peripheral reactions described in the previous chapters, forming mainly catechol (1,2-dihydroxybenzene), protocatechuate (3,4-dihydroxybenzoate), or their fluorinated derivatives. This convergence or channeling of various substrates into few central intermediates is an efficient strategy for degradation of a wide diversity of aromatic compounds (Fig. 4).
Under anaerobic conditions, β-hydroxyphenols and benzoyl-CoA are belonging to this class of central metabolites.
Catechol
Fluorine removal during the initial phase of degradation (“initial dehalogenation”) avoids many of the dangers associated with the handling of fluorinated catechols and their ring-fission products, such as toxic effects and accumulation of dead-end metabolites. Depending on the regioselectivity of the respective enzymes, initial mono- or dioxygenation of substrates like 2-fluorophenol, 2-fluorobenzoate, and fluorobenzene may solely or predominantly produce unsubstituted catechol.
The degradative pathways of catechol are well established and are initiated by fission of the aromatic ring between (“ortho”) or adjacent (“meta”) to the vicinal hydroxyl groups.
Ortho fission by catechol 1,2-dioxygenase (“ortho pyrocatechase,” see Fig. 1) produces cis,cis-muconic acid, which is cycloisomerized to muconolactone. Shifting of the double bond by muconolactone isomerase yields 3-oxoadipate-enol-lactone, which is hydrolyzed to 3-oxoadipate. Coenzyme A then is fused to the carboxyl group in 1-position, thus activating the keto group. Thiolase catalyzes the following elimination of acetyl-CoA and formation of succinyl-CoA, which is transferring CoA to the newly incoming 3-oxoadipate, completing this small circle. Both acetyl-CoA and succinate are substrates of the TCA cycle (Harwood and Parales 1996).
Meta fission by catechol 2,3-dioxygenase (“meta pyrocatechase”) generates 2-hydroxymuconate semi-aldehyde, whose formation is often visible to the naked eye as a yellow coloration of the culture medium due to the intermediate’s absorption maximum at λ = 375 nm. It is transformed into the enol form of 4-oxalocrotonic acid by dehydrogenation and then tautomerized to the keto form. By decarboxylation, 2-oxopent-4-enoic acid is formed. As an alternative, 2-hydroxymuconate semi-aldehyde can be hydrolysed and decarboxylated in a single step to form 2-oxopent-4-enoic acid. Hydration to 4-hydroxy-2-oxopentanoic acid follows, which is finally cleaved to pyruvate and acetaldehyde by aldolase (Harayama and Rekik 1990).
Protocatechuic acid
Unsubstituted protocatechuate may be formed during degradation of 4-fluorobenzoate by replacement of the fluorine substituent by a hydroxyl moiety, followed by another hydroxylation step.
Protocatechuic acid can be degraded via ortho and meta pathways as well. In the latter case, two types—distal (between C-4 and C-5) and proximal (between C-2 and C-3) cleavage—are to be distinguished.
Ortho ring fission gives 3-carboxy-cis,cis-muconic acid which is cycloisomerized to 4-carboxymuconolactone in bacteria. Decarboxylation yields 3-oxoadipate-enol-lactone, which is hydrolysed to 3-oxoadipate and further metabolized along the route described for catechol degradation. In eukaryotes, 3-carboxy-cis,cis-muconic acid is frequently cycloisomerized to 3-carboxymuconolactone. Decarboxylation and hydrolysis then leads to 3-oxoadipate (Harwood and Parales 1996).
During degradation of 4-fluorocinnamic acid by Rhodococcus sp. S2, trans,trans-muconic acid was claimed to be detected in the medium. A degradation pathway via protocatechuate was proposed: as an alternative to the ortho pathway described above, 3-carboxy-cis,cis-muconic acid might be decarboxylated and form trans,trans-muconic acid, which could then be transformed into 3-oxoadipate. After ortho cleavage, decarboxylation would yield trans,trans-muconic acid, which could be metabolized to form 3-oxoadipate (Amorim et al. 2014a). This pathway would be highly unusual since muconate cycloisomerases show nearly no activity even with cis,trans-muconic acid and no activity at all with the trans,trans isomer (Sistrom and Stanier 1954; Schmidt et al. 1980).
The distal meta fission of protocatechuate leads to 2-hydroxy-4-carboxymuconsemi-aldehyde. By a hydrolase, formate and 2-oxo-5-carboxypent-5-enoic acid are produced. Further hydration yields 4-hydroxy-4-carboxy-2-oxopentanoic acid. From this metabolite, aldolase forms two molecules of pyruvate (Dagley et al. 1968). As an alternative, the semi-aldehyde may be oxidated to 2-hydroxy-4-carboxymuconic acid. Further conversion then leads to oxaloacetate and pyruvate (Dennis et al. 1973). Proximal meta cleavage of protocatechuate has been reported as well (Wolgel et al. 1993).
Halogenated catechol derivatives: 3-fluorocatechol
Formation of 3-fluorocatechol has been observed during degradation of fluorobenzene and 2- or 3-fluorosubstituted benzoates and phenols, respectively. However, these pathways are commonly regarded as unproductive due to the formation of dead-end metabolites and the toxicity of 3-fluorocatechol itself. An overview of hypothetical degradative pathways for 3-fluorocatechol is given in Fig. 5. In general, 2-fluoromuconate was—nearly as a dogma—recognized to be the principal and final dead-end metabolite (Schreiber et al. 1980; Schmidt and Knackmuss 1984; Engesser et al. 1990b; Boersma et al. 2004).
Hypothetical degradation pathways of 3-fluorocatechol. Ortho fission of 3-fluorocatechol yields 2-fluoromuconic acid which is a dead-end metabolite in most organisms investigated. Lactonization probably is the critical step. The isomerization of 2-fluoromuconolactone to exocyclic 5-fluoromuconolactone may be due to an “isomerase” or catalyzed by the 2-fluoromuconate cycloisomerazing enzyme, opening and closing again the lactone ring like in 4-methylmuconolactone isomerization (Pieper et al. 1990; Erb et al. 1998). C12O, catechol 1,2-dioxygenase; CC12O, chlorocatechol 1,2-dioxygenase; MCY, muconate cyclase; MCI, muconate cycloisomerase; CMCI, chloromuconate cycloisomerase; MLI, muconolactone isomerase; CMLI, chloromuconolactone isomerase; DLH, dienelactone isomerase; MAR, maleylacetate reductase. Enzymes labeled with a question mark (“?”) have not actually been shown to convert the respective fluorinated substrates but are suggested based on their activity with the chlorinated analogs (see Pieper et al. 2010)
Ortho fission of 3-fluorocatechol yields the quite stable 2-fluoro-cis,cis-muconic acid (Schreiber et al. 1980; Engesser et al. 1990a; Solyanikova et al. 2003; Strunk and Engesser 2013). This reaction is best catalyzed by chlorocatechol 1,2-dioxygenase (pyrocatechase type II, Dorn and Knackmuss 1978b), albeit at a substantially lower rate compared to dioxygenation of 4-fluorocatechol. Dioxygenation by the type I pyrocatechase was even slower (Dorn and Knackmuss 1978a). Due to these low transformation rates, 3-fluorocatechol tends to accumulate. At neutral or alkaline pH, the culture is poisoned during autooxidation and oligomerization/polymerization processes, indicated by browning and blackening of the medium followed by formation of precipitates (Clarke et al. 1975; Engesser et al. 1980; Schmidt and Knackmuss 1984; Wunderwald et al. 2000). Therefore, the toxic effects are caused by chemical transformation products rather than 3-fluorocatechol itself.
2-Fluoromuconic acid is untoxic, but is usually not further converted by muconate or chloromuconate cycloisomerases and accumulates as a dead-end product (Engesser et al. 1980; Schreiber et al. 1980; Schmidt and Knackmuss 1984; Engesser et al. 1990a; Solyanikova et al. 1995; Vollmer et al. 1998, 1999).
A very slow conversion of 2-fluoromuconate was achieved by Gaal and Neujahr (1980) using the lactonizing enzyme cis,cis-muconate cyclase (which is a cycloisomerase) from T. cutaneum (7 % compared to cis,cis-muconate conversion = 100 %), and by Solyanikova et al. (2003) using purified chloromuconate cycloisomerase from R. opacus 1cp (2 % compared to 2-chloromuconate conversion = 100 %). The resulting 5-fluoromuconolactone was claimed to be defluorinated by chloromuconolactone dehalogenase, forming cis-dienelactone (Solyanikova et al. 2003). Growth, however, could not be observed.
A dienelactone and 2-fluoromuconic acid were identified as intermediates during degradation of 3-fluorocatechol by activated sludge (Chaojie et al. 2007a). A two-component monooxygenase from Rhodococcus sp. strain FP1 produced solely 3-fluorocatechol from 2-fluorophenol. No fluoride anion, which would originate from hydroxylation at the C-2 instead of the C-6 position, was detected as a product. The strain utilized both 2-fluorophenol and 3-fluorocatechol as growth substrates; however, a brown coloration of the medium indicated partial autooxidation of 3-fluorocatechol (Duque et al. 2012). A mutant of B. fungorum strain FLU100 defective in the fluorobenzene degradation pathway excreted almost exclusively 3-fluorocatechol (98.8 %) during conversion of fluorobenzene. The strain’s type II ortho pyrocatechase cleaved 3-fluorocatechol at a rate of >20 % compared to unsubstituted catechol or 3-chlorocatechol. Both 3-fluorocatechol and 2-fluoromuconic acid served as growth substrates with a fluorine elimination of >90 % (Strunk and Engesser 2013). A final elucidation of the fluorine elimination step occurring during or after fluorolactone formation, however, was not presented to date.
It is unclear whether the enzyme activities found for conversion of 2-fluoromuconate actually may suffice to support microbial growth. R. opacus 1cp does not grow with 2-fluorophenol as the sole source of carbon and energy (Finkelstein et al. 2000). Since 2-fluoromuconic acid competitively inhibits cycloisomerization of 2-chloromuconolactone, it can be assumed that the fluoro substituent does not interfere with substrate binding to chloromuconate cycloisomerase, and that formation of the lactone ring is the limiting step of 2-fluoromuconate conversion (Schmidt et al. 1980). Of the hypothetical lactonization products, only the isomer carrying an exocyclic fluorine substituent seems to be eligible for further degradation by muconolactone isomerase (see Fig. 5). However, a possible further transformation along the “normal” route for muconolactones cannot be excluded. In this case, fluorosuccinate may be an intermediate, which potentially should be defluorinated allowing normal TCA cycle enzymes to continue operations. Another alternative would be a fluoromuconolactone isomerase, approaching the favorable exocyclic fluorolactone and thereby entering the presumably productive pathway via dienelactone formation. This is however somewhat speculative, being researched at the moment in the authors’ lab to clarify this point.
In the case of 2-chloromuconolactone, formation of protoanemonin under elimination of HCl and CO2 has been observed (Pieper et al. 2010). However, it is unknown whether an analogous reaction might occur with the fluorinated substrate. In any case, protoanemonin is unlikely to be an intermediate of a productive degradative pathway due to its toxic properties.
Meta ring cleavage of 3-fluorocatechol is clearly unproductive, too. Catechol 2,3-dioxygenase from P. putida mt-2 is irreversibly inhibited by 3-halocatechols. Meta fission in proximal position relative to the halo substituent produces 5-haloformyl derivatives of 2-hydroxypenta-2,4-dienoic acid. These highly reactive acyl halides react with the enzyme, inactivating it (Bartels et al. 1984). As the inactivation rate of catechol 2,3-dioxygenase by 3-fluorocatechol was decreased in the presence of 2-mercaptoethanol, mannitol, formate, superoxide dismutase or catalase, the formation of reactive oxygen species such as the superoxide anion, hydroxyl radical or hydrogen peroxide may in addition contribute to enzyme inactivation. A chemically stable acylation product of meta pyrocatechase consistently could not be shown. While inactivation-“resistant” meta-pyrocatechases have been found among 3-chlorocatechol cleaving enzymes (Göbel et al. 2004), no such type was found for 3-fluorocatechol (Mars et al. 1997; and own unpublished results). The fact that this resistance in essence has been an improved tolerance against inactivation only indicated that no actual mechanism-dependent resistance has been newly evolved.
Ring fission in 1,6-position (distal relative to the halo substituent) would avoid formation of acyl halides. This type of dioxygenation reaction was observed when 3-chlorocatechol was incubated with 2,3-dihydroxybiphenyl 1,2-dioxygenase from Sphingomonas strain BN6 (Riegert 2001). However, the enzyme exhibited a much lower activity with 3-fluorocatechol.
Due to the common inapplicability of meta fission pathways for haloaromatics degradation, the simultaneous conversion of alkylated and halogenated aromatic hydrocarbons is problematic: alkyl substituted aromatics are commonly degraded via the meta pathway since ortho fission produces dead-end metabolites at the muconolactone level. Mixtures of halo- and methylaromatics may induce both meta and ortho cleaving enzymes in a bacterium, which leads to misrouting of the catecholic intermediates into unproductive pathways, thereby wasting energy and producing toxic metabolites. This situation is avoided by modified ortho pathways that include an additional isomerization step after muconolactone formation, causing a formal “shift” of methyl group to the 3-position of the muconolactone (Pieper et al. 1985; Rojo et al. 1987).
Halogenated catechol derivatives: 4-fluorocatechol
4-Fluorocatechol is the main catecholic intermediate formed during productive degradation of 3- and 4-fluorosubstituted aromatic hydrocarbons such as benzoates, phenols, and anilines, and may be formed from unsubstituted fluorobenzene as well. An overview of the various bacterial pathways suggested for its degradation is presented in Fig. 6. Cutting-edge experiments were undertaken in the lab of D. Pieper and especially M. Schlömann in order to clarify the foundations of the 4-fluorocatechol degradation mystery, set up a long time ago by the work of Harper and Blakley.
Diversity of different bacterial pathways for 4-fluorocatechol degradation as suggested by Harper and Blakley (1971b) (paths (a) and (b)), Schlömann et al. (1990a) (path (b)) and Schreiber et al. (1980) (path (c)). C12O, catechol 1,2-dioxygenase; CC12O, chlorocatechol 1,2-dioxygenase; MCI, muconate cycloisomerase; CMCI, chloromuconate cycloisomerase; DLH, dienelactone hydrolase; ELH, enol-lactone isomerase; MAR, maleylacetate reductase
The ortho ring fission of 4-fluorocatechol to 3-fluoro-cis,cis-muconic acid is catalyzed by both catechol 1,2-dioxygenase and chlorocatechol 1,2-dioxygenase (pyrocatechase I and II, Dorn and Knackmuss 1978a). Although the type II enzyme—being specialized for halocatechol turnover—has a more relaxed substrate specificity and shows a higher activity for 4-fluorocatechol, it is not necessarily induced during degradation of fluoroaromatics via 4-fluorocatechol (Schlömann et al. 1990b). Schreiber and coworkers found a seven-fold higher specific activity of pyrocatechase I in 4-fluorobenzoate-grown cells of Pseudomonas sp. B13 compared to benzoate-grown cells. Type I enzyme overproduction thus prevented accumulation of the toxic intermediate as an alternative to the induction of the type II enzyme (Schreiber et al. 1980).
3-Fluoro-cis,cis-muconic acid is converted into fluoromuconolactone by muconate or chloromuconate cycloisomerases (Solyanikova et al. 1995; Vollmer et al. 1998, 1999). Depending on which carboxyl group is involved in lactone formation, different products are formed. The regioselectivity of lactonization depends on the enzyme as well as on the halo substituent: Muconate lactonizing enzyme from T. cutaneum transformed 3-chloro- and 3-bromomuconic acid into the corresponding 3-halomuconolactones; 3-fluoromuconic acid was lactonized to 4-fluoromuconolactone. Cyclization of 3-halomuconates catalyzed by chloromuconate cycloisomerase from Pseudomonas B13 proceeded consistently by carboxylate addition to C-3 of the 3-halomuconate. Only the fluorinated 4-halomuconolactone, however, was detectable. The reaction of the cycloisomerase with 3-chloro- and 3-bromomuconates led to formation of cis-4-carboxymethylenebut-2-enolide (“cis-dienelactone”) (Mazur et al. 1994).
Harper and Blakley detected 4-fluoromuconolactone as well as unsubstituted (-)-muconolactone during degradation of 4-fluorobenzoic acid via 4-fluorocatechol (Harper and Blakley 1971b). They proposed two branches of 4-fluorocatechol degradation which are both initiated by ortho fission of the aromatic ring, producing 3-fluoro-cis,cis-muconic acid. In the first pathway lactonization to 4-fluoromuconolactone follows. Hydrolysis yields an unstable gem fluorohydroxy compound which eliminates HF, forming maleylacetate. By reduction, 3-oxoadipate is formed, a metabolite of the common catechol pathway. Since this branch could not explain the detection of unsubstituted muconolactone, lactonization of 3-fluoromuconic acid with concomitant defluorination, yielding (cis or trans) dienelactone, was postulated as a second pathway. Subsequent reduction would give muconolactone. As an alternative, dienelactone might be hydrolyzed to form maleylacetate. These alternatives remained speculative and had to be clarified later.
In contrast, Schreiber and coworkers postulated 4-fluoromuconolactone to be unstable and spontaneously eliminate HF, forming mainly cis-dienelactone (Schreiber et al. 1980; see Fig. 6). The suggestion was apparently supported by observations of a chemical transformation of 3-fluoromuconic acid at acidic pH, producing the cis- and trans-dienelactones (Schmidt et al. 1980; Schmidt and Knackmuss 1980).
This interpretation was questioned by Schlömann and coworkers. The majority of 4-fluorobenzoate-degrading strains produced a type I dienelactone hydrolase with high activity toward trans-dienelactone but low activity toward the cis-isomer (Schlömann et al. 1990b). Moreover, both muconate cycloisomerase and dichloromuconate cycloisomerase produced predominantly 4-fluoromuconolactone from 3-fluoromuconic acid. Cis- and trans-dienelactone were detected in comparatively small amounts; the lack of a preference in the formation of the isomers indicated that they resulted from spontaneous reactions of 3-fluoro-cis,cis-muconate. 4-Fluoromuconolactone proved to be relatively stable at acidic and neutral pH. The main product of its chemical decomposition was maleylacetate, whereas the amount of dienelactones formed did not exceed 1 % of the initial 4-fluoromuconolactone concentration. The authors therefore proposed enzymatic degradation of 4-fluoromuconolactone, possibly by dienelactone hydrolase or 3-oxoadipate-enol-lactone hydrolase (Schlömann et al. 1990a).
Small amounts of cis-dienelactone were detected as a dead-end metabolite during growth of Rhizobiales strain F11 on fluorobenzene (Carvalho et al. 2006), complying with its role as a minor byproduct rather than a true intermediate of productive 4-fluorocatechol degradation (a possible participation of trans-dienelactone was not addressed). Consistently with this interpretation, no cis- or trans-dienelactone transforming activity was detectable in cells of the fluorobenzoate-degrading strain FLB300 (Engesser et al. 1990a).
Type I (trans-) and III (cis/trans-) dienelactone hydrolases have been shown to convert 4-fluoromuconolactone as well (Schlömann et al. 1990b; Schlömann 1994). Based on a mechanism suggested for dienelactone hydrolysis by cis/trans-dienelactone hydrolase of Pseudomonas sp. strain B13 (Cheah et al. 1993), a mechanism for the hydrolysis of 4-chloromuconolactone by Pseudomonas sp. strain MT1 trans-dienelactone hydrolase was proposed (Nikodem et al. 2003): Binding of the enzyme in C-1 position facilitates fission of the lactone ring by attachment of a nucleophile, resulting in an unstable gem chlorohydroxy compound, which eliminates chloride before it is hydrolytically released from the enzyme, forming maleylacetate. This mechanism might also be able to eliminate the halide ion from 4-fluoromuconolactone, with a gem fluorohydroxy compound as an intermediate as proposed by Harper and Blakley (1971b).
Maleylacetate is further transformed by maleylacetate reductase into 3-oxoadipate (Seibert et al. 1993, 2004). At this point, the 4-fluorocatechol degradation pathway converges with the ortho fission pathway for the degradation of unsubstituted catechol. Maleylacetate reductase also converts maleylacetates halogenated at the C-2 carbon. In the first step, the halide ion is eliminated under formation of unsubstituted maleylacetate. The same enzyme then catalyzes reduction to 3-oxoadipate. The conversion of halogenated maleylacetates requires 2 mol of NADH per mole of substrate, double the amount needed for conversion of maleylacetate (Kaschabek and Reineke 1995).
Meta ring cleavage of 4-fluorocatechol does not directly lead to toxic products as does (proximal) meta fission of 3-halocatechols. Although meta-pyrocatechases have been shown to convert 4-fluorocatechol (Heiss et al. 1997; Kaschabek et al. 1998), this type of ring fission has rarely been reported to play a role in fluoroaromatics degradation via 4-fluorocatechol.
Only catechol 2,3-dioxygenase activity was detected in cell lysates of Ralstonia sp. strain FD-1 utilizing 4-fluoroaniline as a growth substrate. The 4-fluorocatechol formed as an intermediate was cleaved in meta position proximal relative to the fluoro substituent and the resulting semi-aldehyde was oxidized to form 2-hydroxy-5-fluoromuconic acid. Subsequent decarboxylation and hydration yielded 5-fluoro-2-oxo-4-hydroxypentanoic acid. This compound was probably cleaved to form pyruvate and fluoroacetate (Song et al. 2014). Fluoroacetate is highly toxic to aerobic organisms since it is transformed into fluorocitrate, a potent inhibitor of the TCA cycle enzyme aconitase (“lethal synthesis,” Peters 1952). However, several enzymes catalyzing the cleavage of the C-F bond in fluoroacetate have been identified (Goldman 1965; Kurihara et al. 2003). Ralstonia sp. FD-1 degraded 500 mg/l 4-fluoroaniline completely and under stoichiometric release of fluoride, indicating that it is able to efficiently detoxify fluoroacetate.
Halogenated catechol derivatives: Fluoroprotocatechuic acids
2-Fluoroprotocatechuatic acid was detected as a metabolite from the oxidation of 2-fluoro-4-nitrobenzoic acid by 4-nitrobenzoate-grown cells of N. erythropolis strain CA4. It was probably cleaved in ortho position and decarboxylated after formation of fluoro-γ-carboxymuconolactone, forming 2-fluoro-3-oxoadipate. Cleavage of this intermediate yielded succinate and fluoroacetate, which could not be defluorinated by this strain (Cain et al. 1968).
2-Fluoroprotocatechuate and 5-fluoroprotocatechuate were formed during conversion of 3-fluorophthalic acid by mutants of P. testosteroni NH1000 (Martin et al. 1987).
5-Fluoroprotocatechuate, as well as its chloro and bromo analogs, was shown to be a substrate for productive meta ring fission (Fig. 7). The 5-haloprotocatechuates were cleaved by protocatechuate 4,5-dioxygenase, forming the respective acyl halides. No suicide inactivation of the enzyme occurred; instead, 2-pyrone-4,6-dicarboxylate was formed by spontaneous reaction and under elimination of the halide ion. Further degradation proceeded by hydrolysis of the pyrone ring. Finally, pyruvate and oxaloacetate were formed (Kersten et al. 1985).
Elimination of fluorine by a meta fission pathway in P. testosteroni (Kersten et al. 1985). The aromatic ring of 5-fluoroprotocatechuate is cleaved in proximal position relative to the fluoro substituent. The resulting acyl halide does not react with the meta pyrocatechase, causing suicide inactivation of the enzyme. Instead, the ring fission product rearranges under spontaneous elimination of HF and formation of a pyrone derivative
Protoanemonin as a toxic byproduct of halocatechol degradation
During the degradation of chloroaromatics via 4-chlorocatechol and 3-chloro-cis,cis-muconic acid, the antibacterial agent protoanemonin, which is ordinarily derived from plants, may be formed. It is a major product of 3-chloro-cis,cis-muconic acid conversion by the muconate cycloisomerase of the ubiquitous 3-oxoadipate pathway, causing cell death. In contrast, 3-chloro-cis,cis-muconic acid conversion by chloromuconate cycloisomerase produces only cis-dienelactone which can be further degraded (Blasco et al. 1995).
During degradation of fluoroaromatics, formation of protoanemonin seems possible as well but has scarcely been observed. The major product of 4-fluoromuconolactone transformation with enol-lactone hydrolase from P. putida A3.12 was tentatively identified by its UV-spectroscopic properties as protoanemonin (Schlömann 1988; Blasco et al. 1995; Fig. 6). Other enol-lactone hydrolases tested, however, did not catalyze the formation of this compound. A trans- or cis/trans-dienelactone hydrolase activity has been proposed to prevent protoanemonin formation (Brückmann et al. 1998; Nikodem et al. 2003). Since these types of dienelactone hydrolase may play an important role in 4-fluoromuconolactone conversion (see above) and thus be expressed by many fluoroarene degraders, protoanemonin accumulation may fortuitously be avoided in these strains.
Side chain fluorinated aromatics
Like fluoro substituents, the trifluoromethyl (-CF3) group exerts a strong electron withdrawing effect on the aromatic nucleus and impedes the electrophilic attack of oxygen. Even in the case of successful ring fission, it usually remains intact.
The effect of the trifluoromethyl group on bacterial metabolism of aromatics was investigated by Engesser and coworkers using toluic acid grown cells of P. putida mt-2 and Rhodococcus rubropertinctus N657 and their crude extracts (Engesser et al. 1988a). Both strains transformed 3-(trifluoromethyl)benzoate by initial dioxygenation and dehydrogenation to 3-(trifluoromethyl)catechol. The catechol 2,3-dioxygenase of P. putida mt-2 converted this intermediate to a semi-aldehyde, 2-hydroxy-6-oxo-7,7,7-trifluoro-hepta-2,4-dienoic acid (7-TFHOD). Further hydrolytic degradation, however, was totally impaired by the trifluoro-substitution. The catechol 1,2-dioxygenase of R. rubropertinctus N657 was unable to cleave 3-(trifluoromethyl)catechol. Compared to a methyl group, the trifluoromethyl substituent significantly reduced the turnover rates of the enzymes from the upper toluic acid degradative pathway (Engesser et al. 1988a). Interestingly, the dihydroxylation of the CF3-arene dramatically increased its instability against hydrolysis. Consistently, 3-CF3-catechol (~3 mM) quantitatively hydrolyzed overnight at 30 °C and pH 7.5 to 2,3-dihydroxybenzoate (Engesser 1982). A possible mechanism is given in Fig. 8.
Similar observations were made during cometabolic conversion of 4-(trifluoromethyl)benzoate by 4-isopropylbenzoate and 4-ethylbenzoate degrading P. putida JT strains. The substrate was dioxygenated in 2,3-position, dehydrogenated to the corresponding catechol and cleaved by 2,3-dihydroxybenzoate-3,4-dioxygenase. The resulting semi-aldehyde was spontaneously decarboxylated to 7-TFHOD. Hydrolysis would produce 2-hydroxypenta-2,4-dienoate and trifluoroacetic acid. All enzymatic reactions, but not the chemical decarboxylation step, were slowed down when substrates carrying a trifluoromethyl group instead of isopropyl were supplied (Engesser et al. 1988b).
The initial hydroxylation of various other trifluoromethyl substituted substrates may lead to the formation of 3-(trifluoromethyl)catechol or 7-TFHOD as well. Fluorobenzene or benzonitrile induced cells of Pseudomonas sp. T-12 produced 3-(trifluoromethyl)catechol from benzotrifluoride (Renganathan and Johnston 1989) and, as the main catecholic metabolite, from 3-fluoro-benzotrifluoride (Renganathan 1989). 7-TFHOD accumulated in benzoate-grown cells of the bacterial strain V-1 during conversion of 3-(trifluoromethyl)benzoate (Taylor 1993) and during transformation of 2-(trifluoromethyl)phenol by thermophilic B. thermoleovorans A2 resting cells grown with LB (Reinscheid et al. 1998). Temperature dependent chemical hydrolysis of 2- and 4-(trifluoromethyl)phenol under release of fluoride was observed by the latter authors.
The 2-hydroxy-6-oxo-hepta-2,4-dienoic acid hydrolases of numerous bacterial strains turned out to be unable to hydrolyze 7-TFHOD, suggesting this intermediate to be a general dead-end metabolite during metabolism of trifluoromethyl substituted compounds via meta cleavage pathways (Engesser et al. 1988a, b, 1990b; Taylor 1993; Reinscheid et al. 1998). It was, however, reported to be hydrolyzed by sunlight (Taylor 1993; Reinscheid et al. 1998). Findings regarding a release of fluoride ion during photolysis were inconsistent among these two work groups. Trifluoroacetic acid, possibly originating from hydrolysis of 7-TFHOD, was detected during conversion of benzotrifluoride by Rhodococcus sp. 065240 (Yano et al. 2015). Partial defluorination was reported by these authors as well; however, only 2.5 % of the total fluorine supplied were recovered as fluoride.
Conversion of 2-(trifluoromethyl)benzoate by enzymes of the phthalate catabolic pathway ceased after formation of 2-(trifluoromethyl)protocatechuate which is not a substrate for the following 3,4-dihydroxyphthalate 2-decarboxylase (Eaton 2001).
Trifluoromethyl substituents are widely used in drug and agrochemicals design due to their effects on physical and biochemical properties (Theodoridis 2006; Purser et al. 2008). Biotransformation by, e.g., hydroxylation, dealkylation or hydrolysis has been reported for many examples of trifluoromethylated drugs, insecticides and herbicides. However, the fate of the trifluoromethyl substituted aromatic ring and, in particular, the substituent itself usually remains unclear.
Herath and Khan screened 40 strains for degradation of the anti-cancer drug flutamide (2-methyl-N-[4-nitro-3-(trifluoromethyl)phenyl]-propanamide). Up to three metabolites were identified, the major being the hydrolysis product 4-nitro-3-(trifluoromethyl)aniline in all flutamide transforming strains (Herath and Khan 2010). This metabolite was produced by C. elegans, too, along with 2-hydroxyflutamide and the product of nitro reduction. Formation of a sulfate conjugate, representing a phase II metabolite, was shown as well (Amadio and Murphy 2011).
Transformation of the insecticide fipronil ((RS)-5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-4-(trifluoromethylsulfinyl)-1H-pyrazole-3-carbonitrile) in soil yielded metabolites modified in the sulfinyl group (Zhu et al. 2004; Mandal et al. 2013).
Bellinaso and coworkers isolated nine strains able to transform the herbicide trifluralin (2,6-dinitro-N,N-dipropyl-4-(trifluoromethyl)aniline) at a concentration of 50 mg/l in a medium supplemented with yeast extract and succinate. Up to 25 % of the initial substrate was converted during 30 days (Bellinaso et al. 2003).
The herbicide fluometuron (1,1-dimethyl-3-[3-(trifluoromethyl)phenyl]urea) was degraded by six cyanobacterial species at concentrations of up to 1.4 g/l within 5 days (Mansy and El‐Bestawy 2002). Transformation of the trifluoromethyl group was shown during a degradation experiment in soil with fluometuron 14C-labeled at the trifluoromethyl group. Within 72 days of incubation, 4.4 % of the initial radioactivity was volatilized and shown to at least partially consist of 14CO2 (Bozarth and Funderburk 1971).
Conclusions
The aerobic conversion of monocyclic aromatic compounds carrying a single fluoro substitution in the aromatic ring to central catecholic metabolites has been well established. In the case of more complex molecules and substrates carrying more than one fluorine substituent or even a trifluoromethyl group, the degree of mineralization and particularly the fate of the fluorine substituent(s) often remain unclear. Obviously, multiple fluorosubstitution substantially retards or inhibits metabolism in a much more pronounced way compared to chlorosubstituted compounds. Since many of these fluorinated compounds are widely used as pharmaceuticals or agrochemicals, further research regarding their degradability—especially under real environmental conditions—seems inevitable. This is especially true for its bacterial, fungal, or mammalian, i.e., human, metabolites.
Recalcitrance depends clearly on the number and position of fluorine substituents at the ring. For example, whereas plenty of information is available for 4-fluorocatechol as a central metabolite of catabolism via ortho pathways, very few examples are claimed for 3-fluorocatechol turnover, questioning the dogma of microbial infallibility since a long time. Its ortho ring fission was therefore thought to be unproductive due to formation of non-metabolizable cis,cis-2-fluoromuconate, representing a real hard dead-end metabolite. Recent findings, however, indicate that further biodegradation of the ring cleavage product indeed may be feasible. To date, however, no details regarding the defluorination step occurring during or after lactonization are known. This is at least valid for the two strains reported to utilize 3-fluorocatechol for growth.
A further “dogma,” i.e., meta ring fission of 3-halocatechols is completely unproductive, has clearly been refuted in the case of 3-chlorocatechol. For conversion of 3-fluorocatechol, however, despite many efforts no productive metabolism could be shown, reinforcing its inertness in the meta pathway: even these specialized catechol-2,3-dioxygenases easily transforming 3-chlorocatechol are strongly inhibited or inactivated by 3-fluorocatechol.
Questions remain regarding alternative degradational strategies as well. The formation of acyl halides during proximal meta cleavage may be circumvented by meta cleavage in distal position. This type of ring fission has been observed with 3-chlorocatechol and seems possible with 3-fluorocatechol as well. In individual cases, a fluorine substituent may act as a replacement of an OH moiety and facilitate ring fission by enzymes that usually require an ortho- or para-dihydroxylated substrate.
References
Adjei MD, Heinze TM, Deck J, Freeman JP, Williams AJ, Sutherland JB (2006) Transformation of the antibacterial agent norfloxacin by environmental mycobacteria. Appl Environ Microbiol 72:5790–5793. doi:10.1128/AEM.03032-05
Ali DA, Callely AG, Hayes M (1962) Ability of a Vibrio grown on Benzoate to oxidize para-Fluorobenzoate. Nature 196:194–195. doi:10.1038/196194a0
Altenschmidt U, Oswald B, Fuchs G (1991) Purification and characterization of benzoate-coenzyme A ligase and 2-aminobenzoate-coenzyme A ligases from a denitrifying Pseudomonas sp. J Bacteriol 173:5494–5501
Altenschmidt U, Oswald B, Steiner E, Herrmann H, Fuchs G (1993) New aerobic benzoate oxidation pathway via benzoyl-coenzyme A and 3-hydroxybenzoyl-coenzyme A in a denitrifying Pseudomonas sp. J Bacteriol 175:4851–4858
Amadio J, Murphy CD (2010) Biotransformation of fluorobiphenyl by Cunninghamella elegans. Appl Microbiol Biotechnol 86:345–351. doi:10.1007/s00253-009-2346-4
Amadio J, Murphy CD (2011) Production of human metabolites of the anti-cancer drug flutamide via biotransformation in Cunninghamella species. Biotechnol Lett 33:321–326. doi:10.1007/s10529-010-0425-3
Amorim CL, Carvalho MF, Afonso CM, Castro PM (2013) Biodegradation of fluoroanilines by the wild strain Labrys portucalensis. Int Biodeterior Biodegrad 80:10–15. doi:10.1016/j.ibiod.2013.02.001
Amorim CL, Ferreira AC, Carvalho MF, Afonso CM, Castro PM (2014a) Mineralization of 4-fluorocinnamic acid by a Rhodococcus strain. Appl Microbiol Biotechnol 98:1893–1905. doi:10.1007/s00253-013-5149-6
Amorim CL, Moreira IS, Maia AS, Tiritan ME, Castro PM (2014b) Biodegradation of ofloxacin, norfloxacin, and ciprofloxacin as single and mixed substrates by Labrys portucalensis F11. Appl Microbiol Biotechnol 98:3181–3190. doi:10.1007/s00253-013-5333-8
Anders HJ, Kaetzke A, Kämpfer P, Ludwig W, Fuchs G (1995) Taxonomic position of aromatic-degrading denitrifying pseudomonad strains K 172 and KB 740 and their description as new members of the genera Thauera, as Thauera aromatica sp. nov., and Azoarcus, as Azoarcus evansii sp. nov., respectively, members of the beta subclass of the Proteobacteria. Int J Syst Bacteriol 45:327–333
Bae HS, Lee JM, Lee S (1996) Biodegradation of 4-chlorophenol via a hydroquinone pathway by Arthrobacter ureafaciens CPR706. FEMS Microbiol Lett 145:125–129. doi:10.1111/j.1574-6968.1996.tb08566.x
Bartels I, Knackmuss HJ, Reineke W (1984) Suicide inactivation of catechol 2,3-dioxygenase from Pseudomonas putida mt-2 by 3-halocatechols. Appl Environ Microbiol 47:500–505
Battaini G, Monzani E, Casella L, Lonardi E, Tepper AW, Canters GW, Bubacco L (2002) Tyrosinase-catalyzed oxidation of fluorophenols. J Biol Chem 277:44606–44612. doi:10.1074/jbc.M207829200
Bellinaso M, Greer CW, do Carmo Peralba M, Pêgas Henriques JA, Gaylarde CC (2003) Biodegradation of the herbicide trifluralin by bacteria isolated from soil. FEMS Microbiol Ecol 43:191–194. doi:10.1111/j.1574-6941.2003.tb01058.x
Biegert T, Fuchs G (1995) Anaerobic oxidation of toluene (analogues) to benzoate (analogues) by whole cells and by cell extracts of a denitrifying Thauera sp. Arch Microbiol 163:407–417. doi:10.1007/BF00272129
Biegert T, Altenschmidt U, Eckerskorn C, Fuchs G (1993) Enzymes of anaerobic metabolism of phenolic compounds. 4-hydroxybenzoate-CoA ligase from a denitrifying Pseudomonas species. Eur J Biochem 213:555–561
Biegert T, Altenschmidt U, Eckerskorn C, Fuchs G (1995) Purification and properties of benzyl alcohol dehydrogenase from a denitrifying Thauera sp. Arch Microbiol 163:418–423
Biegert T, Fuchs G, Heider J (1996) Evidence that anaerobic oxidation of toluene in the denitrifying bacterium Thauera aromatica is initiated by formation of benzylsuccinate from toluene and fumarate. Eur J Biochem 238:661–668. doi:10.1111/j.1432-1033.1996.0661w.x
Blasco R, Wittich R, Mallavarapu M, Timmis KN, Pieper DH (1995) From xenobiotic to antibiotic, formation of protoanemonin from 4-chlorocatechol by enzymes of the 3-oxoadipate pathway. J Biol Chem 270:29229–29235. doi:10.1074/jbc.270.49.29229
Boersma MG, Dinarieva TY, Middelhoven WJ, van Berkel WJ, Doran J, Vervoort J, Rietjens IM (1998) 19F nuclear magnetic resonance as a tool to investigate microbial degradation of fluorophenols to fluorocatechols and fluoromuconates. Appl Environ Microbiol 64:1256–1263
Boersma FGH, McRoberts WC, Cobb SL, Murphy CD (2004) A 19F NMR study of fluorobenzoate biodegradation by Sphingomonas sp. HB-1. FEMS Microbiol Lett 237:355–361. doi:10.1111/j.1574-6968.2004.tb09717.x
Bondar VS, Boersma MG, Golovlev EL, Vervoort J, van Berkel WJ, Finkelstein ZI, Solyanikova IP, Golovleva LA, Rietjens IM (1998) 19F NMR study on the biodegradation of fluorophenols by various Rhodococcus species. Biodegradation 9:475–486. doi:10.1023/A:1008391906885
Bondar VS, Boersma MG, van Berkel WJ, Finkelstein ZI, Golovlev EL, Baskunov BP, Vervoort J, Golovleva LA, Rietjens IM (1999) Preferential oxidative dehalogenation upon conversion of 2-halophenols by Rhodococcus opacus 1G. FEMS Microbiol Lett 181:73–82
Bozarth GA, Funderburk HH Jr (1971) Degradation of fluometuron in sandy loam soil. Weed Sci 19:691–695
Brooks SJ, Doyle EM, Hewage C, Malthouse JPG, Duetz W, O’ Connor KE (2004) Biotransformation of halophenols using crude cell extracts of Pseudomonas putida F6. Appl Microbiol Biotechnol 64:486–492. doi:10.1007/s00253-003-1488-z
Brückmann M, Blasco R, Timmis KN, Pieper DH (1998) Detoxification of protoanemonin by dienelactone hydrolase. J Bacteriol 180:400–402
Cain RB, Tranter EK, Darrah JA (1968) The utilization of some halogenated aromatic acids by Nocardia. Oxidation and metabolism. Biochem J 106:211–227
Carvalho MF, Alves CCT, Ferreira MIM, De Marco P, Castro PML (2002) Isolation and initial characterization of a bacterial consortium able to mineralize fluorobenzene. Appl Environ Microbiol 68:102–105
Carvalho MF, Ferreira Jorge R, Pacheco CC, De Marco P, Castro PML (2005) Isolation and properties of a pure bacterial strain capable of fluorobenzene degradation as sole carbon and energy source. Environ Microbiol 7:294–298. doi:10.1111/j.1462-2920.2004.00714.x
Carvalho MF, Ferreira MIM, Moreira IS, Castro PML, Janssen DB (2006) Degradation of fluorobenzene by Rhizobiales strain F11 via ortho cleavage of 4-fluorocatechol and catechol. Appl Environ Microbiol 72:7413–7417. doi:10.1128/AEM.01162-06
Carvalho MF, De Marco P, Duque AF, Pacheco CC, Janssen DB, Castro PML (2008) Labrys portucalensis sp. nov., a fluorobenzene-degrading bacterium isolated from an industrially contaminated sediment in northern Portugal. Int J Syst Evol Microbiol 58:692–698. doi:10.1099/ijs.0.65472-0
Cass AE, Ribbons DW, Rossiter JT, Williams SR (1987) Biotransformation of aromatic compounds monitoring fluorinated analogues by NMR. FEBS Lett 220:353–357. doi:10.1016/0014-5793(87)80845-1
Cerniglia CE, Miller DW, Yang SK, Freeman JP (1984) Effects of a fluoro substituent on the fungal metabolism of 1-fluoronaphthalene. Appl Environ Microbiol 48:294–300
Chaojie Z, Qi Z, Ling C, Yuan Y, Hui Y (2007a) Degradation of mono-fluorophenols by an acclimated activated sludge. Biodegradation 18:51–61. doi:10.1007/s10532-005-9035-5
Chaojie Z, Qi Z, Ling C, Zhichao W, Bin X (2007b) Biodegradation of meta-fluorophenol by an acclimated activated sludge. J Hazard Mater 141:295–300. doi:10.1016/j.jhazmat.2006.07.002
Cheah E, Ashley GW, Gary J, Ollis D (1993) Catalysis by dienelactone hydrolase: a variation on the protease mechanism. Proteins 16:64–78. doi:10.1002/prot.340160108
Chen S, Dong YH, Chang C, Deng Y, Zhang XF, Zhong G, Song H, Hu M, Zhang L (2013) Characterization of a novel cyfluthrin-degrading bacterial strain Brevibacterium aureum and its biochemical degradation pathway. Bioresour Technol 132:16–23. doi:10.1016/j.biortech.2013.01.002
Clarke KF, Callely AG, Livingstone A, Fewson CA (1975) Metabolism of monofluorobenzoates by Acinetobacter calcoaceticus N.C.I.B. 8250. Formation of monofluorocatechols. Biochim Biophys Acta 404:169–179
Coulombel L, Nolan LC, Nikodinovic J, Doyle EM, O’Connor KE (2011) Biotransformation of 4-halophenols to 4-halocatechols using Escherichia coli expressing 4-hydroxyphenylacetate 3-hydroxylase. Appl Microbiol Biotechnol 89:1867–1875. doi:10.1007/s00253-010-2969-5
Crawford RL, Olson PE, Frick TD (1979) Catabolism of 5-chlorosalicylate by a Bacillus isolated from the Mississippi River. Appl Environ Microbiol 38:379–384
Creaser C, dos Santos LF, Lamarca DG, New A, Wolff J (2002) Biodegradation studies of 4-fluorobenzoic acid and 4-fluorocinnamic acid: an evaluation of membrane inlet mass spectrometry as an alternative to high performance liquid chromatography and ion chromatography. Anal Chim Acta 454:137–145. doi:10.1016/S0003-2670(01)01514-8
Dagley S, Geary PJ, Wood JM (1968) The metabolism of protocatechuate by Pseudomonas testosteroni. Biochem J 109:559–568. doi:10.1042/bj1090559
Dennis DA, Chapman PJ, Dagley S (1973) Degradation of protocatechuate in Pseudomonas testosteroni by a pathway involving oxidation of the product of meta-fission. J Bacteriol 113:521–523
Dobslaw D, Engesser K (2012) Degradation of 2-chlorotoluene by Rhodococcus sp. OCT 10. Appl Microbiol Biotechnol 93:2205–2214. doi:10.1007/s00253-011-3543-5
Dorn E, Knackmuss HJ (1978a) Chemical structure and biodegradability of halogenated aromatic compounds. Substituent effects on 1,2-dioxygenation of catechol. Biochem J 174:85–94
Dorn E, Knackmuss HJ (1978b) Chemical structure and biodegradability of halogenated aromatic compounds. Two catechol 1,2-dioxygenases from a 3-chlorobenzoate-grown pseudomonad. Biochem J 174:73–84
Drzyzga O, Jannsen S, Blotevogel K (1994) Mineralization of monofluorobenzoate by a diculture under sulfate-reducing conditions. FEMS Microbiol Lett 116:215–219. doi:10.1111/j.1574-6968.1994.tb06703.x
Duque AF, Bessa VS, Carvalho MF, Castro PM (2011) Bioaugmentation of a rotating biological contactor for degradation of 2-fluorophenol. Bioresour Technol 102:9300–9303. doi:10.1016/j.biortech.2011.07.003
Duque AF, Hasan SA, Bessa VS, Carvalho MF, Samin G, Janssen DB, Castro PML (2012) Isolation and characterization of a Rhodococcus strain able to degrade 2-fluorophenol. Appl Microbiol Biotechnol 95:511–520. doi:10.1007/s00253-011-3696-2
Duque AF, Bessa VS, Castro PM (2014) Bacterial community dynamics in a rotating biological contactor treating 2-fluorophenol-containing wastewater. J Ind Microbiol Biotechnol 41:97–104. doi:10.1007/s10295-013-1381-4
Eaton RW (2001) Plasmid-encoded phthalate catabolic pathway in Arthrobacter keyseri 12B. J Bacteriol 183:3689–3703. doi:10.1128/JB.183.12.3689-3703.2001
Emanuelsson MAE, Osuna MB, Ferreira Jorge RM, Castro PML (2009) Isolation of a Xanthobacter sp. degrading dichloromethane and characterization of the gene involved in the degradation. Biodegradation 20:235–244. doi:10.1007/s10532-008-9216-0
Engesser KH (1982) Der Einfluss der Trifluormethylgruppe auf die biologische Abbaubarkeit von Aromaten. Dissertation, University of Göttingen
Engesser KH, Fischer P (1991) Degradation of haloaromatic compounds. In: Robards AW, Betts WB (eds) Biodegradation. Springer, London, pp 15–54
Engesser KH, Schulte P (1989) Degradation of 2-bromo-, 2-chloro- and 2-fluorobenzoate by Pseudomonas putida CLB 250. FEMS Microbiol Lett 60:143–147
Engesser KH, Schmidt E, Knackmuss HJ (1980) Adaptation of Alcaligenes eutrophus B9 and Pseudomonas sp. B13 to 2-fluorobenzoate as growth substrate. Appl Environ Microbiol 39:68–73
Engesser KH, Cain RB, Knackmuss HJ (1988a) Bacterial metabolism of side chain fluorinated aromatics: cometabolism of 3-trifluoromethyl(TFM)-benzoate by Pseudomonas putida (arvilla) mt-2 and Rhodococcus rubropertinctus N657. Arch Microbiol 149:188–197
Engesser KH, Rubio MA, Ribbons DW (1988b) Bacterial metabolism of side chain fluorinated aromatics: cometabolism of 4-trifluoromethyl(TFM)-benzoate by 4-isopropylbenzoate grown Pseudomonas putida JT strains. Arch Microbiol 149:198–206
Engesser KH, Auling G, Busse J, Knackmuss H (1990a) 3-Fluorobenzoate enriched bacterial strain FLB 300 degrades benzoate and all three isomeric monofluorobenzoates. Arch Microbiol 153:193–199
Engesser KH, Rubio MA, Knackmuss HJ (1990b) Bacterial metabolism of side-chain-fluorinated aromatics: unproductive meta-cleavage of 3-trifluoromethylcatechol. Appl Microbiol Biotechnol 32(5):600–8. doi:10.1007/BF00173734
Erb RW, Timmis KN, Pieper DH (1998) Characterization of a gene cluster from Ralstonia eutropha JMP134 encoding metabolism of 4-methylmuconolactone. Gene 206:53–62. doi:10.1016/S0378-1119(97)00565-9
Ferreira MIM, Marchesi JR, Janssen DB (2008) Degradation of 4-fluorophenol by Arthrobacter sp. strain IF1. Appl Microbiol Biotechnol 78:709–717. doi:10.1007/s00253-008-1343-3
Ferreira MIM, Iida T, Hasan SA, Nakamura K, Fraaije MW, Janssen DB, Kudo T (2009) Analysis of two gene clusters involved in the degradation of 4-fluorophenol by Arthrobacter sp. Strain IF1. Appl Environ Microbiol 75:7767–7773. doi:10.1128/AEM.00171-09
Fetzner S, Müller R, Lingens F (1992) Purification and some properties of 2-halobenzoate 1,2-dioxygenase, a two-component enzyme system from Pseudomonas cepacia 2CBS. J Bacteriol 174:279–290
Fewson CA, Kennedy SI, Livingstone A (1968) Metabolism of monofluorobenzoates by Bacterium N.C.I.B. 8250. Biochem J 109:6P–7P
Finkelstein ZI, Baskunov BP, Boersma MG, Vervoort J, Golovlev EL, van Berkel WJH, Golovleva LA, Rietjens IMCM (2000) Identification of fluoropyrogallols as new intermediates in biotransformation of monofluorophenols in Rhodococcus opacus 1cp. Appl Environ Microbiol 66:2148–2153. doi:10.1128/AEM.66.5.2148-2153.2000
Finkelstein ZI, Baskunov BP, Rietjens IM, Boersma MG, Vervoort J, Golovleva LA (2001) Transformation of the insecticide teflubenzuron by microorganisms. J Environ Sci Health B 36:559–567. doi:10.1081/PFC-100106185
Franco AR, Ferreira AC, Castro PM (2014) Co-metabolic degradation of mono-fluorophenols by the ectomycorrhizal fungi Pisolithus tinctorius. Chemosphere 111:260–265. doi:10.1016/j.chemosphere.2014.03.094
Freitas dos Santos LM, Spicq A, New AP, Lo Biundo G, Wolff J, Edwards A (2001) Aerobic biotransformation of 4-fluorocinnamic acid to 4-fluorobenzoic acid. Biodegradation 12:23–29. doi:10.1023/A:1011973824171
Fuchs G, Boll M, Heider J (2011) Microbial degradation of aromatic compounds—from one strategy to four. Nat Rev Microbiol 9:803–816. doi:10.1038/nrmicro2652
Gaal A, Neujahr HY (1980) cis,cis-Muconate cyclase from Trichosporon cutaneum. Biochem J 191:37–43
Gibson DT, Koch JR, Schuld CL, Kallio RE (1968) Oxidative degradation of aromatic hydrocarbons by microorganisms. II. Metabolism of halogenated aromatic hydrocarbons. Biochemistry 7:3795–3802. doi:10.1021/bi00851a003
Göbel M, Kranz OH, Kaschabek SR, Schmidt E, Pieper DH, Reineke W (2004) Microorganisms degrading chlorobenzene via a meta-cleavage pathway harbor highly similar chlorocatechol 2,3-dioxygenase-encoding gene clusters. Arch Microbiol 182:147–156. doi:10.1007/s00203-004-0681-5
Goldman P (1965) The enzymatic cleavage of the carbon-fluorine bond in fluoroacetate. J Biol Chem 240:3434–3438
Goldman P, Milne G, Pignataro MT (1967) Fluorine containing metabolites formed from 2-fluorobenzoic acid by Pseudomonas species. Arch Biochem Biophys 118:178–184. doi:10.1016/0003-9861(67)90295-0
Green N, Meharg A, Till C, Troke J, Nicholson J (1999a) Degradation of 4-fluorobiphenyl in soil investigated by 19F NMR spectroscopy and 14C radiolabelling analysis. Chemosphere 38:1085–1101. doi:10.1016/S0045-6535(98)00351-8
Green NA, Meharg AA, Till C, Troke J, Nicholson JK (1999b) Degradation of 4-fluorobiphenyl by mycorrhizal fungi as determined by (19)F nuclear magnetic resonance spectroscopy and (14)C radiolabelling analysis. Appl Environ Microbiol 65:4021–4027
Gribble GW (2002) Naturally occurring organofluorines. In: Neilson AH (ed) Organofluorines. Springer-Verlag, Berlin, pp 121–136
Gros M, Cruz-Morato C, Marco-Urrea E, Longrée P, Singer H, Sarrà M, Hollender J, Vicent T, Rodriguez-Mozaz S, Barceló D (2014) Biodegradation of the X-ray contrast agent iopromide and the fluoroquinolone antibiotic ofloxacin by the white rot fungus Trametes versicolor in hospital wastewaters and identification of degradation products. Water Res 60:228–241. doi:10.1016/j.watres.2014.04.042
Guzik U, Hupert-Kocurek K, Wojcieszysk D (2013) Intradiol dioxygenases — The key enzymes in xenobiotics degradation. In: Chamy R (ed) Biodegradation of hazardous and special products. InTech, Rijeka, pp 129–153
Häggblom M, Knight V, Kerkhof L (2000) Anaerobic decomposition of halogenated aromatic compounds. Environ Pollut 107:199–207. doi:10.1016/S0269-7491(99)00138-4
Harayama S, Rekik M (1990) The meta cleavage operon of TOL degradative plasmid pWW0 comprises 13 genes. Mol Gen Genet 221:113–120
Harper DB, Blakley ER (1971a) The metabolism of p-fluorophenylacetic acid by a Pseudomonas sp. I. Isolation and identification of intermediates in degradation. Can J Microbiol 17:635–644. doi:10.1139/m71-103
Harper DB, Blakley ER (1971b) The metabolism of p-fluorobenzoic acid by a Pseudomonas sp. Can J Microbiol 17:1015–1023
Harper DB, Blakley ER (1971c) The metabolism of p-fluorophenylacetic acid by a Pseudomonas sp. II. The degradative pathway. Can J Microbiol 17:645–650
Harwood CS, Parales RE (1996) The β-ketoadipate pathway and the biology of self-identity. Annu Rev Microbiol 50:553–590. doi:10.1146/annurev.micro.50.1.553
Hasan SA, Ferreira MIM, Koetsier MJ, Arif MI, Janssen DB (2011) Complete biodegradation of 4-fluorocinnamic acid by a consortium comprising Arthrobacter sp. Strain G1 and Ralstonia sp. Strain H1. Appl Environ Microbiol 77:572–579. doi:10.1128/AEM.00393-10
Hatta T, Fujii E, Takizawa N (2012) Analysis of two gene clusters involved in 2,4,6-trichlorophenol degradation by Ralstonia pickettii DTP0602. Biosci Biotechnol Biochem 76:892–899. doi:10.1271/bbb.110843
Heiss G, Müller C, Altenbuchner J, Stolz A (1997) Analysis of a new dimeric extradiol dioxygenase from a naphthalenesulfonate-degrading sphingomonad. Microbiology (Reading, Engl) 143(Pt 5):1691–1699
Herath W, Khan IA (2010) Microbial metabolism. Part 11. Metabolites of flutamide. Chem Pharm Bull 58:562–564. doi:10.1248/cpb.58.562
Hickey WJ, Focht DD (1990) Degradation of mono-, di-, and trihalogenated benzoic acids by Pseudomonas aeruginosa JB2. Appl Environ Microbiol 56:3842–3850
Higson FK, Focht DD (1990) Degradation of 2-bromobenzoic acid by a strain of Pseudomonas aeruginosa. Appl Environ Microbiol 56:1615–1619
Hintner J, Reemtsma T, Stolz A (2004) Biochemical and molecular characterization of a ring fission dioxygenase with the ability to oxidize (substituted) salicylate(s) from Pseudaminobacter salicylatoxidans. J Biol Chem 279:37250–37260. doi:10.1074/jbc.M313500200
Hiyama T (2000) Organofluorine compounds. Chemistry and applications. Springer, Berlin
Hofrichter M, Bublitz F, Fritsche W (1994) Unspecific degradation of halogenated phenols by the soil fungus Penicillium frequentans Bi 7/2. J Basic Microbiol 34:163–172. doi:10.1002/jobm.3620340306
Hongsawat P, Vangnai AS (2011) Biodegradation pathways of chloroanilines by Acinetobacter baylyi strain GFJ2. J Hazard Mater 186:1300–1307. doi:10.1016/j.jhazmat.2010.12.002
Hoskeri RS, Mulla SI, Shouche YS, Ninnekar HZ (2011) Biodegradation of 4-chlorobenzoic acid by Pseudomonas aeruginosa PA01 NC. Biodegradation 22:509–516. doi:10.1007/s10532-010-9423-3
Hu GP, Zhao Y, Song FQ, Liu B, Vasseur L, Douglas C, You MS (2014) Isolation, identification and cyfluthrin-degrading potential of a novel Lysinibacillus sphaericus strain, FLQ-11-1. Res Microbiol 165:110–118. doi:10.1016/j.resmic.2013.11.003
Hughes D, Clark BR, Murphy CD (2011) Biodegradation of polyfluorinated biphenyl in bacteria. Biodegradation 22:741–749. doi:10.1007/s10532-010-9411-7
Husain M, Entsch B, Ballou DP, Massey V, Chapman PJ (1980) Fluoride elimination from substrates in hydroxylation reactions catalyzed by p-hydroxybenzoate hydroxylase. J Biol Chem 255:4189–4197
Karl W, Schneider J, Wetzstein H (2006) Outlines of an "exploding" network of metabolites generated from the fluoroquinolone enrofloxacin by the brown rot fungus Gloeophyllum striatum. Appl Microbiol Biotechnol 71:101–113. doi:10.1007/s00253-005-0177-5
Karns JS, Kilbane JJ, Duttagupta S, Chakrabarty AM (1983) Metabolism of halophenols by 2,4,5-trichlorophenoxyacetic acid-degrading Pseudomonas cepacia. Appl Environ Microbiol 46:1176–1181
Kaschabek SR, Reineke W (1995) Maleylacetate reductase of Pseudomonas sp. strain B13: specificity of substrate conversion and halide elimination. J Bacteriol 177:320–325
Kaschabek SR, Kasberg T, Müller D, Mars AE, Janssen DB, Reineke W (1998) Degradation of chloroaromatics: purification and characterization of a novel type of chlorocatechol 2,3-dioxygenase of Pseudomonas putida GJ31. J Bacteriol 180:296–302
Kersten PJ, Chapman PJ, Dagley S (1985) Enzymatic release of halogens or methanol from some substituted protocatechuic acids. J Bacteriol 162:693–697
Kieltsch I (2008) Elektrophile Trifluormethylierung—Anwendung von hypervalenten Iodverbindungen. Dissertation, ETH Zürich
Kim E, Jeon J, Kim Y, Murugesan K, Chang Y (2010) Mineralization and transformation of monofluorophenols by Pseudonocardia benzenivorans. Appl Microbiol Biotechnol 87:1569–1577. doi:10.1007/s00253-010-2647-7
Kim D, Heinze TM, Kim B, Schnackenberg LK, Woodling KA, Sutherland JB (2011) Modification of norfloxacin by a Microbacterium sp. strain isolated from a wastewater treatment plant. Appl Environ Microbiol 77:6100–6108. doi:10.1128/AEM.00545-11
Kodama N, Takenaka S, Murakami S, Shinke R, Aoki K (1997) Production of methyl, dimethyl, ethyl, chloro-, fluoro-, and hydroxyl derivatives of catechol from thirteen aromatic amines by the transpositional mutant B-9 of the aniline-assimilating Pseudomonas species AW-2. J Ferment Bioeng 84:232–235. doi:10.1016/S0922-338X(97)82060-0
Kozlovsky SA, Zaitsev GM, Kunc F, Gabriel J, Boronin AM (1993) Degradation of 2-chlorobenzoic and 2,5-dichlorobenzoic acids in pure culture by Pseudomonas stutzeri. Folia Microbiol 38:371–375. doi:10.1007/BF02898758
Kramer C, Kreisel G, Fahr K, Kassbohrer J, Schlosser D (2004) Degradation of 2-fluorophenol by the brown-rot fungus Gloeophyllum striatum. Evidence for the involvement of extracellular Fenton chemistry. Appl Microbiol Biotechnol 64:387–395. doi:10.1007/s00253-003-1445-x
Kuntze K, Kiefer P, Baumann S, Seifert J, von Bergen M, Vorholt JA, Boll M (2011) Enzymes involved in the anaerobic degradation of meta-substituted halobenzoates. Mol Microbiol 82:758–769. doi:10.1111/j.1365-2958.2011.07856.x
Kurihara T, Yamauchi T, Ichiyama S, Takahata H, Esaki N (2003) Purification, characterization, and gene cloning of a novel fluoroacetate dehalogenase from Burkholderia sp. FA1. J Mol Catal B Enzym 23:347–355. doi:10.1016/S1381-1177(03)00098-5
Laempe D, Jahn M, Breese K, Schägger H, Fuchs G (2001) Anaerobic metabolism of 3-hydroxybenzoate by the denitrifying bacterium Thauera aromatica. J Bacteriol 183:968–979. doi:10.1128/JB.183.3.968-979.2001
Löffler F, Lingens F, Müller R (1995) Dehalogenation of 4-chlorobenzoate. Biodegradation 6:203–212. doi:10.1007/BF00700458
Londry KL, Fedorak PM (1993) Fluorophenols and 3-fluorobenzoate in phenol-degrading methanogenic cultures. Arch Microbiol 160:137–143. doi:10.1007/BF00288716
Maeda T, Okamura D, Yokoo M, Yamashita E, Ogawa HI (2007) Fluorine elimination from 4-fluorobenzyl alcohol by Pseudomonas spp. J Environ Biotechnol 7:45–53
Maia AS, Ribeiro AR, Amorim CL, Barreiro JC, Cass QB, Castro PM, Tiritan ME (2014) Degradation of fluoroquinolone antibiotics and identification of metabolites/transformation products by liquid chromatography-tandem mass spectrometry. J Chromatogr A 1333:87–98. doi:10.1016/j.chroma.2014.01.069
Mandal K, Singh B, Jariyal M, Gupta VK (2013) Microbial degradation of fipronil by Bacillus thuringiensis. Ecotoxicol Environ Saf 93:87–92. doi:10.1016/j.ecoenv.2013.04.001
Mansy AER, El-Bestawy E (2002) Toxicity and biodegradation of fluometuron by selected cyanobacterial species. World J Microbiol Biotechnol 18:125–131. doi:10.1023/A:1014490811121
Marr J, Kremer S, Sterner O, Anke H (1996) Transformation and mineralization of halophenols by Penicillium simplicissimum SK9117. Biodegradation 7:165–171. doi:10.1007/BF00114628
Mars AE, Kasberg T, Kaschabek SR, van Agteren MH, Janssen DB, Reineke W (1997) Microbial degradation of chloroaromatics: use of the meta-cleavage pathway for mineralization of chlorobenzene. J Bacteriol 179:4530–4537
Martens R, Wetzstein HG, Zadrazil F, Capelari M, Hoffmann P, Schmeer N (1996) Degradation of the fluoroquinolone enrofloxacin by wood-rotting fungi. Appl Environ Microbiol 62:4206–4209
Martin RE, Baker PB, Ribbons DW (1987) Biotransformations of fluoroaromatic compounds: accumulation of hydroxylated products from 3-fluorophthalic acid using mutant strains of Pseudomonas testosteroni. Biocatal Biotransfor 1:37–46. doi:10.3109/10242428709040129
Mazur P, Pieken WA, Budihas SR, Williams SE, Wong S, Kozarich JW (1994) Cis,cis-muconate lactonizing enzyme from Trichosporon cutaneum: evidence for a novel class of cycloisomerases in eucaryotes. Biochemistry 33:1961–1970. doi:10.1021/bi00173a045
Mc Cullar MV, Koh S, Focht DD (2002) The use of mutants to discern the degradation pathway of 3,4'-dichlorobiphenyl in Pseudomonas acidovorans M3GY. FEMS Microbiol Ecol 42:81–87. doi:10.1111/j.1574-6941.2002.tb00997.x
Mechichi T, Stackebrandt E, Gad'on N, Fuchs G (2002) Phylogenetic and metabolic diversity of bacteria degrading aromatic compounds under denitrifying conditions, and description of Thauera phenylacetica sp. nov., Thauera aminoaromatica sp. nov., and Azoarcus buckelii sp. nov. Arch Microbiol 178:26–35. doi:10.1007/s00203-002-0422-6
Meckenstock RU, Morasch B, Griebler C, Richnow HH (2004) Stable isotope fractionation analysis as a tool to monitor biodegradation in contaminated acquifers. J Contam Hydrol 75:215–255. doi:10.1016/j.jconhyd.2004.06.003
Milne GW, Goldman P, Holtzman JL (1968) The metabolism of 2-fluorobenzoic acid. II. Studies with 18O2. J Biol Chem 243:5374–5376
Misiak K, Casey E, Murphy CD (2011) Factors influencing 4-fluorobenzoate degradation in biofilm cultures of Pseudomonas knackmussii B13. Water Res 45:3512–3520. doi:10.1016/j.watres.2011.04.020
Moonen MJH, Rietjens IMCM, van Berkel WJH (2001) 19F NMR study on the biological Baeyer-Villiger oxidation of acetophenones. J Ind Microbiol Biotechnol 26:35–42. doi:10.1038/sj.jim.7000071
Moreira IS, Amorim CL, Carvalho MF, Castro PML (2012) Degradation of difluorobenzenes by the wild strain Labrys portucalensis. Biodegradation 23:653–662. doi:10.1007/s10532-012-9541-1
Moreira IS, Amorim CL, Carvalho MF, Ferreira AC, Afonso CM, Castro PML (2013) Effect of the metals iron, copper and silver on fluorobenzene biodegradation by Labrys portucalensis. Biodegradation 24:245–255. doi:10.1007/s10532-012-9581-6
Mouttaki H, Nanny MA, McInerney MJ (2009) Metabolism of hydroxylated and fluorinated benzoates by Syntrophus aciditrophicus and detection of a fluorodiene metabolite. Appl Environ Microbiol 75:998–1004. doi:10.1128/AEM.01870-08
Murphy CD (2006) Fluorophenol oxidation by a fungal chloroperoxidase. Biotechnol Lett 29:45–49. doi:10.1007/s10529-006-9207-3
Murphy CD (2010) Biodegradation and biotransformation of organofluorine compounds. Biotechnol Lett 32:351–359. doi:10.1007/s10529-009-0174-3
Murphy CD, Quirke S, Balogun O (2008) Degradation of fluorobiphenyl by Pseudomonas pseudoalcaligenes KF707. FEMS Microbiol Lett 286:45–49. doi:10.1111/j.1574-6968.2008.01243.x
Murphy CD, Clark BR, Amadio J (2009) Metabolism of fluoroorganic compounds in microorganisms: impacts for the environment and the production of fine chemicals. Appl Microbiol Biotechnol 84:617–629. doi:10.1007/s00253-009-2127-0
Natarajan R, Azerad R, Badet B, Copin E (2005) Microbial cleavage of CF bond. J Fluor Chem 126:424–435. doi:10.1016/j.jfluchem.2004.12.001
Neilson AH, Allard A (2002) Degradation and transformation of organic fluorine compounds. In: Neilson AH (ed) Organofluorines. Springer-Verlag, Berlin, pp 137–202
New AP, Freitas dos Santos LM, Lo Biundo G, Spicq A (2000) Analytical techniques used for monitoring the biodegradation of fluorinated compounds in waste streams from pharmaceutical production. J Chromatogr A 889:177–184. doi:10.1016/S0021-9673(00)00571-9
Nikodem P, Hecht V, Schlömann M, Pieper DH (2003) New bacterial pathway for 4- and 5-chlorosalicylate degradation via 4-chlorocatechol and maleylacetate in Pseudomonas sp. strain MT1. J Bacteriol 185:6790–6800
Nimmo WB, Wilde D, Pieter C, Verloop A (1984) The degradation of diflubenzuron and its chief metabolites in soils. Part I: hydrolytic cleavage of diflubenzuron. Pestic Sci 15:574–585. doi:10.1002/ps.2780150608
Nimmo WB, Joustra KD, Willems AGM (1990) The degradation of diflubenzuron and its chief metabolites in soils. Part III. Fate of 2,6-difluorobenzoic acid. Pestic Sci 29:39–45. doi:10.1002/ps.2780290106
Oltmanns RH, Müller R, Otto MK, Lingens F (1989) Evidence for a new pathway in the bacterial degradation of 4-fluorobenzoate. Appl Environ Microbiol 55:2499–2504
Osborne RL, Raner GM, Hager LP, Dawson JH (2006) C. fumago chloroperoxidase is also a dehaloperoxidase: oxidative dehalogenation of halophenols. J Am Chem Soc 128:1036–1037. doi:10.1021/ja056213b
Parshikov IA, Freeman JP, Lay JO, Beger RD, Williams AJ, Sutherland JB (1999) Regioselective transformation of ciprofloxacin to N-acetylciprofloxacin by the fungus Mucor ramannianus. FEMS Microbiol Lett 177:131–135. doi:10.1111/j.1574-6968.1999.tb13723.x
Parshikov IA, Freeman JP, Lay JO, Beger RD, Williams AJ, Sutherland JB (2000) Microbiological transformation of enrofloxacin by the Fungus Mucor ramannianus. Appl Environ Microbiol 66:2664–2667. doi:10.1128/AEM.66.6.2664-2667.2000
Parshikov IA, Freeman JP, Lay JO, Moody JD, Williams AJ, Beger RD, Sutherland JB (2001a) Metabolism of the veterinary fluoroquinolone sarafloxacin by the fungus Mucor ramannianus. J Ind Microbiol Biotechnol 26:140–144. doi:10.1038/sj/jim/7000077
Parshikov IA, Heinze TM, Moody JD, Freeman JP, Williams AJ, Sutherland JB (2001b) The fungus Pestalotiopsis guepini as a model for biotransformation of ciprofloxacin and norfloxacin. Appl Microbiol Biotechnol 56:474–477
Peelen S, Rietjens IMCM, Boersma MG, Vervoort J (1995) Conversion of phenol derivatives to hydroxylated products by phenol hydroxylase from Trichosporon cutaneum. A comparison of regioselectivity and rate of conversion with calculated molecular orbital substrate characteristics. Eur J Biochem 227:284–291. doi:10.1111/j.1432-1033.1995.tb20386.x
Peng X, Shindo K, Kanoh K, Inomata Y, Choi S, Misawa N (2005) Characterization of Sphingomonas aldehyde dehydrogenase catalyzing the conversion of various aromatic aldehydes to their carboxylic acids. Appl Microbiol Biotechnol 69:141–150. doi:10.1007/s00253-005-1962-x
Perez-Pantoja D, Donoso RA, Sanchez MA, Gonzalez B (2009) Genuine genetic redundancy in maleylacetate-reductase-encoding genes involved in degradation of haloaromatic compounds by Cupriavidus necator JMP134. Microbiology 155:3641–3651. doi:10.1099/mic.0.032086-0
Peters RA (1952) Croonian lecture: lethal synthesis. Proc R Soc Lond Ser B Biol Sci 139:143–170. doi:10.1098/rspb.1952.0001
Pieper DH, Engesser K, Don RH, Timmis KN, Knackmuss H (1985) Modified ortho-cleavage pathway in Alcaligenes eutrophus JMP134 for the degradation of 4-methylcatechol. FEMS Microbiol Lett 29:63–67. doi:10.1111/j.1574-6968.1985.tb00836.x
Pieper DH, Stadler-Fritzsche K, Knackmuss HJ, Engesser KH, Bruce NC, Cain RB (1990) Purification and characterization of 4-methylmuconolactone methylisomerase, a novel enzyme of the modified 3-oxoadipate pathway in the gram-negative bacterium Alcaligenes eutrophus JMP 134. Biochem J 271:529–534
Pieper DH, González B, Cámara B, Pérez-Pantoja D, Reineke W (2010) Aerobic degradation of chloroaromatics. In: Timmis KN (ed) Handbook of hydrocarbon and lipid microbiology. Springer, Berlin, pp 839–864
Prenafeta-Boldú FX, Luykx DM, Vervoort J, de Bont JA (2001) Fungal metabolism of toluene: monitoring of fluorinated analogs by (19)F nuclear magnetic resonance spectroscopy. Appl Environ Microbiol 67:1030–1034. doi:10.1128/AEM.67.3.1030-1034.2001
Prieto A, Möder M, Rodil R, Adrian L, Marco-Urrea E (2011) Degradation of the antibiotics norfloxacin and ciprofloxacin by a white-rot fungus and identification of degradation products. Bioresour Technol 102:10987–10995. doi:10.1016/j.biortech.2011.08.055
Purser S, Moore PR, Swallow S, Gouverneur V (2008) Fluorine in medicinal chemistry. Chem Soc Rev 37:320–330. doi:10.1039/b610213c
Rapp P, Gabriel-Jürgens LHE (2003) Degradation of alkanes and highly chlorinated benzenes, and production of biosurfactants, by a psychrophilic Rhodococcus sp. and genetic characterization of its chlorobenzene dioxygenase. Microbiology 149:2879–2890. doi:10.1099/mic.0.26188-0
Reineke W, Knackmuss H (1978) Chemical structure and biodegradability of halogenated aromatic compounds. Substituent effects on 1,2-dioxygenation of benzoic acid. Biochim Biophys Acta Gen Subj 542:412–423. doi:10.1016/0304-4165(78)90372-0
Reineke W, Knackmuss HJ (1988) Microbial degradation of haloaromatics. Annu Rev Microbiol 42:263–287. doi:10.1146/annurev.mi.42.100188.001403
Reinscheid UM, Bauer MP, Müller R (1996) Biotransformation of halophenols by a thermophilic Bacillus sp. Biodegradation 7:455–461. doi:10.1007/BF00115292
Reinscheid UM, Zuilhof H, Müller R, Vervoort J (1998) Biological, thermal and photochemical transformation of 2-trifluoromethylphenol. Biodegradation 9:487–499. doi:10.1023/A:1008394115008
Renganathan V (1989) Possible involvement of toluene-2,3-dioxygenase in defluorination of 3-fluoro-substituted benzenes by toluene-degrading Pseudomonas sp. Strain T-12. Appl Environ Microbiol 55:330–334
Renganathan V, Johnston J (1989) Catechols of novel substrates produced using the toluene ring oxidation pathway of Pseudomonas sp. strain T-12. Appl Microbiol Biotechnol 31(4):419–424. doi:10.1007/BF00257615
Riegert U (2001) Eine neuartige bakterielle Dioxygenase mit der Fähigkeit zur extradiolen Spaltung von chlorierten Brenzkatechinen. Herbert Utz Verlag, Munich
Rojo F, Pieper DH, Engesser KH, Knackmuss HJ, Timmis KN (1987) Assemblage of ortho cleavage route for simultaneous degradation of chloro- and methylaromatics. Science 238:1395–1398
Rossiter JT, Williams SR, Cass AE, Ribbons DW (1987) Aromatic biotransformations 2: production of novel chiral fluorinated 3,5-cyclohexadiene-cis-1,2-diol-1-carboxylates. Tetrahedron Lett 28:5173–5174. doi:10.1016/S0040-4039(00)95620-X
Saikia N, Gopal M (2004) Biodegradation of β-cyfluthrin by fungi. J Agric Food Chem 52:1220–1223. doi:10.1021/jf0349580
Saikia N, Das SK, Patel BKC, Niwas R, Singh A, Gopal M (2005) Biodegradation of beta-cyfluthrin by Pseudomonas stutzeri strain S1. Biodegradation 16:581–589. doi:10.1007/s10532-005-0211-4
Satsuma K, Hayashi O, Sato K, Hashimura M, Kato Y (2000) Microbial degradation of herbicide pentoxazone in soils. J Pestic Sci 25:201–206
Schennen U, Braun K, Knackmuss HJ (1985) Anaerobic degradation of 2-fluorobenzoate by benzoate-degrading, denitrifying bacteria. J Bacteriol 161:321–325
Schlömann M (1988) Die verschiedenen Typen der Dienlacton-Hydrolase und ihre Rolle beim bakteriellen Abbau von 4-Fluorbenzoat. Dissertation, University of Göttingen
Schlömann M (1994) Evolution of chlorocatechol catabolic pathways. Conclusions to be drawn from comparisons of lactone hydrolases. Biodegradation 5:301–321
Schlömann M, Fischer P, Schmidt E, Knackmuss HJ (1990a) Enzymatic formation, stability, and spontaneous reactions of 4-fluoromuconolactone, a metabolite of the bacterial degradation of 4-fluorobenzoate. J Bacteriol 172:5119–5129
Schlömann M, Schmidt E, Knackmuss HJ (1990b) Different types of dienelactone hydrolase in 4-fluorobenzoate-utilizing bacteria. J Bacteriol 172:5112–5118
Schmidt E, Knackmuss HJ (1980) Chemical structure and biodegradability of halogenated aromatic compounds. Conversion of chlorinated muconic acids into maleoylacetic acid. Biochem J 192:339–347
Schmidt E, Knackmuss H (1984) Production of cis,cis-muconate from benzoate and 2-fluoro-cis,cis-muconate from 3-fluorobenzoate by 3-chlorobenzoate degrading bacteria. Appl Microbiol Biotechnol 20:351–355. doi:10.1007/BF00270599
Schmidt E, Remberg G, Knackmuss HJ (1980) Chemical structure and biodegradability of halogenated aromatic compounds. Halogenated muconic acids as intermediates. Biochem J 192:331–337
Schmidt S, Wittich RM, Erdmann D, Wilkes H, Francke W, Fortnagel P (1992) Biodegradation of diphenyl ether and its monohalogenated derivatives by Sphingomonas sp. strain SS3. Appl Environ Microbiol 58:2744–2750
Schmidt S, Fortnagel P, Wittich RM (1993) Biodegradation and transformation of 4,4'- and 2,4-dihalodiphenyl ethers by Sphingomonas sp. strain SS33. Appl Environ Microbiol 59:3931–3933
Schreiber A, Hellwig M, Dorn E, Reineke W, Knackmuss HJ (1980) Critical reactions in fluorobenzoic acid degradation by Pseudomonas sp. B13. Appl Environ Microbiol 39:58–67
Seeger M, Cámara B, Hofer B (2001) Dehalogenation, denitration, dehydroxylation, and angular attack on substituted biphenyls and related compounds by a biphenyl dioxygenase. J Bacteriol 183:3548–3555. doi:10.1128/JB.183.12.3548-3555.2001
Seibert V, Stadler-Fritzsche K, Schlömann M (1993) Purification and characterization of maleylacetate reductase from Alcaligenes eutrophus JMP134(pJP4). J Bacteriol 175:6745–6754
Seibert V, Thiel M, Hinner I, Schlömann M (2004) Characterization of a gene cluster encoding the maleylacetate reductase from Ralstonia eutropha 335T, an enzyme recruited for growth with 4-fluorobenzoate. Microbiology 150:463–472
Selesi D, Meckenstock RU (2009) Anaerobic degradation of the aromatic hydrocarbon biphenyl by a sulfate-reducing enrichment culture. FEMS Microbiol Ecol 68:86–93. doi:10.1111/j.1574-6941.2009.00652.x
Sharak Genthner BR, Townsend GT, Chapman PJ (1990) Effect of fluorinated analogues of phenol and hydroxybenzoates on the anaerobic transformation of phenol to benzoate. Biodegradation 1:65–74. doi:10.1007/BF00117052
Sistrom WR, Stanier RY (1954) The mechanism of formation of β-ketoadipic acid by bacteria. J Biol Chem 210:821–836
Solyanikova IP, Maltseva OV, Vollmer MD, Golovleva LA, Schlömann M (1995) Characterization of muconate and chloromuconate cycloisomerase from Rhodococcus erythropolis 1CP: indications for functionally convergent evolution among bacterial cycloisomerases. J Bacteriol 177:2821–2826
Solyanikova IP, Moiseeva OV, Boeren S, Boersma MG, Kolomytseva MP, Vervoort J, Rietjens IMCM, Golovleva LA, van Berkel WJH (2003) Conversion of 2-fluoromuconate to cis-dienelactone by purified enzymes of Rhodococcus opacus 1cp. Appl Environ Microbiol 69:5636–5642. doi:10.1128/AEM.69.9.5636-5642.2003
Song B, Palleroni NJ, Haggblom MM (2000) Isolation and characterization of diverse halobenzoate-degrading denitrifying bacteria from soils and sediments. Appl Environ Microbiol 66:3446–3453. doi:10.1128/AEM.66.8.3446-3453.2000
Song B, Palleroni NJ, Kerkhof LJ, Häggblom MM (2001) Characterization of halobenzoate-degrading, denitrifying Azoarcus and Thauera isolates and description of Thauera chlorobenzoica sp. nov. Int J Syst Evol Microbiol 51:589–602
Song E, Wang M, Shen D (2014) Isolation, identification and characterization of a novel Ralstonia sp. FD-1, capable of degrading 4-fluoroaniline. Biodegradation 25:85–94. doi:10.1007/s10532-013-9642-5
Strunk N, Engesser K (2013) Degradation of fluorobenzene and its central metabolites 3-fluorocatechol and 2-fluoromuconate by Burkholderia fungorum FLU100. Appl Microbiol Biotechnol 97:5605–5614. doi:10.1007/s00253-012-4388-2
Sun X, Man F, Pang L, Gao G, Li X, Qi X, Li F (2009) Fungal biotransformation of mosapride by Cunninghamella elegans. J Mol Catal B Enzym 59:82–89. doi:10.1016/j.molcatb.2009.01.009
Taylor B (1993) Degradation of meta-trifluoromethylbenzoate by sequential microbial and photochemical treatments. FEMS Microbiol Lett 110:213–216. doi:10.1016/0378-1097(93)90468-H
Taylor BF, Hearn WL, Pincus S (1979) Metabolism of monofluoro- and monochlorobenzoates by a denitrifying bacterium. Arch Microbiol 122:301–306. doi:10.1007/BF00411295
Theodoridis G (2006) Chapter 4 Fluorine-containing agrochemicals: an overview of recent developments. In: Alain Tressaud (ed) Advances in fluorine science: fluorine and the environment agrochemicals, archaeology, green chemistry & water. Elsevier, pp 121–175
Travkin V, Solyanikova I, Rietjens I, Vervoort J, van Berkel W, Golovleva L (2003) Degradation of 3,4-dichloro- and 3,4-difluoroaniline by Pseudomonas fluorescens 26-K. J Environ Sci Health B 38:121–132. doi:10.1081/PFC-120018443
Uotila JS, Kitunen VH, Saastamoinen T, Coote T, Häggblom MM, Salkinoja-Salonen MS (1992) Characterization of aromatic dehalogenases of Mycobacterium fortuitum CG-2. J Bacteriol 174:5669–5675
Uotila JS, Kitunen VH, Coote T, Saastamoinen T, Salkinoja-Salonen M, Apajalahti JH (1995) Metabolism of halohydroquinones in Rhodococcus chlorophenolicus PCP-1. Biodegradation 6:119–126
van der Bolt FJ, van den Heuvel RH, Vervoort J, van Berkel WJ (1997) 19F NMR study on the regiospecificity of hydroxylation of tetrafluoro-4-hydroxybenzoate by wild-type and Y385F p-hydroxybenzoate hydroxylase: evidence for a consecutive oxygenolytic dehalogenation mechanism. Biochemistry 36:14192–14201. doi:10.1021/bi971213c
Vargas C, Song B, Camps M, Häggblom MM (2000) Anaerobic degradation of fluorinated aromatic compounds. Appl Microbiol Biotechnol 53:342–347
Vollmer MD, Hoier H, Hecht HJ, Schell U, Gröning J, Goldman A, Schlömann M (1998) Substrate specificity of and product formation by muconate cycloisomerases: an analysis of wild-type enzymes and engineered variants. Appl Environ Microbiol 64:3290–3299
Vollmer MD, Schell U, Seibert V, Lakner S, Schlömann M (1999) Substrate specificities of the chloromuconate cycloisomerases from Pseudomonas sp. B13, Ralstonia eutropha JMP134 and Pseudomonas sp. P51. Appl Microbiol Biotechnol 51:598–605
Vora KA, Singh C, Modi VV (1988) Degradation of 2-fluorobenzoate by a pseudomonad. Curr Microbiol 17:249–254. doi:10.1007/BF01571323
Walker MC, Chang MCY (2014) Natural and engineered biosynthesis of fluorinated natural products. Chem Soc Rev 43:6527–6536. doi:10.1039/c4cs00027g
Wang HZ, Zuo HG, Ding YJ, Miao SS, Jiang C, Yang H (2014) Biotic and abiotic degradation of pesticide Dufulin in soils. Environ Sci Pollut Res Int 21:4331–4342. doi:10.1007/s11356-013-2380-8
Wetzstein HG, Schmeer N, Karl W (1997) Degradation of the fluoroquinolone enrofloxacin by the brown rot fungus Gloeophyllum striatum: identification of metabolites. Appl Environ Microbiol 63:4272–4281
Wetzstein HG, Stadler M, Tichy HV, Dalhoff A, Karl W (1999) Degradation of ciprofloxacin by basidiomycetes and identification of metabolites generated by the brown rot fungus Gloeophyllum striatum. Appl Environ Microbiol 65:1556–1563
Wetzstein H, Schneider J, Karl W (2006) Patterns of metabolites produced from the fluoroquinolone enrofloxacin by basidiomycetes indigenous to agricultural sites. Appl Microbiol Biotechnol 71:90–100. doi:10.1007/s00253-005-0178-4
Wetzstein H, Schneider J, Karl W (2012) Metabolite proving fungal cleavage of the aromatic core part of a fluoroquinolone antibiotic. AMB Express 2:3. doi:10.1186/2191-0855-2-3
Wolgel SA, Dege JE, Perkins-Olson PE, Jaurez-Garcia CH, Crawford RL, Münck E, Lipscomb JD (1993) Purification and characterization of protocatechuate 2,3-dioxygenase from Bacillus macerans: a new extradiol catecholic dioxygenase. J Bacteriol 175:4414–4426
Wunderwald U, Hofrichter M, Kreisel G, Fritsche W (1997) Transformation of difluorinated phenols by Penicillium frequentans Bi 7/2. Biodegradation 8:379–385. doi:10.1023/A:1008230926973
Wunderwald U, Kreisel G, Braun M, Schulz M, Jäger C, Hofrichter M (2000) Formation and degradation of a synthetic humic acid derived from 3-fluorocatechol. Appl Microbiol Biotechnol 53:441–446
Yano K, Wachi M, Tsuchida S, Kitazume T, Iwai N (2015) Degradation of benzotrifluoride via the dioxygenase pathway in Rhodococcus sp. 065240. Biosci Biotechnol Biochem 79:1–9. doi:10.1080/09168451.2014.982502
Zaitsev GM, Uotila JS, Tsitko IV, Lobanok AG, Salkinoja-Salonen MS (1995) Utilization of halogenated benzenes, phenols, and benzoates by Rhodococcus opacus GM-14. Appl Environ Microbiol 61:4191–4201
Zeyer J, Wasserfallen A, Timmis KN (1985) Microbial mineralization of ring-substituted anilines through an ortho-cleavage pathway. Appl Environ Microbiol 50:447–453
Zhanel GG, Ennis K, Vercaigne L, Walkty A, Gin AS, Embil J, Smith H, Hoban DJ (2002) A critical review of the fluoroquinolones. Drugs 62:13–59. doi:10.2165/00003495-200262010-00002
Zhang H, Hanada S, Shigematsu T, Shibuya K, Kamagata Y, Kanagawa T, Kurane R (2000) Burkholderia kururiensis sp. nov., a trichloroethylene (TCE)-degrading bacterium isolated from an aquifer polluted with TCE. Int J Syst Evol Microbiol 50:743–749. doi:10.1099/00207713-50-2-743
Zhang X, Lai T, Kong RY (2012) Biology of fluoro-organic compounds. In: Horváth IT (ed) Fluorous chemistry. Springer, Berlin, pp 365–404
Zhang X, Feng H, Shan D, Shentu J, Wang M, Yin J, Shen D, Huang B, Ding Y (2014) The effect of electricity on 2–fluoroaniline removal in a bioelectrochemically assisted microbial system (BEAMS). Electrochim Acta 135:439–446. doi:10.1016/j.electacta.2014.05.033
Zhao Z, Feng Y, Feng H, Ghulam A, Su Y, Shen D (2014) Anaerobic biotransformation of fluoronitrobenzenes and microbial communities in methanogenic systems. J Environ Sci Health A 49:1187–1197. doi:10.1080/10934529.2014.897537
Zhao Z, Tian B, Zhang X, Ghulam A, Zheng T, Shen D (2015) Aerobic degradation study of three fluoroanilines and microbial community analysis: the effects of increased fluorine substitution. Biodegradation 26:1–14. doi:10.1007/s10532-014-9704-3
Zhou L, Marks TS, Poh RPC, Smith RJ, Chowdhry BZ, Smith ARW (2004) The purification and characterisation of 4-chlorobenzoate:CoA ligase and 4-chlorobenzoyl CoA dehalogenase from Arthrobacter sp. strain TM-1. Biodegradation 15:97–109
Zhu G, Wu H, Guo J, Kimaro FME (2004) Microbial degradation of fipronil in clay loam soil. Water Air Soil Pollut 153:35–44. doi:10.1023/B:WATE.0000019928.67686.b1
Ziegler K, Braun K, Böckler A, Fuchs G (1987) Studies on the anaerobic degradation of benzoic acid and 2-aminobenzoic acid by a denitrifying Pseudomonas strain. Arch Microbiol 149:62–69. doi:10.1007/BF00423138
Ziegler K, Buder R, Winter J, Fuchs G (1989) Activation of aromatic acids and aerobic 2-aminobenzoate metabolism in a denitrifying Pseudomonas strain. Arch Microbiol 151:171–176. doi:10.1007/BF00414434
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kiel, M., Engesser, KH. The biodegradation vs. biotransformation of fluorosubstituted aromatics. Appl Microbiol Biotechnol 99, 7433–7464 (2015). https://doi.org/10.1007/s00253-015-6817-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6817-5