Abstract
Polychlorinated biphenyls (PCBs) pose potential risks to human and environmental health because they are carcinogenic, persistent, and bioaccumulative. In this study, we investigated bacterial communities in soil microcosms spiked with PCB 52, 77, and 153. Switchgrass (Panicum virgatum) was employed to improve overall PCB removal, and redox cycling (i.e., sequential periods of flooding followed by periods of no flooding) was performed in an effort to promote PCB dechlorination. Lesser chlorinated PCB transformation products were detected in all microcosms, indicating the occurrence of PCB dechlorination. Terminal restriction fragment length polymorphism (T-RFLP) and clone library analysis showed that PCB spiking, switchgrass planting, and redox cycling affected the microbial community structure. Putative organohalide-respiring Chloroflexi populations, which were not found in unflooded microcosms, were enriched after 2 weeks of flooding in the redox-cycled microcosms. Sequences classified as Geobacter sp. were detected in all microcosms and were most abundant in the switchgrass-planted microcosm spiked with PCB congeners. The presence of possible organohalide-respiring bacteria in these soil microcosms suggests that they play a role in PCB dechlorination therein.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polychlorinated biphenyls (PCBs), widely used in industry in the last century, encompasses a set of 209 congeners. Although their production has been banned since the 1970s, they remain persistent in the environment and pose potential risk to public health because they are toxic and carcinogenic and bioaccumulate in the food web (Ross 2004). The bioremediation of PCB-contaminated soils and sediments is challenging because of the tendency of PCB congeners to be recalcitrant in the environment (Agarwal et al. 2007; Amend and Lederman 1992). Traditional methods such as soil excavation and dredging with subsequent landfill processing or high temperature incineration are energy-consuming and are thus considered not sustainable (Vasilyeva and Strijakova 2007).
Microorganisms can degrade and detoxify PCBs via two major pathways—anaerobic PCB dechlorination and aerobic PCB oxidation (Borja et al. 2005; Gibson and Parales 2000; Pieper and Seeger 2008; Wiegel and Wu 2000). Under anaerobic conditions, certain bacteria use PCB congeners as electron acceptors, which lead to reduction of the PCB molecule and removal of chlorine atoms from the biphenyl ring. Anaerobic reductive dechlorination of PCBs can be growth supporting or co-metabolic (Wiegel and Wu 2000; Wu et al. 1998). All confirmed PCB dechlorinators are members of the phylum Chloroflexi, including the Dehalococcoides spp. and the o-17/DF-1 group (Adrian et al. 2009; Fagervold et al. 2007; Fennell et al. 2004; Wang et al. 2014; Yan et al. 2006). Reductive dechlorination of PCBs by Dehalobacter was confirmed in an enrichment culture but not yet in pure culture (Yoshida et al. 2009). Under aerobic conditions, certain microorganisms oxidize PCBs via the upper biphenyl pathway, generating chlorobenzoates (Gibson and Parales 2000; Macková et al. 2010). Chlorobenzoates can be further metabolized by other microbial community members (Pavlů et al. 1999).
Several studies have demonstrated the effect of plants on enhancing PCB degradation and increasing microbial PCB-degrading populations (Leigh et al. 2006; Macková et al. 2006; Slater et al. 2011). Plants play an important role in promoting oxygen diffusion and regulating soil water in the rhizosphere (Schnoor et al. 1995). Plant-released compounds such as flavonoids and terpenes were found to support PCB-degrading microbial populations and induce PCB degradation (de Cárcer et al. 2007; Donnelly et al. 1994; Gilbert and Crowley 1997; Hernandez et al. 1997; Leigh et al. 2002). Some plant species, such as Austrian pine (Pinus nigra) and goat willow (Salix caprea), were able to increase the abundance of microbial PCB degraders in their root zones (Leigh et al. 2006).
However, most studies have focused on how plants influence aerobic PCB degradation while anaerobic dechlorination in the root zone is rarely investigated. There is a recent report of PCB dechlorination in the switchgrass rhizosphere (Meggo and Schnoor 2013); however, the microbial community and its potential roles in PCB dechlorination was not investigated. In the present study, we developed an independent set of soil microcosms and aimed to characterize microbial communities in switchgrass planted and unplanted PCB-dechlorinating soil microcosms, identify potential PCB-dechlorinating community members, and investigate the effect of plants and soil zone water flooding on PCB-dechlorinating communities.
From a remediation technologies perspective, plant-promoted PCB degradation could be useful in instances where PCB-contaminated sediments are dredged from freshwater lakes, rivers, estuaries, and coastal marine areas. These sediments are typically transferred to a confined disposal facility (CDF) for long-term storage (Liang et al. 2014a). Planting dredged, PCB-contaminated sediments with hardy plant species such as switchgrass could acceralate remediation and facilitate reclamation of these sediments for beneficial purposes.
Materials and methods
Soil microcosm setup
The soil microcosm setup procedure has been described previously (Liang et al. 2014b; Meggo and Schnoor 2013). Briefly, a mixture of PCB 52, PCB 77, and PCB 153 (99 % pure) (Accustandard Inc., New Haven, CT) was dissolved in hexane and then applied to the PCB-free soil (Nodaway series, silt loam from Middle Amana, Iowa, USA) at a target concentration of 500 ng g−1 each (wet weight). The three congeners were selected because they are considered more difficult to degrade in comparison to other congeners (Bedard et al. 1986). PCB 52 and PCB 77 are tetrachlorinated, and PCB 153 is hexachlorinated and thus relatively more resistant to aerobic oxidation than lesser chlorinated congeners (Bedard et al. 1986). The ortho chlorines in PCB 52 and PCB 153 are difficult for enzymes to attack (Dai et al. 2002). PCB 77 has a dioxin-like structure and is one of the most toxic congeners (Van den Berg et al. 2006). Finally, the three congeners are frequently detected in the environment (Lammel and Stemmler 2012; Martinez et al. 2010). The PCB-spiked soil was homogenized and aged for 8 weeks at 25 °C in the sealed tubs to facilitate sequestration of PCB congeners into the soil matrix.
Four soil microcosms were constructed by filling plastic containers (33.8 cm × 21.6 cm × 211.9 cm) with 2,500 g of PCB-spiked soil each, prepared as described above. Two microcosms remained unplanted (UP), and the remaining two microcosms were planted with switchgrass (Panicum virgatum) (SG) seeds (Adams-Briscoe Seed Co., Jackson, GA) (Figure S1). Besides the four soil microcosms, an unplanted “blank” microcosm (BLK), which contained 2,500 g of soil that was not spiked with PCBs, was also prepared. All microcosms were covered with aluminum foil and incubated for 8 weeks in a plant growth chamber at 25 °C and 60 % humidity. Each day consisted of a 16-h light period (200 mmol m−2 s−1 intensity) and an 8-h dark period. After the initial 8-week incubation, one UP microcosm and one SG microcosm was subjected to alternating cycles of complete soil saturation (i.e., “flooding”—hereafter referred to UPF and SGF microcosms). Two weeks of flooding with deionized water was followed by 2 weeks where extra water was withdrawn with a sterile syringe, and soil drying was allowed to occur (i.e., non-flooding). Four complete flooding cycles were performed during the experiment. This flooding/non-flooding procedure caused cycling of the redox potential in the soils (Fig. S2) and is thus hereafter referred to as “redox cycling.”
Volumetric soil moisture content was monitored in each microcosm with Vernier soil moisture sensors interfaced with the Vernier LabQuest data collection system (Vernier Software & Technology, Beaverton, OR). The redox potential was measured with a portable pH/ORP meter (Hanna Instruments, Woonsocket, RI). A sterile syringe (5 ml) with the narrow tip removed was used to sample vertical soil cores randomly from the microcosms. The core samples were homogenized before PCB congener and microbial community analysis. Soil samples for PCB congener analysis were taken at week 12 and week 24. Soil samples for microbial community analysis were taken every 2 weeks starting at week 8.
PCB congener analysis
The PCB extraction method, described previously (Liang et al. 2014b; Meggo and Schnoor 2013), was performed in triplicate for each soil sample. Briefly, soil (5 g) was mixed with 1:1 hexane/acetone (3 ml g−1). The surrogate standards including PCB14, deuterated PCB65, and PCB166 (Cambridge Isotope Laboratories, Inc.) were added into the samples (50 ng of each surrogate) to determine the extraction recovery. Surrogate recoveries were 101.8 ± 17.5 % (PCB 14), 100.4 ± 15.2 % (deuterated PCB 65), and 99.8 ± 18.9 % (PCB 166).
The soil and hexane/acetone mixture was sonicated for 1 h and then subjected to two rounds of centrifugation (1,500 × g, 5 min). The combined supernatant was evaporated to dryness by rotary evaporation and dissolved in hexane. Lipids and other polar substances were removed by double extraction with concentrated sulfuric acid and hexane. A nitrogen stream was used to concentrate the hexane extract down to 0.5 ml. The concentrate was eluted with 10 ml hexane through a filter consisting of 0.1 g of silica (70–230 mesh, Fisher Scientific, Inc.), 0.1 g of anhydrous sodium sulfate, and 0.9 g silica gel acidified with sulfuric acid (silica/sulfuric acid 2:1).
The PCB congener analysis procedure was described previously (Hu et al. 2010). PCB 204 (2,2′,3,4,4′,5,6,6′-octachlorobiphenyl; 100 ng) was added to the concentrated extracts as an internal standard. PCB congeners were analyzed and quantified via a modified EPA method 1668 A gas chromatography with mass selective detection (GC-MS/MS) method (US EPA 1999). An Agilent 6,890 N gas chromatograph was used with an Agilent 7,683 series autosampler coupled to a Waters Micromass Quattro micro GC mass spectrometer (Milford, MA, USA). The mass spectrometer was operated under electron impact (EI) positive mode at 70 eV and multiple reaction monitoring (MRM), and the trap current was 200 μA. The retention windows were defined by PCB parent/daughter ion pairs from mono- to deca-homologs which were 188/152, 222/152.10, 255.96/186, 291.92/222, 325.88/255.90, 359.84/289.90, 393.80/323.90, 427.76/357.80, 461.72/391.83, 497.68/427.70, respectively. For each congener, the limit of quantification was defined as six times the standard deviation from three samples from the blank microcosm. Congeners with a concentration lower than the limit of quantification (as determined by analysis of the blank) were removed from the PCB profiles in PCB-spiked microcosms. The molar percentage of initial concentration for PCB 52, 77, and 153 after 12 and 24 weeks of incubation in the microcosms is shown in Fig. S3. The unplanted and switchgrass-planted PCB congener data (Fig. S3) was previously presented in Liang et al. (2014b) and is shown here for the purposes of comparison with the other treatments.
Nucleic acid extraction and quantitative PCR (qPCR)
DNA was extracted from 2-g soil (wet weight) immediately after sampling using the DNA Elution Accessory Kit attached with the RNA PowerSoil kit (Mobio, Carlsbad, CA) and stored at −80 °C before further analysis. The abundances of total bacteria, Geobacteraceae, and putative dechlorinating Chloroflexi were estimated with qPCR targeting bacterial 16S ribosomal RNA (rRNA) gene (primer set 16SU f/r) (Nadkarni et al. 2002), Geobacteraceae 16S rRNA genes (Wei and Finneran 2011), and putative dechlorinating Chloroflexi 16S rRNA genes (primer set dhc793f/946r) (Yoshida et al. 2005), respectively (Table S1). PCR conditions were as follows: 10 min at 95 °C, 40 cycles of 15 s at 95 °C, and 1 min at 60 °C followed by a dissociation step. Each 25 μl qPCR contained 12.5 μl Power SYBR Green PCR Master Mix (Invitrogen, Carlsbad, CA), various amount of primers, and DNA templates (Table S2). Bovine serum albumin (500 ng) was added to reduce possible PCR inhibition (Kreader 1996).
For qPCR targeting total bacterial 16S rRNA genes, the standard DNA template was PCR amplified from Burkholderia xenovorans strain LB400 with primer set 8F/1492R (Grabowski et al. 2005). For qPCR targeting putative dechlorinating Chloroflexi 16S rRNA gene and Geobacteraceae 16S rRNA genes, the standard curves were prepared from pCR 2.1-TOPO vectors containing a PCR product amplified from soil DNA with primer set dhc793f/946r and Geo494f/825r, respectively. All qPCRs were performed in triplicate or replicate with an ABI 7000 Sequence Detection System (Applied Biosystems, Grand Island, NY), and fluorescence data was analyzed by ABI 7000 System SDS Software (Applied Biosystems, Grand Island, NY) at the Iowa Institute of Human Genetics Genomics Division. With each primer set, the target gene was not detected in no template (DI water) controls (Ct value >35). Additional qPCR information, including qPCR linear range, qPCR efficiency range of the standard curves, and y-intercepts are provided in Table S2, in accordance with MIQE guidelines (Bustin et al. 2009).
qPCR quality assurance
Several published qPCR primer sets targeting putative dechlorinating Chloroflexi 16S rRNA gene and Geobacteraceae were tested. Primer sets chl348f/884r (Fagervold et al. 2005), dhc1f/264r (Grostern and Edwards 2006), dhc793f/946r (Yoshida et al. 2005), dhc1154f/1286r (Krzmarzick et al. 2012), and Geo494f/825r (Wei and Finneran 2011) each yielded a single band of expected size with the soil DNA template. To verify the specificity of these primer sets, clone libraries were constructed from the amplification products of DNA extracted from soil with redox cycling. From the dhc793f/946r PCR product clone library, five unique sequences were obtained from 12 clones and 92 % of these sequences were identified as Dehalogenimonas sp. by RDP classifier (Table S3) (Cole et al. 2007). Primer sets chl348f/884r, dhc1f/264r, and dhc1154f/1286r also amplified sequences from the class Anaerolineae (phylum Chloroflexi) and from the Actinobacteria, both of which are not associated with dechlorination (Table S3). From the Geo494f/825r PCR product clone library, 12 unique sequences were obtained from 14 clones. Seventy nine percent of these sequences were identified as Geobacteraceae sp. and the rest as unclassified Deltaproteobacteria by RDP classifier (Table S3). Thus, the primer set dhc793f/946r and Geo494f/825r was used to quantify the putative dechlorinating Chloroflexi 16S rRNA gene and Geobacteraceae 16S rRNA gene in soil samples. Primer set dhc793f/946r is not expected to amplify sequences from theo-17/DF-1 group. However, the primer set 14f/dehal1265r, which targets the o-17/DF-1 group, did not generate amplicons, suggesting that the o-17/DF-1 group was not present in our soil samples (Watts et al. 2005). The specificity of the SYBR green-based qPCR was also validated by dissociation curve analysis, which showed similar melting temperatures for each qPCR experiment with these primers.
Terminal-restriction fragment length polymorphism (T-RFLP) analysis
T-RFLP analysis was performed on the bacterial 16S rRNA gene as described previously (Liang et al. 2014a). Briefly, PCR was performed with fluorescent-labeled primer set 6-FAM 8Fm/533R (Schütte et al. 2008) with 1 ng DNA as template (Table S1). The restriction enzyme HaeIII (New England BioLabs, Inc., Ipswich, MA) was used to digest PCR products. Digested DNA was precipitated, recovered by centrifugation, resuspended in distilled water, and sent to the Iowa Institute of Human Genetics Genomics Division at the University of Iowa for sizing.
Terminal restriction fragment (TRF) sizes were estimated by Peak Scanner software (Applied Biosystems, Carlsbad, CA). The TRF size matrix containing 84 samples (rows) and 439 unique 16S rRNA gene TRFs (columns) was produced by T-REX software after filtering background peak noises and rounding fragment sizes to the nearest whole number (Culman et al. 2009). TRF profiles were analyzed by non-metric multidimensional scaling (NMDS) in R (da C Jesus et al. 2009). The Bray-Curtis dissimilarity index was calculated with a random starting configuration, and a two-dimensional solution was reached after two runs. The final stress was 12.77. The TRF composition differences among groups were assessed by multiresponse permutation procedure (MRPP), which evaluates grouping with real and randomized data based on Bray-Curtis distances and 5,000 permutations, and permutational multivariate analyses of variance (PERMANOVA) based on Bray-Curtis distances with 5,000 permutations.
PCR amplification, cloning, and sequencing of bacterial 16S rRNA genes
Clone libraries were constructed with bacterial 16S rRNA genes amplified from unplanted soil, switchgrass-planted soil, unplanted and cycled soil, and switchgrass planted and cycled soil at 24 weeks. The cloning procedure was described previously (Liang et al. 2014a). Bacterial 16S rRNA genes were amplified using primer set 8F/1492R (Grabowski et al. 2005) (Table S1). Purified PCR products were cloned into the pCR 2.1-TOPO vector using the TOPO TA cloning Kit (Invitrogen Corp., Carlsbad, CA) and transformed into One Shot TOP10 chemically competent Escherichia coli cells (Invitrogen Corp., Carlsbad, CA). Recombinant E. coli were plated on Luria Broth agar containing kanamycin (50 mg l−1) and X-gal (0.4 mg plate−1) and incubated overnight at 37 °C to check for successful DNA insertion via blue-white screening. Clones were partially Sanger-sequenced at the Iowa Institute of Human Genetics Genomics Division initially with M13F primers (Table S1). A second round of sequencing was performed with M13R primers (Table S1) if the M13F sequence revealed that the 16S gene was cloned in the opposite orientation. Chimeric sequence identification was performed by DECIPHER (Wright et al. 2012). Sequence identification and classification were performed by RDP classifier (Cole et al. 2007). The sequences were subjected to in silico TRF production using TRiFLE (Junier et al. 2008). Sequences collected in this study were submitted to Genbank (Accession Nos. KJ766128–KJ766299).
Statistical analyses
PCB congener data was analyzed with independent sample t test using R. The total bacterial 16S rRNA gene copies, putative dechlorinating Chloroflexi 16S rRNA gene copies, and Geobacteraceae 16S rRNA gene copies were analyzed with a two-factor analysis of variance after log transformation using R. Pearson’s r was calculated in R to evaluate the correlation between gene abundances and environmental variables (Rieu and Powers 2009).
Results
Soil PCB congener analysis
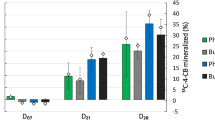
Parent PCB congener concentrations decreased in all microcosms by 30–40 % after 24 weeks of incubation with greater overall removal of PCB 77 and PCB 153 than PCB 52 observed (Fig. S3). The occurrence of PCB dechlorination was further verified by detecting the expected transformation products of PCB52, PCB77, and PCB153. These included PCB3, PCB15, PCB 26/29, PCB 35, PCB48, and PCB 118 in all soil microcosms (unplanted (UP), switchgrass planted (SG), and redox-cycled soils (UPF and SGF)) (Figs. 1 and 2). Redox potential measurements (Fig. S2) suggest that soil microcosms that were not subjected to periods of flooding were still possibly experiencing “anoxic” conditions (<350 mV) (Setter and Waters 2003). PCB 101 and PCB 118 are plausible dechlorination products of PCB 153 (Fig. 2). PCB 101 could be further transformed into PCB 52 by para chlorine removal, and PCB 118 can be further dechlorinated to PCB 77 by ortho chlorine removal (Fig. 2). PCB 95 and 110/115 were also detected at low concentrations in PCB-spiked soils but not in the blank soil (i.e., soil without PCB spiking) (Fig. 1). PCB 35 is a plausible dechlorination product of PCB 77 by para chlorine removal, and PCB 26 could be generated from PCB 52 by ortho chlorine removal (Fig. 2). PCB dechlorination in a similar system has been previously reported (Meggo and Schnoor 2013), but the mediating microbial community was not investigated. Therefore, in this study, we focused on comparative microbial community analysis and searched for possible PCB dechlorinators in the microcosms in an effort to delineate the role of microorganisms in the observed biotransformations.
Transformation products detected after 12 and 24 weeks of incubation in unplanted microcosm (UP), switchgrass-planted microcosm (SG), unplanted microcosm with redox cycling (UPF), and switchgrass-planted microcosm with redox cycling (SGF). Error bars indicate the standard deviation of three soil subsamples from the same microcosm
Influence of PCB spiking, switchgrass planting, and redox cycling on microbial community structure in microcosms
With evidence of PCB dechlorination, we next sought to better characterize the microbial communities in the microcosms. We hypothesized that a comparative microbial community analysis would allow identification of potentially important community members that might be contributing to PCB dechlorination in the soil microcosms. To achieve this, T-RFLP analysis was performed to delineate the overall microbial community structure of the microcosms and track changes in the communities with time in response to different treatments. NMDS ordination of the T-RFLP profiles revealed that at the beginning of the experiment, the BLK and UP microcosms shared similar bacterial community structures (Fig. 3). Interestingly, the subsequent soil treatments resulted in community structures that diverged significantly from the original community structure. The combined effect of PCB spiking, switchgrass planting, and periodic flooding appeared to have the greatest impact (Fig. 3). The differences between T-RFLP profiles in microcosms with different treatments were confirmed by MRPP and PERMANOVA (p = 0.001).
NMDS ordination of T-RFLP profiles from the blank microcosm (BLK), unplanted microcosm (UP), switchgrass-planted microcosm (SG), unplanted microcosm with redox cycling (UPF), and switchgrass-planted microcosm with redox cycling (SGF). The symbols are shaded with respect to incubation time, with lighter shading indicating longer incubation time at sampling. The final stress was 12.77
The composition of the bacterial community in soil microcosms with different treatments after 24 weeks of incubation was assessed by sequencing a total of 162 clones from four 16S rRNA gene clone libraries (UP, SG, UPF, and SGF). Each of the soil communities were dominated by Proteobacteria (47.7 ± 12.9 % of total clones) and Acidobacteria (15.4 ± 7.8 % of total clones) (Fig. 4). The remaining phylotypes detected were affiliated with Bacteroidetes, Actinobacteria, Chloroflexi, Chloroplasts, Firmicutes, Gemmatimonadetes, Nitrospira, OP11, Planctomycetes, and Verrucomicrobia.
Redox cycling had significant effects on the microbial community structure in comparison to the other treatments (Fig. 3). Analysis of clone libraries from UPF and SGF microcosms indicated that Proteobacteria increased by 21.2 %, and in particular, Gammaproteobacteria increased by 18.1 % in comparison to the UP and SG microcosms. Conversely, Acidobacteria abundance decreased in the UPF and SGF microcosms by 11.7 %. The 203 and 215 bp TRFs, which were more abundant in switchgrass-planted soils (p < 0.05; Figure S4), corresponded to Geobacter sp. as determined by T-RFLP of the clones themselves (Table 1). Of the 42 clones sequenced from the UPF microcosm, two were classified as Geobacter sp., a genus containing known sulfate, sulfur, and Fe(III) reducers (Methe et al. 2003) as well as dechlorinators (Sung et al. 2006). Another clone that would produce a 203-bp TRF is from the same order of Desulfuromonadales (Table 1). Cloned 16S rRNA gene sequences that generated 288 and 298-bp TRFs were classified as Clostridium sp. These TRFs comprised 0.3 and 0.5 % of all TRFs detected in each microcosm (Figure S4).
Sequences classified as Geobacter sp. and Clostridium sp. were not highly abundant in the four clone libraries (both sequences were only 1.2 % of the total sequences retrieved), although these libraries were not extensively sequenced (162 clones sequenced in total). Members of the Chloroflexi detected in 16S rRNA gene clone libraries from the SG, UPF, and SGF microcosms were classified as members of the Anaerolineae and Caldilineae (Table 1), both of which are not currently associated with reductive dechlorination processes (Gupta et al. 2013).
qPCR analysis of total bacterial 16S rRNA genes, putative dechlorinating Chloroflexi, and Geobacteraceae 16S rRNA genes in soil microcosms
Because the Geobacteraceae have previously been implicated in reductive dechlorination processes (Sung et al. 2006) and certain members of the Chloroflexi are the only confirmed anaerobic PCB dechlorinators (Adrian et al. 2009; Fennell et al. 2004; Yan et al. 2006), we quantified putative dechlorinating Chloroflexi and Geobacteraceae 16S rRNA genes in all microcosms using more specific qPCR primers (Table S1). We also quantified total 16S bacterial rRNA gene abundance over the course of the experiment (Table S1, Fig. S6). We found that switchgrass treatment significantly increased the total bacterial 16S rRNA gene abundance; however, this parameter was not affected by redox cycling (p > 0.05) (Figure S6).
Initially, putative dechlorinating Chloroflexi 16S rRNA gene abundance was below the qPCR quantification limit (<30 genes per reaction) in all microcosms and remained below quantification in the BLK, UP, and SG microcosms for the duration of the experiment. However, after 2 weeks of flooding, putative dechlorinating Chloroflexi 16S rRNA gene abundance increased to between 105 and 106 genes per gram soil in the UPF and SGF microcosms before leveling off at week 12 (Fig. 5). Despite repeated flooding and subsequent draining periods, putative dechlorinating Chloroflexi 16S rRNA gene abundance remained stable for the remainder of the experiment and was not correlated to redox potential or moisture content change (Pearson’s r, p > 0.05). Interestingly, the abundance of putative dechlorinating Chloroflexi 16S rRNA genes in the SGF microcosm was significantly higher than in the UPF microcosm (p < 0.05).
qPCR analysis of putative dechlorinating Chloroflexi 16S rRNA gene in unplanted microcosm with redox cycling (UPF) and switchgrass-planted microcosm with redox cycling (SGF). Error bars indicate the range of two soil subsamples from the same microcosm. Putative dechlorinating Chloroflexi 16S rRNA gene abundance in unplanted (UP) and switchgrass-planted microcosms (SG) were below the quantification limit
Clone libraries were constructed with the UPF and SGF qPCR products in an effort to identify the Chloroflexi members we detected by qPCR. Each of the 12 sequences obtained from the clone libraries were classified as Dehalogenimonas sp. (Table S3). Our sequences share 98–100 % similarity with the Dehalogenimonas sequence retrieved from a PCB-dechlorinating enrichment culture CG3, which can couple its growth with PCB dechlorination (Wang and He 2013b), as visualized by phylogenetic tree analysis (Figure S5). Overall, putative dechlorinating Chloroflexi 16S rRNA genes were not abundant with respect to total bacterial 16S rRNA gene abundance (0.0028 ± 0.0008 % in UPF and 0.0029 ± 0.0025 % in SGF).
PCB addition did not significantly affect Geobacteraceae abundance (p > 0.05, Fig. 6), while the highest Geobacteraceae 16S rRNA gene abundances were observed in the SG microcosm (Fig. 6). Redox cycling treatment did not affect Geobacteraceae abundance significantly (p > 0.05). Overall, the Geobacteraceae and putative dechlorinating Chloroflexi represented only a small fraction of the total bacteria 16S rRNA genes (1.01 to 1.43 % in the four microcosms, respectively).
qPCR analysis of Geobacteraceae 16S rRNA genes in the blank microcosm (BLK), unplanted microcosm (UP), switchgrass-treated microcosm (SG), unplanted microcosm with redox cycling (UPF), and switchgrass-treated microcosm with redox cycling (SGF). Error bars indicate the range of two soil subsamples from the same microcosm
Discussion
Detection of PCB congener transformation products verified the occurrence of PCB dechlorination in the soil microcosms. PCB dechlorination is typically observed in more strongly reducing anaerobic environments, as typified by a higher negative redox potential (Bedard et al. 1996; Krumins et al. 2009; Quensen et al. 1990). However, it is plausible that within soil microenvironments, pockets of strongly reducing conditions existed that favored the utilization of PCB congeners as alternative electron acceptors by microorganisms.
The detection of PCB 118 was notable, because it requires ortho chlorine removal from PCB 153 which is known to be difficult in other systems (Bedard et al. 2006; Quensen et al. 1990). In this system, PCB 153 is potentially dechlorinated to PCB 52 and 77, congeners which were also spiked into the soils initially. The reduction of PCB 153 to PCB 52 is more likely, which can be achieved by removing two para-chlorines, while PCB 77 production requires ortho dechlorination, which is considered more difficult (Tiedje et al. 1993).
PCB 95 and 110/115 are not expected dechlorination products of the three spiked PCB congeners and were possibly produced by chlorine rearrangement, although the mechanism is unclear. Chlorine rearrangement of PCB congeners has been observed previously in poplars, switchgrass, and maize (Liu et al. 2009; Wang et al. 2011).
Overall, the PCB transformation products were less than 5 % on a molar basis with respect to the amount of parent PCB congeners added and did not accumulate significantly, possibly because of further sequential dechlorination and subsequent aerobic oxidation (Master et al. 2001). Evidence that supports the occurrence of aerobic PCB oxidation in these systems (Figure S3) was also presented previously (Liang et al. 2014b). Our results were also somewhat in contrast to a similar study, which indicated greater accumulation of dechlorination products in redox-cycled soils (Meggo and Schnoor 2013). The variability in PCB dechlorination among similarly constructed and treated microcosms is not clear but might have resulted by a differential development of soil microbial communities between the two experiments.
Proteobacteria are commonly the dominant bacteria in soils and sediments (de Cárcer et al. 2007; Petric et al. 2011; Spain et al. 2009). Many known aerobic PCB degraders are Proteobacteria (e.g., Pseudomonas, Sphingomonas, Acinetobacter, Comamonas, and Burkholderia (Nováková et al. 2002)); thus, it is possible that these same groups could participate in aerobic PCB degradation in the soil microcosms. In a previous study, we quantified the biphenyl dioxygenase alpha subunit gene (bphA) from the UP and SG microcosms (Liang et al. 2014b). The BphA gene encodes the enzyme catalyzing the first step of the upper biphenyl pathway. Additional potential aerobic PCB degraders other than Proteobacteria, such as Bacillus and Rhodococcus sp., were also identified (Masai et al. 1995; Shimura et al. 1999). A high abundance of Acidobacteria was previously noted in other PCB-contaminated soils and sediments (Nogales et al. 1999; Petric et al. 2011); however, there is no evidence linking them to PCB degradation.
It is possible that the bacterial groups represented by the TRFs identified as important in the UP microcosm are stimulated by PCB spiking and play a role in PCB degradation in the soil. The relatively low abundance of these groups with respect to the total abundance of the microbial community, plus the relative low coverage of the clone libraries (162 clones) are probably what prevented us from identifying these TRFs.
Putative dechlorinating Chloroflexi were not detected in our bacterial 16S clone libraries, possibly because of their low relative abundance in the microcosms with respect to total bacterial 16S rRNA gene sequences. However, using a different set of qPCR primers, we were able to verify the presence of putative dechlorinating Chloroflexi and identify them as probable Dehalogenimonas sp. Strains of Dehalogenimonas are reported to reductively dechlorinate polychlorinated aliphatic alkanes (Maness et al. 2012; Yan et al. 2009). A Dehalogenimonas strain was recently implicated in growth-coupled PCB dechlorination in the sediment-free culture, CG3 (Wang and He 2013b). Dechlorinating Chloroflexi, while found in our microcosms, were more abundant in previously reported PCB-dechlorinating sediment-free cultures. For example, Dehalococcoides was 3.74 % of total bacterial community in highly enriched sediment-free JN culture (Bedard et al. 2006). Dehalogenimonas sp. represented 2.16 % of a sediment-free culture that dechlorinated Aroclor 1260 (Wang and He 2013b). Despite this, the apparent growth of Dehalogenimonas sp. in the UPF and SGF microcosms strongly suggests that they played a role in PCB dechlorination therein.
Although we observed putative dechlorinating Chloroflexi in soil microcosms via qPCR, they were only present in the UPF and SGF microcosms at relatively low abundance. This suggests that additional microbial groups were involved in PCB dechlorination in the soil microcosms (UP and SG especially). Within the Proteobacteria, the delta- and gamma-proteobacteriac ontain some strains known to degrade chlorinated compounds via organohalide respiration or co-metabolic reductive dehalogenation, such as Desulfuromonas spp. (Krumholz 1997), Geobacterlovleyi (Sung et al. 2006), and Shewanella putrefaciens (Picardal et al. 1995). Certain Geobacter sp. can dechlorinate trichloroacetic acid and tetrachloroethene (de Wever et al. 2000; Strycharz et al. 2008; Sung et al. 2006) and have been found in sediment-free PCB-dechlorinating enrichment cultures (Bedard et al. 2006; Bedard et al. 2007; Holoman et al. 1998). Notably, a recent study found that Geobacteraceae enriched in cultures that completely degraded trichloroethene under iron-reducing conditions, suggesting that Geobacter may be able to dechlorinate under less strict anaerobic conditions (Wei and Finneran 2011). Our results suggest that the presence of switchgrass enriched for certain Geobacter sp. or related Desulfuromonadales (Figure S4), perhaps by releasing root exudates as carbon and energy sources (Bais et al. 2006; Baudoin et al. 2003)
Clostridium is another genus we identified as responding to redox cycling with the potential to participate in PCB dechlorination. For instance, certain Clostridia dechlorinate tetrachloroethene (Chang et al. 2000; Okeke et al. 2001). Clostridium sp. were detected in a PCB-dechlorinating enrichment culture derived from St. Lawrence River sediment (Oh et al. 2008) and sediment-free PCB-dechlorinating culture AD14 (Wang and He 2013a). In another study, Clostridium sp. were abundant in sediments where para- and meta-PCB dechlorination was occurring (Hou and Dutta 2000). Clostridium sp. can also produce hydrogen, an important and common electron donor for reductive dechlorination processes (Bowman et al. 2009; Nandi and Sengupta 1998). The presence of such species in soil microcosms where PCB dechlorination had occurred indicates that their possible role in PCB degradation should be considered in future studies.
In this study, we investigated the microbial communities in soils displaying PCB dechlorination. The occurrence of dechlorination was verified by the detection of transformation products of parent PCB congeners in all soil microcosms, some of which were under anoxic conditions. Putative dechlorinating Chloroflexi populations were enriched after the first 2 weeks of flooding of the redox cycling treatment, which sequences were classified as Dehalogenimonas sp., a potential PCB-dechlorinating species. Geobacter were identified in all soil samples and were more abundant in switchgrass-planted soil with PCB spiking. Overall, the potential organohalide-respiring bacteria were about 1 % of the total bacteria, but their presence in the soils suggests a dechlorination potential of the indigenous microbial community and their role in PCB dechlorination.
References
Adrian L, Dudková V, Demnerová K, Bedard DL (2009) "Dehalococcoides" sp. strain CBDB1 extensively dechlorinates the commercial polychlorinated biphenyl mixture Aroclor 1260. Appl Environ Microbiol 75(13):4516–4524
Agarwal S, Al-Abed SR, Dionysiou DD (2007) In situ technologies for reclamation of PCB-contaminated sediments: current challenges and research thrust areas. J Environ Eng Asce 133(12):1075–1078
Amend LJ, Lederman PB (1992) Critical-evaluation of PCB remediation technologies. Environ Prog 11(3):173–177
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57(1):233–266
Baudoin E, Benizri E, Guckert A (2003) Impact of artificial root exudates on the bacterial community structure in bulk soil and maize rhizosphere. Soil Biol Biochem 35(9):1183–1192
Bedard DL, Bailey JJ, Reiss BL, Jerzak GV (2006) Development and characterization of stable sediment-free anaerobic bacterial enrichment cultures that dechlorinate Aroclor 1260. Appl Environ Microbiol 72(4):2460–2470
Bedard DL, Bunnell SC, Smullen LA (1996) Stimulation of microbial para-dechlorination of polychlorinated biphenyls that have persisted in Housatonic River sediment for decades. Environ Sci Technol 30(2):687–694
Bedard DL, Ritalahti KA, Löffler FE (2007) The Dehalococcoides population in sediment-free mixed cultures metabolically dechlorinates the commercial polychlorinated biphenyl mixture Aroclor 1260. Appl Environ Microbiol 73(8):2513–2521
Bedard DL, Unterman R, Bopp LH, Brennan MJ, Haberl ML, Johnson C (1986) Rapid assay for screening and characterizing microorganisms for the ability to degrade polychlorinated biphenyls. Appl Environ Microbiol 51(4):761–768
Borja J, Taleon DM, Auresenia J, Gallardo S (2005) Polychlorinated biphenyls and their biodegradation. Process Biochem 40(6):1999–2013
Bowman KS, Rainey FA, Moe WM (2009) Production of hydrogen by Clostridium species in the presence of chlorinated solvents. Fems Microbiol Lett 290(2):188–194
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55(4):611–622
Chang YC, Hatsu M, Jung K, Yoo YS, Takamizawa K (2000) Isolation and characterization of a tetrachloroethylene dechlorinating bacterium, Clostridium bifermentans DPH-1. J Biosci Bioeng 89(5):489–491
Cole JR, Chai B, Farris RJ, Wang Q, Kulam-Syed-Mohideen AS, McGarrell DM, Bandela AM, Cardenas E, Garrity GM, Tiedje JM (2007) The ribosomal database project (RDP-II): introducing myRDP space and quality controlled public data. Nucleic Acids Res 35:169–172
Culman SW, Bukowski R, Gauch HG, Cadillo-Quiroz H, Buckley DH (2009) T-REX: software for the processing and analysis of T-RFLP data. BMC Bioinformat 10:171
da C Jesus E, Marsh TL, Tiedje JM, de S Moreira FM (2009) Changes in land use alter the structure of bacterial communities in Western Amazon soils. ISME J 3(9):1004–1011
Dai S, Vaillancourt FH, Maaroufi H, Drouin NM, Neau DB, Snieckus V, Bolin JT, Eltis LD (2002) Identification and analysis of a bottleneck in PCB biodegradation. Nat Struct Biol 9(12):934–939
de Cárcer DA, Martín M, Karlson U, Rivilla R (2007) Changes in bacterial populations and in biphenyl dioxygenase gene diversity in a polychlorinated biphenyl-polluted soil after introduction of willow trees for rhizoremediation. Appl Environ Microbiol 73(19):6224–6232
de Wever H, Cole JR, Fettig MR, Hogan DA, Tiedje JM (2000) Reductive dehalogenation of trichloroacetic acid by Trichlorobacter thiogenes gen. nov., sp. nov. Appl Environ Microbiol 66(6):2297–2301
Donnelly PK, Hegde RS, Fletcher JS (1994) Growth of PCB-degrading bacteria on compounds from photosynthetic plants. Chemosphere 28(5):981–988
Fagervold SK, May HD, Sowers KR (2007) Microbial reductive dechlorination of Aroclor 1260 in Baltimore harbor sediment microcosms is catalyzed by three phylotypes within the phylum Chloroflexi. Appl Environ Microbiol 73(9):3009–3018
Fagervold SK, Watts JE, May HD, Sowers KR (2005) Sequential reductive dechlorination of meta-chlorinated polychlorinated biphenyl congeners in sediment microcosms by two different Chloroflexi phylotypes. Appl Environ Microbiol 71(12):8085–8090
Fennell DE, Nijenhuis I, Wilson SF, Zinder SH, Häggblom MM (2004) Dehalococcoides ethenogenes strain 195 reductively dechlorinates diverse chlorinated aromatic pollutants. Environ Sci Technol 38(7):2075–2081
Gibson DT, Parales RE (2000) Aromatic hydrocarbon dioxygenases in environmental biotechnology. Curr Opin Biotechnol 11(3):236–243
Gilbert ES, Crowley DE (1997) Plant compounds that induce polychlorinated biphenyl biodegradation by Arthrobacter sp. strain B1B. Appl Environ Microbiol 63(5):1933–1938
Grabowski A, Nercessian O, Fayolle F, Blanchet D, Jeanthon C (2005) Microbial diversity in production waters of a low-temperature biodegraded oil reservoir. FEMS Microbiol Ecol 54(3):427–443
Grostern A, Edwards EA (2006) Growth of Dehalobacter and Dehalococcoides spp. during degradation of chlorinated ethanes. Appl Environ Microbiol 72(1):428–436
Gupta RS, Chander P, George S (2013) Phylogenetic framework and molecular signatures for the class Chloroflexi and its different clades; proposal for division of the class Chloroflexi class. nov into the suborder Chloroflexineae subord. nov., consisting of the emended family Oscillochloridaceae and the family Chloroflexaceae fam. nov., and the suborder Roseiflexineae subord. nov., containing the family Roseiflexaceae fam. nov. Antonie Van Leeuwenhoek 103(1):99–119
Hernandez BS, Koh SC, Chial M, Focht DD (1997) Terpene-utilizing isolates and their relevance to enhanced biotransformation of polychlorinated biphenyls in soil. Biodegradation 8(3):153–158
Holoman TR, Elberson MA, Cutter LA, May HD, Sowers KR (1998) Characterization of a defined 2,3,5, 6-tetrachlorobiphenyl-ortho-dechlorinating microbial community by comparative sequence analysis of genes coding for 16S rRNA. Appl Environ Microbiol 64(9):3359–3367
Hou LH, Dutta SK (2000) Phylogenetic characterization of several para- and meta-PCB dechlorinating Clostridium species: 16s rDNA sequence analyses. Lett Appl Microbiol 30(3):238–243
Hu DF, Lehmler HJ, Martinez A, Wang K, Hornbuckle KC (2010) Atmospheric PCB congeners across Chicago. Atmos Environ 44(12):1550–1557
Junier P, Junier T, Witzel KP (2008) TRiFLe, a program for in silico terminal restriction fragment length polymorphism analysis with user-defined sequence sets. Appl Environ Microbiol 74(20):6452–6456
Kreader CA (1996) Relief of amplification inhibition in PCR with bovine serum albumin or T4 gene 32 protein. Appl Environ Microbiol 62(3):1102–1106
Krumholz LR (1997) Desulfuromonas chloroethenica sp. nov. uses tetrachloroethylene and trichloroethylene as electron acceptors. Int J Sys Bacteriol 47(4):1262–1263
Krumins V, Park JW, Son EK, Rodenburg LA, Kerkhof LJ, Häggblom MM, Fennell DE (2009) PCB dechlorination enhancement in Anacostia River sediment microcosms. Water Res 43(18):4549–4558
Krzmarzick MJ, Crary BB, Harding JJ, Oyerinde OO, Leri AC, Myneni SCB, Novak PJ (2012) Natural niche for organohalide-respiring Chloroflexi. Appl Environ Microbiol 78(2):393–401
Lammel G, Stemmler I (2012) Fractionation and current time trends of PCB congeners: evolvement of distributions 1950–2010 studied using a global atmosphere-ocean general circulation model. Atmos Chem Phys 12(5):11699–11731
Leigh MB, Fletcher JS, Fu XO, Schmitz FJ (2002) Root turnover: an important source of microbial substrates in rhizosphere remediation of recalcitrant contaminants. Environ Sci Technol 36(7):1579–1583
Leigh MB, Prouzová P, Macková M, Macek T, Nagle DP, Fletcher JS (2006) Polychlorinated biphenyl (PCB)-degrading bacteria associated with trees in a PCB-contaminated site. Appl Environ Microbiol 72(4):2331–2342
Liang Y, Martinez A, Hornbuckle KC, Mattes TE (2014a) Potential for polychlorinated biphenyl biodegradation in sediments from Indiana Harbor and Ship Canal. Int Biodeter Biodegr 89:50–57
Liang Y, Meggo R, Hu D, Schnoor JL, Mattes TE (2014b) Enhanced polychlorinated biphenyl removal in a switchgrass rhizosphere by bioaugmentation with Burkholderia xenovorans LB400. Ecol Eng 71:215–222
Liu JY, Hu DF, Jiang GB, Schnoor JL (2009) In vivo biotransformation of 3,3 ',4,4 '-tetrachlorobiphenyl by whole plants—poplars and switchgrass. Environ Sci Technol 43(19):7503–7509
Macková M, Barriault D, Francová K, Sylvestre M, Möder M, Vrchotová B, Lovecká P, Najmanová J, Demnerová K, Nováková M (2006) Phytoremediation of polychlorinated biphenyls Phytoremediation rhizoremediation. Springer, pp 143–167
Macková M, Uhlík O, Lovecká P, Viktorova J, Nováková M, Demnerová K, Sylvestre M, Macek T (2010) Bacterial degradation of polychlorinated biphenyls. In: Barton LL, Mandl M, Loy A (eds) Geomicrobiology: molecular and environmental perspective. Springer, Netherlands, pp 347–366
Maness AD, Bowman KS, Yan J, Rainey FA, Moe WM (2012) Dehalogenimonas spp. can reductively dehalogenate high concentrations of 1,2-dichloroethane, 1,2-dichloropropane, and 1,1,2-trichloroethane. AMB Expr 2(1):1–7
Martinez A, Norstrom K, Wang K, Hornbuckle KC (2010) Polychlorinated biphenyls in the surficial sediment of Indiana Harbor and Ship Canal, Lake Michigan. Environ Int 36(8):849–854
Masai E, Yamada A, Healy JM, Hatta T, Kimbara K, Fukuda M, Yano K (1995) Characterization of biphenyl catabolic genes of Gram-positive polychlorinated biphenyl degrader Rhodococcus sp strain RHA1. Appl Environ Microbiol 61(6):2079–2085
Master ER, Lai VWM, Kuipers B, Cullen WR, Mohn WW (2001) Sequential anaerobic−aerobic treatment of soil contaminated with weathered Aroclor 1260. Environ Sci Technol 36(1):100–103
Meggo RE, Schnoor JL (2013) Rhizospere redox cycling and implications for rhizosphere biotransformation of selected polychlorinated biphenyl (PCB) congeners. Ecol Eng 57:285–292
Methe BA, Nelson KE, Eisen JA, Paulsen IT, Nelson W, Heidelberg JF, Wu D, Wu M, Ward N, Beanan MJ, Dodson RJ, Madupu R, Brinkac LM, Daugherty SC, DeBoy RT, Durkin AS, Gwinn M, Kolonay JF, Sullivan SA, Haft DH, Selengut J, Davidsen TM, Zafar N, White O, Tran B, Romero C, Forberger HA, Weidman J, Khouri H, Feldblyum TV, Utterback TR, Van Aken SE, Lovley DR, Fraser CM (2003) Genome of Geobacter sulfurreducens: metal reduction in subsurface environments. Science 302(5652):1967–1969
Nadkarni MA, Martin FE, Jacques NA, Hunter N (2002) Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiol Sgm 148:257–266
Nandi R, Sengupta S (1998) Microbial production of hydrogen: an overview. Crit Rev Microbiol 24(1):61–84
Nogales B, Moore ERB, Abraham WR, Timmis KN (1999) Identification of the metabolically active members of a bacterial community in a polychlorinated biphenyl polluted moorland soil. Environ Microbiol 1(3):199–212
Nováková H, Vošahlı́ková M, Pazlarová J, Macková M, Burkhard J, Demnerová K (2002) PCB metabolism by Pseudomonas sp P2. Int Biodeter Biodegr 50(1):47–54
Oh KH, Ostrofsky EB, Cho YC (2008) Molecular characterization of polychlorinated biphenyl-dechlorinating populations in contaminated sediments. J Microbiol 46(2):165–173
Okeke BC, Chang YC, Hatsu M, Suzuki T, Takamizawa K (2001) Purification, cloning, and sequencing of an enzyme mediating the reductive dechlorination of tetrachloroethylene (PCE) from Clostridium bifermentans DPH-1. Can J Microbiol 47(5):448–456
Pavlů L, Vosáhlová J, Klierová H, Prouza M, Demnerová K, Brenner V (1999) Characterization of chlorobenzoate degraders isolated from polychlorinated biphenyl-contaminated soil and sediment in the Czech Republic. J Appl Microbiol 87(3):381–386
Petric I, Bru D, Udikovic-Kolic N, Hrsak D, Philippot L, Martin-Laurent F (2011) Evidence for shifts in the structure and abundance of the microbial community in a long-term PCB-contaminated soil under bioremediation. J Haz Mat 195:254–260
Picardal F, Arnold RG, Huey BB (1995) Effects of electron-donor and acceptor conditions on reductive dehalogenation of tetrachloromethane by Shewanella putrefaciens 200. Appl Environ Microbiol 61(1):8–12
Pieper DH, Seeger M (2008) Bacterial metabolism of polychlorinated biphenyls. J Mol Microbiol Biotechnol 15(2–3):121–138
Quensen JF, Boyd SA, Tiedje JM (1990) Dechlorination of four commercial polychlorinated biphenyl mixtures (Aroclors) by anaerobic microorganisms from sediments. Appl Environ Microbiol 56(8):2360–2369
Rieu I, Powers SJ (2009) Real-time quantitative RT-PCR: design, calculations, and statistics. Plant Cell 21(4):1031–1033
Ross G (2004) The public health implications of polychlorinated biphenyls (PCBs) in the environment. Ecotoxicol Environ Saf 59(3):275–291
Schnoor JL, Licht LA, Mccutcheon SC, Wolfe NL, Carreira LH (1995) Phytoremediation of organic and nutrient contaminants. Environ Sci Technol 29(7):A318–A323
Schütte UME, Abdo Z, Bent SJ, Shyu C, Williams CJ, Pierson JD, Forney LJ (2008) Advances in the use of terminal restriction fragment length polymorphism (T-RFLP) analysis of 16S rRNA genes to characterize microbial communities. Appl Microbiol Biotechnol 80(3):365–380
Setter TL, Waters I (2003) Review of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant Soil 253(1):1–34
Shimura M, Mukerjee-Dhar G, Kimbara K, Nagato H, Kiyohara H, Hatta T (1999) Isolation and characterization of a thermophilic Bacillus sp JF8 capable of degrading polychlorinated biphenyls and naphthalene. Fems Microbiol Lett 178(1):87–93
Slater H, Gouin T, Leigh MB (2011) Assessing the potential for rhizoremediation of PCB contaminated soils in northern regions using native tree species. Chemosphere 84(2):199–206
Spain AM, Krumholz LR, Elshahed MS (2009) Abundance, composition, diversity and novelty of soil Proteobacteria. ISME J 3(8):992–1000
Strycharz SM, Woodard TL, Johnson JP, Nevin KP, Sanford RA, Loffler FE, Lovley DR (2008) Graphite electrode as a sole electron donor for reductive dechlorination of tetrachlorethene by Geobacter lovleyi. Appl Environ Microbiol 74(19):5943–5947
Sung Y, Fletcher KF, Ritalaliti KM, Apkarian RP, Ramos-Hernandez N, Sanford RA, Mesbah NM, Loffler FE (2006) Geobacter lovleyi sp nov strain SZ, a novel metal-reducing and tetrachloroethene-dechlorinating bacterium. Appl Environ Microbiol 72(4):2775–2782
Tiedje JM, Quensen JF 3rd, Chee-Sanford J, Schimel JP, Boyd SA (1993) Microbial reductive dechlorination of PCBs. Biodegradation 4(4):231–240
Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson RE (2006) The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci 93(2):223–241
U.S. EPA (1999) Method 1668, Revision A: Chlorinated biphenyl congeners in water, soil, sediment, and tissue by HRGC/HRMS. Bibliogov, Washington, DC
Vasilyeva G, Strijakova E (2007) Bioremediation of soils and sediments contaminated by polychlorinated biphenyls. Microbiology 76(6):639–653
Wang S, Chng KR, Wilm A, Zhao S, Yang K-L, Nagarajan N, He J (2014) Genomic characterization of three unique Dehalococcoides that respire on persistent polychlorinated biphenyls. Proc Natl Acad Sci U S A 111(33):12103–12108
Wang S, He J (2013a) Dechlorination of commercial PCBs and other multiple halogenated compounds by a sediment-free culture containing Dehalococcoides and Dehalobacter. Environ Sci Technol 47(18):10526–10534
Wang S, Zhang SZ, Huang HL, Zhao MM, Lv JT (2011) Uptake, translocation and metabolism of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in maize (Zea mays L.). Chemosphere 85(3):379–385
Wang SQ, He JZ (2013b) Phylogenetically distinct bacteria involve extensive dechlorination of Aroclor 1260 in sediment-free cultures. PLoS One 8(3):e59178
Watts JEM, Fagervold SK, May HD, Sowers KR (2005) A PCR-based specific assay reveals a population of bacteria within the Chloroflexi associated with the reductive dehalogenation of polychlorinated biphenyls. Microbiology 151(6):2039–2046
Wei N, Finneran KT (2011) Influence of ferric iron on complete dechlorination of trichloroethylene (TCE) to ethene: Fe(III) reduction does not always inhibit complete dechlorination. Environ Sci Technol 45(17):7422–7430
Wiegel J, Wu Q (2000) Microbial reductive dehalogenation of polychlorinated biphenyls. FEMS Microbiol Ecol 32(1):1–15
Wright ES, Yilmaz LS, Noguera DR (2012) DECIPHER, a search-based approach to chimera identification for 16S rRNA sequences. Appl Environ Microbiol 78(3):717–725
Wu Q, Sowers KR, May HD (1998) Microbial reductive dechlorination of aroclor 1260 in anaerobic slurries of estuarine sediments. Appl Environ Microbiol 64(3):1052–1058
Yan J, Rash BA, Rainey FA, Moe WM (2009) Isolation of novel bacteria within the Chloroflexi capable of reductive dechlorination of 1,2,3-trichloropropane. Environ Microbiol 11(4):833–843
Yan T, LaPara TM, Novak PJ (2006) The reductive dechlorination of 2,3,4,5-tetrachlorobiphenyl in three different sediment cultures: evidence for the involvement of phylogenetically similar Dehalococcoides-like bacterial populations. FEMS Microbiol Ecol 55(2):248–261
Yoshida N, Takahashi N, Hiraishi A (2005) Phylogenetic characterization of a polychlorinated-dioxin-dechlorinating microbial community by use of microcosm studies. Appl Environ Microbiol 71(8):4325–4334
Yoshida N, Ye L, Baba D, Katayama A (2009) Reductive dechlorination of polychlorinated biphenyls and dibenzo-p-dioxins in an enrichment culture containing Dehalobacter species. Microbes Environ 24(4):343–346
Acknowledgments
This research was funded by the National Institute of Environmental Health Sciences (NIEHS) Superfund Research Program (SRP) Grant No. P42ES13661 and by a fellowship for YL from the Center for Biocatalysis and Bioprocessing at the University of Iowa.
Conflict of interest
The authors declare that they have no conflict of interest pertaining to this work.
Ethical statement
This work complies with the Ethical Standards of the Committee on Publication Ethics (COPE).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 588 kb)
Rights and permissions
About this article
Cite this article
Liang, Y., Meggo, R., Hu, D. et al. Microbial community analysis of switchgrass planted and unplanted soil microcosms displaying PCB dechlorination. Appl Microbiol Biotechnol 99, 6515–6526 (2015). https://doi.org/10.1007/s00253-015-6545-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6545-x