Abstract
Extensive studies have been operated on the biosorption of heavy metal using white-rot fungi, whereas information on the stability of the sorbed metal species has never been taken into consideration, which is important for the later disposal of the used biomass. In this study, the growing cells of Phanerochaete chrysosporium were used to remove Pb from the fungal living environment. The bioremoval of Pb proceeded continually until 121 h. The bioremoved Pb was found to be stabilized at the first time P. chrysosporium was exposed to Pb ions. The extractable rate of removed Pb decreased constantly and kept at a stable level around 20 % after 121 h. The results indicated that the growing biomass is efficient for the stabilization of Pb, and the used biomass was suitable to be separated for further disposal at 121 h. With environment scanning electron microscopy coupled with energy-dispersive X-ray analysis (ESEM-EDAX) and X-ray powder diffraction (XRD) analysis, the stabilized Pb species were identified to be lead oxalate and lead chloride phosphate. Further, it is found that the stabilization of Pb by growing P. chrysosporium is not strictly limited in the aspect of pH when pH in the environment is in the range of 4–6.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Discharge and disposal of waste products contaminated with heavy metals have resulted in the pollution of valuable groundwater resources (Hashim et al. 2011). Because heavy metals cannot be degraded and are toxic to biological systems, heavy metals will continue to be an environmental concern for a long time unless they are taken out from the ecosystem. Lead (Pb) is one of the heavy metals which have drawn most attention. Various techniques have been introduced to remediate metal-contaminated waters. One of these techniques is separating metal from waters by using microorganisms (Lesmana et al. 2009; Zheng et al. 2014).
White-rot fungi have been well known for their strong degradation of various xenobiotics (Barr and Aust 1994; Huang et al. 2008), while they have also been investigated with a great interest on the removing of heavy metals from wastewater in recent decades (Jarosz-Wilkołazka et al. 2006; Kahraman et al. 2005; Say et al. 2001; Xu et al. 2012). In those pieces of research, mature fungal mycelium was utilized originally or pretreated with chemical or as the base of biomaterials. In this study, growing cells of Phanerochaete chrysosporium were used for the bioremoval of Pb2+. The actual wastewater, such as municipal sewage, paper waste, etc., always contains not only heavy metals but also various organic matters, which provide a condition for the fungal growth and a possibility for the practical application of growing cells in the treatment of metal-polluted wastewater.
The focus of many studies is on the removal efficiency of heavy metals (Gomes et al. 2014; Wang and Chen 2009). To obtain an effective removal, biomass concentration, time, pH, and sometimes the type of biosorbents were considered. Among all the factors, pH is an important control factor which was always optimized with chemicals. The use of additional chemicals would increase the cost of the operation. The mechanisms for metal bioremoval have also been discussed. It has been reported that binding of heavy metal to fungal mycelia surfaces is the most important way (Baldrian 2003; Xu et al. 2013). The contribution of cell-wall binding, also called biosorption, to metal removal in white-rot fungi has been extensively reported (Gadd 1993; Kahraman et al. 2005). Even with many efforts in those pieces of research, the stability of the bioremoved Pb species has little been taken into account. At the end of the biotreatments, the used biomass would be separated from waters for further disposal. And, at that stage, the less stable and exchangeable Pb adsorbed on the fungal surfaces would be liable to cause secondary dissolution. To supply more detailed information for the biotreatment of heavy metal pollution, studies on the stability of Pb species during the Pb removal process are necessary.
First of all, suitable extracting methods could help to differentiate the exchangeable Pb species. Since acidic solutions could cause deterioration of fungal physicochemical properties, use of chelants is considered to be biologically less harmful. Ethylenediaminetetraacetic acid (EDTA) is one of the most wildly applied chelating reagents, and it is reliable to extract entirely the bioavailable pool of toxic metals (Kim et al. 2003; Lestan and Udovic 2011; Lo and Yang 1999). In this study, EDTA extraction together with atomic absorption spectroscopy (AAS) was utilized to provide quantitative information of Pb species. Environment scanning electron microscopy coupled with energy-dispersive X-ray analysis (ESEM-EDAX) and X-ray powder diffraction (XRD) analysis were well suited to identify the composition and type of Pb species. The present work is expected to supply more information on the mode of Pb removal by white-rot fungi, further promoting the safe disposal of the used biomass and the application of white-rot fungi in the treatment of heavy metal pollution.
Materials and methods
Preparation of inocula and culture condition
The fungus strain P. chrysosporium BKMF-1767 (CCTCC AF96007) was obtained from China Center for type Culture Collection (Wuhan, China). It was maintained on potato dextrose agar (PDA) slants at 4 °C and then transferred to PDA plates at 37 °C for several days. Inocula consisted of spore suspension which was prepared by scraping the spores on the agar surface and then diluting them in sterile distilled water. Spore concentration was measured and adjusted to 2.0 × 106 CFU mL−1. Aliquots (2 mL) of such spore suspension were inoculated into 200 mL of sterile potato dextrose broth (PDB) in 500-mL flasks, at 150 rpm and 30 °C.
Pb2+ removal experiments
The pure culture without Pb addition was terminated at 41 h, which was found to correspond to the accelerated phase of the culture in the preliminary experiment, so that the influence of Pb2+ on spore germination was diminished as far as possible. Then, Pb2+ was added in the form of Pb(NO3)2 to the 41-h-old cultures to concentrations of 50, 100, 200, and 400 mg L−1, which corresponded to group B (50), C (100), D (200), and E (400), respectively. For comparison, control flasks without Pb2+ (group A (0)) and flasks without inoculation (blank group) were also prepared under the same conditions. The Pb2+ exposure period was terminated at the end of the stationary phase of growth at 205 h, in the case of the more easy penetration of Pb into cells or the metal chelation by intracellular substances released from the fungal hyphal autolysis in the decline phase of growth. All experiments were performed in triplicates, and mean values were used in the analysis.
EDTA extraction of bioremoved Pb by P. chrysosporium
After Pb2+ addition, all the fungal mycelia in each treatment were harvested by filtration at the given time, rinsed twice with 200 mL of cold deionized water, and blotted dry between layers of filter paper. Mycelia were then resuspended in 200 mL of 5 mM EDTA (pH 5.35) to remove superficially bound metals (Jarosz-Wilkołazka et al. 2002, 2006). Afterward, the EDTA extract was separated from fungal mycelia and acidified with 3% (v/v) HNO3 for the estimation of Pb concentration, which was determined with AAS (Agilent 3510, USA). The instrument was calibrated with Pb2+ standard solutions. Pb concentration in the culture medium was determined with the same method. Finally, the EDTA-extracted mycelia were washed with sterile water, freeze-dried, and weighed.
ESEM-EDAX analysis
The freeze-dried fungal mycelia before and after EDTA extraction were used for ESEM-EDAX analysis. The fungal mycelia were mounted on aluminum stubs using carbon adhesive tape and sputter-coated with Au and then examined using a Fei Quanta 200 ESEM operating at an accelerating voltage of 20 kV. The ESEM examination gave both the secondary electron (SE) imaging and the backscattered electron (BSE) imaging of the mycelial mat. In SE detection mode, the ESEM can produce images with a well-defined, three-dimensional appearance of a sample surface. BSE is used in analytical ESEM along with the spectra made from a Genesis XM-2 EDAX microanalysis system. BSE images can provide information about the distribution of different elements in the sample.
XRD analysis
The freeze-dried mycelium pellets were powdered for XRD analysis. X-ray diffraction data were acquired using an XRD Siemens D500 equipped with Cu K X-ray tube over 20°–50° 2 theta.
Determination of pH in the culture medium
It is well known that pH in the environment has a significant effect on the immobilization of toxic metals (Horsfall and Spiff 2004). To know more details about the stability of Pb species, the determination of pH in the culture medium was needed. The pH of the culture medium obtained at a given time was measured with a Mettler Toledo FE 20 pH meter.
Results
Effect of Pb2+ on fungal growth
As shown in Fig. 1, biomass weight in the control increased rapidly at the early stage of acceleration phase (41 to 61 h), and the increase became slower from 61 to 121 h. It reached the highest level at 164 h, following a tiny decline at 205 h. The biomass weights in groups with Pb2+ addition display similar variations. It is obvious that the least biomass was detected in group E (400), while the biomass weights in other groups were next to that in group A (0). Those data suggest that the growth of P. chrysosporium was partially affected in the presence of less than 400 mg L−1 of Pb ions and was inhibited in the medium containing 400 mg L−1 of Pb2+.
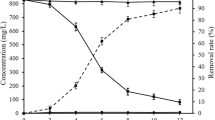
Changes of Pb concentration in the medium and the EDTA extractable rate of removed Pb
Figure 2a represents the time course profile of the bioremoval process of Pb2+ by P. chrysosporium. Pb concentrations in all the groups decreased rapidly from 41 to 61 h (i.e., the early stage of the acceleration phase); then, the decrease slowed down a little from 61 to 121 h (i.e., the latter stage of the acceleration phase). The following Pb contents detected from 121 to 205 h (i.e., the stationary phase) were kept at a stable level in all the groups except group E (400) in which that was still decreasing slowly. The continuous decrease of Pb concentration implies a successive removal of Pb by growing P. chrysosporium.
As shown in Fig. 2b, the EDTA extraction rates in the four groups display similar variations, decreasing from 41 to 121 h and then keeping at a stable level. The decrease of EDTA extraction rate is evidence for the stabilization of Pb species by P. chrysosporium. In the groups except B (50), the extraction rate from 42 to 49 h decreased apparently from up to 85% to less than 60%, and the decrease of extraction rate became slower after 49 h. Those data probably indicate that the presence of more than 50 mg L−1 of Pb2+ may arouse a stronger biological defense system, and the 7 h may be the fungal adaptive phase to higher level of toxic Pb ions. The least extractable ratio of removed Pb was found after 121 h, implying that the bioremoved Pb by P. chrysosporium was most stable and less environmentally harmful at that time.
ESEM-EDAX analysis of the fungal mycelium before EDTA extraction
Morphological observations revealed that the filamentous fungi were always in the form of pellets, with the shape somewhat spherical and bead like. From the SE imaging, it is shown that the mycelia intertwine (Fig. 3a) and form a matrix in the pellets which is with high surface/volume ratio and good mechanical properties for the successful Pb removal. The BSE image of fungal mycelium obtained at 121 h shows that small granular particles distribute on the surface of the fungal mycelium; larger granular particles accumulate around the mycelium and are entrapped in the mycelium matrix (Fig. 3b, c). Since heavy elements (high atomic number) backscatter electrons more strongly than light elements (low atomic number) in the BSE detecting mode, those bright particles in the images probably imply the distribution of heavy elements. The larger particles are likely to be the accumulations of heavy elements, such as heavy metal precipitates. The speculation is verified in the EDAX analysis which demonstrated that the granular particles on the surface of fungal hyphae were rich in lead, carbon, oxygen, phosphorus, and chlorine (Fig. 3d), and the particles entrapped in the fungal mycelium matrix also had Pb-rich composition (Fig. 3e).
ESEM-EDAX analysis of the fungal mycelium after EDTA extraction
Compared with the BSE images of the mycelium without EDTA extraction, the density of light spots in the BSE images of the EDTA-extracted mycelium reduces, especially on the cell wall surface, even when the latter images are displayed in a lower magnification (Figs. 3 and 4). Less light spots in the BSE imaging probably indicate the decrease of heavy elements, such as Pb. With EDAX analysis, it is displayed that there was still Pb adsorbed on the surface of fungal mycelium (Fig. 4d) or entrapped in the fungal mycelium matrix (Fig. 4e). The element composition of the granular particles was similar with that in the mycelium without EDTA extraction, while less Pb was detected in the EDTA-extracted mycelium (Figs. 3 and 4), especially on the surface of the mycelium.
The secondary electron (SE) imaging (a) and the backscattered electron (BSE) imaging (b and c) of the EDTA-extracted fungal mycelium at 121 h, coupled with EDAX spectrum indicating the elemental composition of the small granular particle (d) and the larger granular particle (e) observed in the ESEM image
Identification of the type of stabilized Pb species
Further studies were carried out using XRD, an effective method to investigate the microstructure of crystalline or amorphous materials. The powder patterns of the EDTA-extracted mycelium matched the reference patterns for lead oxalate (ICDD, PDF 14-0803) and the similar pattern of pyromorphite (19-0701) (Fig. 5). The powder patterns give broader peaks, probably indicating that the sizes of Pb species are in the nanometer range and not well crystalline (Chisholm et al. 1987). The amount of EDTA-inaccessible Pb species increased during the previous 20-h contact period between the biomass and Pb2+ (Fig. S1), which excludes their origination from simple chemical reactions in the culture medium.
Changes of pH in the medium during the Pb removal process by P. chrysosporium
Figure 6 shows that the changes of pH in all the groups are within the range of 4–6.5. The different pH values in the medium at 41 h were brought about by the addition of different Pb2+ amounts. There was nearly no distinction for the pH value in the control at 41 h and that at 42 h, whereas the decline of pH in the groups with Pb2+ addition appeared in the first hour. During the first 20-h exposure of P. chrysosporium to Pb2+, pH in the medium descended continually. With more Pb2+ existing, the downward trend was more obvious. At 61 h, pH began to increase and the upward trend lasted until the end of the detection.
Discussion
Effect of Pb2+ on fungal growth
In the biotreatment of heavy metal pollution, biomass weight is an important factor influencing the efficiency and the cost of the operation process. In this study, initial biomass was around 0.4 g L−1 when the growing cells of P. chrysosporium were first exposed to Pb2+. Until the end of the experiment, the biomass in the groups with less than 400 mg L−1 of Pb2+ was around 1.5 g L−1, and that in group E (400) was around 1.2 g L−1. It is demonstrated that the biomass was guaranteed during the exposure period to Pb2+, implying the feasibility of growing cells of P. chrysosporium in the biotreatment of Pb2+ pollution. The fungal growth inhibition is a symptom of Pb2+ toxicity, so it is easy to expect that an elevated Pb2+ concentration resulted in less biomass. Nevertheless, it is interesting to find that the fungal growth cycles in all the groups were affected little. The reason probably is that because of the efficient defense mechanisms in P. chrysosporium, Pb2+ did not cause serious damage to the related DNA.
Changes of bioremoval of Pb and the EDTA extractable rate of removed Pb
The removal of Pb by P. chrysosporium could be accomplished by extracellular and intracellular compounds that are active in complexing and binding the metal (Baldrian 2003). The production of those functional compounds is dependent on fungal metabolism. The different metabolism speeds of the growing cells in different growth phases led maybe to the various Pb removal rates, so that the highest removal speed presented in the early stage of the acceleration phase, with a lower removal speed in the latter stage of the acceleration phase and almost no removal of Pb in the stationary phase.
At 42 h, the Pb concentrations and the extraction rates both decreased, suggesting that Pb began to be bioremoved and stabilized at the first time when the fungus encountered Pb2+. In other words, the removal of Pb (Fig. 2a) and the stabilization of Pb (Fig. 2b) were initiated nearly simultaneously. The EDTA extraction efficiency in this study was different from that reported in previous studies (Jarosz-Wilkołazka et al. 2002, 2006), which may be due to that the metal exposure period in this study was at the fungal growing phase, different from previous stationary phase. The most predominant mechanism for the surface of the fungal cells in the stationary phase lies maybe in the ion exchange process (Say et al. 2001). During the fungal growing period, the metabolism-dependent accumulation probably plays a more important role in the metal removal process. The fungus modified its local microenvironment by creating conditions such as extracellular chemical precipitation of mineral phases, which was with very low solubility and inaccessible to EDTA (Kim et al. 2003; Lo and Yang 1999).
The above observations demonstrated that the growing P. chrysosporium was efficient for the removal and the stabilization of Pb. Considering the fungal growth stage, Pb removal rate, time, and the stability of removed Pb, it is quite a suitable time to separate the used biomass from the Pb-polluted medium for further disposal at 121 h (Fig. 2).
ESEM-EDAX analysis of the fungal mycelium before and after EDTA extraction
The results of ESEM analysis probably illustrate the way for P. chrysosporium to immobilize heavy metals (Figs. 3 and 4). On the one hand, metal ions were immobilized by P. chrysosporium with its mycelial cell wall which consists mostly of polysaccharides, peptides, and pigments with good capacity to bind heavy metals (Huang et al. 2008). As for the insoluble metal precipitates, fungi immobilized it with its mucilaginous extracellular hyphal sheath which is made mostly of polysaccharides. Crystals were reported to be found in the fungal extracellular mucilaginous hyphal sheath (Connolly and Jellison 1995). From the comparison of ESEM analysis of mycelium before and after EDTA extraction (Figs. 3 and 4), it appeared that the Pb ions immobilized with fungal cell wall were more likely to be extracted than the Pb species entrapped in the mycelium matrix.
Identification of the type of stabilized Pb species
During the bioremoval process of Pb ions by growing P. chrysosporium, part of removed Pb ions were stabilized as lead oxalate and pyromorphite (Fig. 5). The formation of those Pb species probably resulted from the biologically induced mineralization (Gadd 2010). When P. chrysosporium was exposed to Pb2+, oxalic acid was secreted and led to the formation of lead oxalate so that active Pb2+ concentrations were reduced (Baldrian 2003; Li et al. 2011). The source of phosphate for the formation of pyromorphite may be derived from the unspecific oxidation of proteins and membrane lipids and DNA injury, which was caused by the reactive oxygen species induced by the stress of Pb ions (Kim et al. 2008). Both the two kinds of Pb species are insoluble and stable, especially pyromorphite, which is geochemically stable over a wide range of pH, ionic strength, and temperature (Nriagu 1974; Rhee Young et al. 2012.
Changes of pH in the medium during the Pb removal process by P. chrysosporium
The decrease of pH (Fig. 6) was prone to dissolve metal, while Pb removal in the medium did not slow down but proceeded successfully (Fig. 2a) and so did the stabilization of Pb species (Fig. 2b). The seemingly contradictory phenomenon could be explained by the secretion of organic acids. These observations suggest that the bioremoval and stabilization of Pb by growing P. chrysosporium is a comprehensive process in which pH is not a strict limiting factor within the pH range from 4 to 6.
In conclusion, this study directly demonstrated that the growing cells of P. chrysosporium were efficient for the stabilization of Pb species. Companioned with a continuous bioremoval of Pb from the medium, the rate of extractable Pb to bioremoved Pb decreased constantly and kept at a stable level around 20% after 121 h. With ESEM-EDAX and XRD analysis of fungal hyphae, the remaining Pb species after EDTA extraction were identified to be oxalate and pyromorphite. Further, it is found that the stabilization of Pb by growing P. chrysosporium was not strictly limited in the aspect of pH when pH in the environment was in the range of 4–6. The present findings are expected to supply more information on the mode of Pb removal by white-rot fungi, providing references for the safe disposal of the used biomass and promoting the practical application of white-rot fungi in the treatment of heavy metal pollution.
References
Baldrian P (2003) Interactions of heavy metals with white-rot fungi. Enzyme Microb Technol 32:78–91
Barr DP, Aust SD (1994) Mechanisms white rot fungi use to degrade pollutants. Environ Sci Technol 28:78A–87A
Chisholm JE, Jones GC, Purvis OW (1987) Hydrated copper oxalate, moolooite, in lichens. Mineral Mag 51:715–718
Connolly JH, Jellison J (1995) Calcium translocation, calcium oxalate accumulation, and hyphal sheath morphology in the white-rot fungus Resinicium bicolor. Can J Bot 73:927–936
Gadd GM (1993) Interactions of fungi with toxic metals. New Phytol 124:25–60
Gadd GM (2010) Metals, minerals and microbes: geomicrobiology and bioremediation. Microbiology 156:609–643
Gomes P, Lennartsson P, Persson NK, Taherzadeh M (2014) Heavy metal biosorption by Rhizopus sp. biomass immobilized on textiles. Water Air Soil Pollut 225:1–10
Hashim MA, Mukhopadhyay S, Sahu JN, Sengupta B (2011) Remediation technologies for heavy metal contaminated groundwater. J Environ Manage 92:2355–2388
Horsfall MJ, Spiff AI (2004) Studies on the effect of pH on the sorption of Pb2+ and Cd2+ ions from aqueous solutions by Caladium bicolor (wild cocoyam) biomass. Electron J Biotechnol 7:313–323
Huang DL, Zeng GM, Feng CL, Hu S, Jiang XY, Tang L, Su FF, Zhang Y, Zeng W, Liu HL (2008) Degradation of lead-contaminated lignocellulosic waste by Phanerochaete chrysosporium and the reduction of lead toxicity. Environ Sci Technol 42:4946–4951
Jarosz-Wilkołazka A, Malarczyk E, Pirszel J, Skowroński T, Leonowicz A (2002) Uptake of cadmium ions in white-rot fungus Trametes versicolor: effect of Cd(II) ions on the activity of laccase. Cell Biol Int 26:605–613
Jarosz-Wilkołazka A, Grąz M, Braha B, Menge S, Schlosser D, Krauss G-J (2006) Species-specific Cd-stress response in the white rot basidiomycetes Abortiporus biennis and Cerrena unicolor. Biometals 19:39–49
Kahraman S, Erdemoglu S, Yesilada O (2005) Biosorption of copper (II) by live and dried biomass of the white rot fungi Phanerochaete chrysosporium and Funalia trogii. Eng Life Sci 5:72–77
Kim C, Lee Y, Ong SK (2003) Factors affecting EDTA extraction of lead from lead-contaminated soils. Chemosphere 51:845–853
Kim SJ, Jeong HJ, Myung NY, Kim M, Lee JH, So H, Park RK, Kim HM, Um JY, Hong SH (2008) The protective mechanism of antioxidants in cadmium-induced ototoxicity in vitro and in vivo. Environ Health Perspect 116:854–862
Lesmana SO, Febriana N, Soetaredjo FE, Sunarso J, Ismadji S (2009) Studies on potential applications of biomass for the separation of heavy metals from water and wastewater. Biochem Eng J 44:19–41
Lestan D, Udovic M (2011) Mobility and availability of toxic metals after soil washing with chelating agents. In: Khan MS, Zaidi A, Goel R, Goel R, Musarrat J (eds) Biomanagement of metal-contaminated soils. 20. Springer, Netherlands, pp 343–364
Li NJ, Zeng GM, Huang DL, Hu S, Feng CL, Zhao MH, Lai C, Huang C, Wei Z, Xie GX (2011) Oxalate production at different initial Pb2+ concentrations and the influence of oxalate during solid-state fermentation of straw with Phanerochaete chrysosporium. Bioresour Technol 102:8137–8142
Lo IC, Yang XY (1999) EDTA extraction of heavy metals from different soil fractions and synthetic soils. Water Air Soil Pollut 109:219–236
Nriagu JO (1974) Lead orthophosphates-IV. Formation and stability of chloropyromorphite at 25 °C. Geochim Cosmochim Acta 37:367–377
Rhee Young J, Hillier S, Gadd Geoffrey M (2012) Lead transformation to pyromorphite by fungi. Curr Biol 22:237–241
Say R, Denizli A, Yakup Arıca M (2001) Biosorption of cadmium (II), lead(II) and copper(II) with the filamentous fungus Phanerochaete chrysosporium. Bioresour Technol 76:67–70
Wang JL, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27:195–226
Xu P, Zeng GM, Huang DL, Lai C, Zhao MH, Wei Z, Li NJ, Huang C, Xie GX (2012) Adsorption of Pb (II) by iron oxide nanoparticles immobilized Phanerochaete chrysosporium: equilibrium, kinetic, thermodynamic and mechanisms analysis. Chem Eng J 203:423–431
Xu P, Zeng GM, Huang DL, Hu S, Feng CL, Lai C, Zhao MH, Huang C, Li NJ, Wei Z (2013) Synthesis of iron oxide nanoparticles and their application in Phanerochaete chrysosporium immobilization for Pb(II) removal. Colloid Surface A 419:147–155
Zheng S, Huang H, Zhang R, Cao L (2014) Removal of Cr(VI) from aqueous solutions by fruiting bodies of the jelly fungus (Auricularia polytricha). Appl Microbiol Biotechnol 98:8729–8736
Acknowledgments
The study was financially supported by the National Natural Science Foundation of China (51039001, 51278176, 51378190, and 51408206), the Environmental Protection Technology Research Program of Hunan (2007185), the Research Fund for the Doctoral Program of Higher Education of China (20100161110012), New Century Excellent Talents in University (NECT-13-0186), the Young Teacher Growth Program of Hunan University, Scientific Research Fund of Hunan Provincial Education Department (521293050), the Fundamental Research Funds for the Central Universities, Hunan University Fund for Multidisciplinary Developing (531107040762), and the Hunan Provincial Innovation Foundation for Postgraduate (CX2014B141).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 163 kb)
Rights and permissions
About this article
Cite this article
Zeng, G., Li, N., Huang, D. et al. The stability of Pb species during the Pb removal process by growing cells of Phanerochaete chrysosporium . Appl Microbiol Biotechnol 99, 3685–3693 (2015). https://doi.org/10.1007/s00253-014-6275-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6275-5