Abstract
In this study, the impact of the hydrogen partial pressure on lactate degradation was investigated in a coculture of Desulfovibrio sp. G11 and Methanobrevibacter arboriphilus DH1. To impose a change of the hydrogen partial pressure, formate was added to the reactor. Hydrogen results from the bioconversion of formate besides lactate in the liquid phase. In the presence of a hydrogen-consuming methanogen, this approach allows for a better estimation of low dissolved hydrogen concentrations than under conditions where hydrogen is supplied externally from the gas phase, resulting in a more accurate determination of kinetic parameters. A change of the hydrogen partial pressure from 1,200 to 250 ppm resulted in a threefold increase of the biomass-specific lactate consumption rate. The 50 % inhibition constant of hydrogen on lactate degradation was determined as 0.692 ± 0.064 μM dissolved hydrogen (831 ± 77 ppm hydrogen in the gas phase). Moreover, for the first time, the maximum biomass-specific lactate consumption rate of Desulfovibrio sp. G11 (0.083 ± 0.006 mol-Lac/mol-XG11/h) and the affinity constant for hydrogen uptake of Methanobrevibacter arboriphilus DH1 (0.601 ± 0.022 μM dissolved hydrogen) were determined. Contrary to the widely established view that the biomass-specific growth rate of a methanogenic coculture is determined by the hydrogen-utilizing partner; here, it was found that the hydrogen-producing bacterium determined the biomass-specific growth rate of the coculture grown on lactate and formate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anaerobic methanogenic digestion processes proceed close to thermodynamic equilibrium (Kleerebezem and Stams 2000; McCarty and Bae 2011; Rodriguez et al. 2006; Schink 1997). One example is syntrophic lactate conversion, in which lactate is converted to acetate, methane, and carbon dioxide. During lactate degradation, 1 mol of lactate can be converted into 1 mol of acetate, 1 mol of bicarbonate, and 2 mol of hydrogen. At high hydrogen partial pressures, lactate degradation becomes thermodynamically unfavorable. However, hydrogen and bicarbonate can be consumed by hydrogenotrophic methanogens, which renders lactate degradation thermodynamically feasible. The syntrophic interactions of the coculture used in this study are shown in Fig. 1.
Experimental evidence for the impact of the hydrogen partial pressure on syntrophic lactate conversion is limited. In particular, no hydrogen inhibition constant has been reported for lactate degradation mediated by Desulfovibrio sp. G11. Pankhania et al. (1988) reported 50 % inhibition of lactate degradation at a hydrogen partial pressure of 2 % (K iH2, Lacox) in a pure culture of Desulfovibrio vulgaris strain Marburg (37 °C, pH 7.2). Willquist et al. (2012) suggested, based on thermodynamic considerations, a higher theoretical K iH2,Lacox of 10 %.
External supply of hydrogen to the reactor headspace suffers from mass transfer limitation towards the liquid phase, especially in view of the low solubility of hydrogen in water. The hydrogen concentration in the reactor liquid may thus be significantly overestimated when hydrogen is simultaneously consumed, resulting in an overestimated K iH2,Lacox. For a more accurate determination of kinetic parameters, mass transfer limitation should be quantified adequately. In the present study, the bioconversion of formate, besides lactate, served as an additional supply of hydrogen in the liquid phase. This allows to impose a change of the hydrogen concentration in the liquid. Moreover, troublesome estimation of the hydrogen concentration in the liquid is avoided as hydrogen did not need to be transferred from the gas to the liquid.
Dolfing et al. (2008) reported syntrophic formate degradation in a coculture of Desulfovibrio sp. G11 and the non-formate utilizing Methanobrevibacter arboriphilus AZ. During syntrophic formate degradation, Desulfovibrio sp. G11 converts 1 mol of formate to 1 mol of hydrogen and 1 mol of bicarbonate, followed by product removal mediated by hydrogenotrophic methanogens.
Here, the impact of the hydrogen partial pressure on syntrophic lactate conversion was studied in a coculture of Desulfovibrio sp. G11 and the non-formate utilizing Methanobrevibacter arboriphilus DH1 (Zeikus and Henning 1975). The proposed method of formate addition was successfully applied in this study to determine several kinetic parameters including K iH2,Lacox, the maximum biomass-specific lactate consumption rate of Desulfovibrio sp. G11 (q Lac,max), and the affinity constant for hydrogen uptake of Methanobrevibacter arboriphilus DH1 (K S,H2). These parameters have not been reported so far.
Material and methods
Precultivation
Pure cultures of Desulfovibrio sp. strain G11 (DSM 7057) and Methanobrevibacter arboriphilus-type strain DH1 (DSM 1125) were obtained from the Laboratory of Microbiology, Wageningen University, The Netherlands, and cultivated in 2 L Schott bottles under sterile anaerobic conditions. The composition of the culture medium and the cultivation conditions for Desulfovibrio sp. G11 are described elsewhere (Junicke et al. 2014). Methanobrevibacter arboriphilus DH1 was cultivated under the same conditions as previously described for Methanospirillum hungatei-type strain JF1 (DSM 864) (Junicke et al. 2014).
The coculture was constructed from pure cultures of Desulfovibrio sp. strain G11 and Methanobrevibacter arboriphilus DH1, both taken from the late exponential phase. The optical density was measured at 660 nm (DR 2800, Hach-Lange, Tiel, The Netherlands) ranging from 0.150 and 0.250 for both pure cultures. The coculture medium was prepared according to Plugge (2005). Additionally, 20 mM sodium lactate and 4.0 mM cysteine hydrochloride were added after autoclaving of the basic medium from sterile stock solutions. The coculture was kept under 80 % N2/20 % CO2 atmosphere, while constantly shaking at 150 rpm. A pH between 7.0 and 7.2, and a temperature of 37 °C were maintained.
Experimental setup
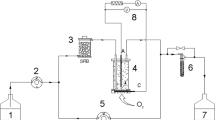
A double-jacket bioreactor (Applikon, Schiedam, The Netherlands) with a working volume of 2 L was used to perform the batch experiment. The bioreactor was filled with basic medium and autoclaved at 121 °C for 30 min. The basic medium was composed of 2.0 mM NH4Cl, 1.0 mM KH2PO4, 0.49 mM MgCl2, 0.68 mM CaCl2, 0.10 mM cysteine hydrochloride, 0.010 g/L yeast extract, 2.2 μM resazurin, and 1.0 mL/L of the acidic and alkaline trace element stock solution, respectively. The acidic and alkaline stock solutions were prepared according to Plugge (2005). After autoclaving, the basic medium was supplemented with the following additives from sterile anaerobic stock solutions: 20 mM sodium lactate, 20 mM sodium formate, 0.20 mM Na2S, and 0.83 mL/L of the vitamin stock solution. The vitamin stock solution was prepared according to Plugge (2005), except for 0.61 g/L pyridoxine hydrochloride instead of 0.50 g/L pyridoxamine. Moreover, the acid and base solutions used for pH control were autoclaved at 121 °C for 30 min. To maintain sterile conditions, a PTFE membrane filter with a pore size of 0.2 μm (Millex-FG50 filter unit, Millipore) and an off-gas filter system consisting of a 50-mL syringe (BD PlastipakTM, BD Drogheda, Ireland) filled with cotton were placed at the gas inlet and at the gas outlet of the reactor, respectively. A sterile sampling system (Applikon) was used and the purity of the coculture was verified by microscopic investigation in course of the experiment.
To maintain anaerobic conditions, the reactor liquid was continuously sparged with nitrogen gas (0.050 LN/min). Traces of oxygen were eliminated by sparging through a 200-mM dithionite solution. A H2S trapping zinc acetate solution was installed after the off-gas measuring equipment.
A temperature of 37 °C was maintained and the pH was kept at 7.1 ± 0.1 by dosing 0.5 M NaOH and 0.5 M HCl. The off-gas temperature was kept at 4 °C using off-gas cooler and cryostat. The temperature, acid/base pumps, stirring speed (400 rpm), pH, and the inflow of the nitrogen gas were controlled via the Biostat B plus biocontroller (Sartorius Systems, Bohemia, NY) and data were recorded by the MFCS/win software program.
The coculture of Methanobrevibacter arboriphilus DH1 and Desulfovibrio sp. G11 served as inoculum. Prior to inoculation, the coculture was transferred several times to establish a fixed ratio between the two species. To avoid a lag phase at the start of the batch experiment, a volume of 600 mL of the coculture was taken from its exponential phase (OD660 = 0.133). The total biomass concentration amounted to 1.691 mM at the start of the batch experiment.
Analytical measurements
The partial pressures of hydrogen, methane, and carbon dioxide in the reactor off-gas were measured continuously via the online Agilent 490 micro gas chromatograph (molsieve channel for H2; PPQ channel for CH4 and CO2; thermal conductivity detector; Argon 5.0 as carrier gas). Liquid samples were taken from the reactor for analysis by high-performance liquid chromatography (HPLC with Animex HPX-87H column from Bio-Rad) after filtration through a 0.45-mm pore size filter (Millex-HV filter, Durapore PVDF membrane).
Carbon and electron balances
At each given liquid sampling point, the carbon and electron balance were determined. The total carbon amount (C-mol) was obtained from the amount of all measured compounds multiplied by the number of carbon atoms per compound. The total electron amount (e-mol) was obtained from the amount of all measured compounds multiplied by the respective degree of reduction (e-mol/mol-compound). The carbon gap in percent was obtained as the difference between the actual total amount of carbon and the initial total amount of carbon, divided by the initial total amount of carbon. The electron gap was determined accordingly.
Stoichiometric yields
The stoichiometric yields of the metabolic reactions considered in this study were estimated according to the Gibbs energy dissipation method proposed by Kleerebezem and Van Loosdrecht (2010). Whereas formate and lactate served as carbon and energy sources for Desulfovibrio sp. G11, carbon dioxide and hydrogen served as carbon and energy source for Methanobrevibacter arboriphilus DH1.
Mass balances
Net gas production rates were derived from continuous off-gas measurements and expressed in millimole per hour. The gas outflow rate was calculated from the nitrogen gas inflow rate, corrected for the mole fractions of all the gases produced. Multiplication of the gas outflow rate and the mole fraction of the respective gas yields the net production rate of the desired gas. Cumulative gas amounts were obtained by integration of the net production rates.
The amounts of lactate, formate, and acetate; the biomass amount of Desulfovibrio sp. G11; and the biomass amount of Methanobrevibacter arboriphilus DH1 were derived from the gas rates and the estimated stoichiometric yields. Here, it was used that
where R H2,produced is the hydrogen production rate, R H2,consumed is the hydrogen consumption rate, and R H2,measured is the measured hydrogen net production rate. The hydrogen consumption rate is equal to the methane production rate (R CH4) divided by the stoichiometric yield of methane on hydrogen for hydrogenotrophic methanogenesis (Y CH4/H2,Hym). For the derivation of the lactate and formate amounts from off-gas data, the measured ratio of produced hydrogen and carbon dioxide was used to attribute the amounts of hydrogen produced to either lactate or formate.
Gas–liquid mass transfer
The gas–liquid transfer of hydrogen, methane, and carbon dioxide was calculated using standard mass transfer theory according to
where MTR is the mass transfer rate (mM/h), k La the mass transfer coefficient (1/h), c the actual gas concentration in the liquid phase, and c* the gas solubility in the liquid. Gas solubility was derived from the partial pressure of either hydrogen, methane, or carbon dioxide in the off-gas and the respective Henry coefficient at 37 °C. The k La was estimated from k La measurements for oxygen at 37 °C and 400 rpm stirring speed, subsequently corrected for the different diffusion coefficients (Cussler 1997; de Kok et al. 2013).
Under pseudo steady-state conditions, MTR equals the net production rate of the respective gas, which allows to derive c/c *, the saturation of each gas in the liquid. All gas concentrations in the liquid were corrected for the effect of oversaturation by multiplication with the derived saturation factor.
Biomass-specific conversion rates
The biomass-specific conversion rates resulted from the off-gas-derived rates divided by the biomass amount of the individual species. At any moment in time, the biomass amount of each species was calculated from the individual biomass amount at the beginning of the experiment and the biomass increase based on off-gas-derived rates and estimated yields. Initial biomass concentrations were calculated from the initial total biomass concentration and the theoretical biomass fractions according to estimated biomass yields. The total biomass concentration at the start of the experiment was obtained from optical density measurement at 660 nm (OD660, triplicates) and a correlation between the biomass concentration and the OD660 of the coculture. To determine the correlation factor, volatile suspended solids (VSS) and the optical density of the coculture were measured in triplicate. The measurement of VSS is described elsewhere (Junicke et al. 2014). The resulting VSS/OD660 correlation factor amounted to 0.522 ± 0.007 g-VSS/L per absorption unit.
Determination of K iH2, Lacox
The inhibition constant K i of a certain reaction is defined as the inhibitory concentration c i at which the reaction is inhibited by 50 %. The non-competitive inhibition constant of hydrogen on lactate degradation (K iH2,Lacox) was determined using the mean hydrogen concentrations in the liquid and the biomass-specific lactate consumption rates (q Lac) of phases 1 and 3, respectively, according to
where q S is the biomass-specific substrate conversion rate, q S,max the maximum biomass-specific substrate conversion rate, K S the affinity constant for substrate uptake, and c S the substrate concentration. Due to the presence of excess lactate, the hyperbolic term including the affinity constant was neglected.
Determination of K S,H2
The K S,H2 of Methanobrevibacter arboriphilus DH1 was first approximated by applying the Michaelis-Menten equation to two different R CH4 and the respective two hydrogen partial pressures. Due to the proximity of the chosen R CH4 values, the biomass increase of Methanobrevibacter arboriphilus DH1 is negligible in this period of time. The maximum biomass-specific methane production rate (q CH4,max) was obtained by dividing the model-based q CH4,max in this regime by the hyperbolic K S,H2 term considering the actual hydrogen partial pressure. The Michaelis-Menten equation was then fitted to the data series in a least-squares sense using the Levenberg-Marquardt algorithm and K S,H2 as a free parameter.
Thermodynamic calculations
It was evaluated whether the partial reactions shown in Table 1 are energetically favorable under the prevailing experimental conditions using the estimated stoichiometric yields. The actual Gibbs energy change, ∆G1, was calculated by means of the measured data according to
∆G 01 denotes the Gibbs energy change at pH 7.0 and 310.15 K. Y i is the stoichiometric coefficient of compound i and a i is the respective activity. The activity was assumed to be equal to the concentration of the ith component. R is the gas constant amounting to 8.314 J/K/mol and T is the temperature expressed in Kelvin.
The reaction stoichiometry of the NADH-dependent hydrogen formation is as follows:
The NADH/NAD+ ratio was calculated at thermodynamic equilibrium (ΔG 1 = 0), using the hydrogen partial pressure during the first two phases of the batch experiment (1,204 ± 31 ppm corrected for 1.8-fold oversaturation of hydrogen in the liquid), ΔG 01 = +18.1 kJ/mol NADH, pH 7.0, and 298.15 K.
Results
In a batch experiment, a coculture of Desulfovibrio sp. G11 and Methanobrevibacter arboriphilus DH1 was grown on 20 mM lactate and 20 mM formate. Desulfovibrio sp. G11 degrades both lactate and formate, whereas Methanobrevibacter arboriphilus DH1 is a hydrogenotrophic methanogen which can utilize hydrogen and carbon dioxide for methane production and growth. The metabolic reactions considered in this study were estimated according to the Gibbs energy dissipation method (Kleerebezem and Van Loosdrecht 2010) and are shown in Table 1.
Figure 2 shows the net production rates of hydrogen, methane, and carbon dioxide obtained from continuous off-gas measurements. The amounts of lactate, acetate, and formate in the liquid phase of the reactor were measured by HPLC (Fig. 3a, symbols) and derived from gas production rates using reaction stoichiometry (Fig. 3a, lines). Individual biomass amounts were derived from gas production rates using reaction stoichiometry (Fig. 3b). Carbon and electron balances showed a gap of less than 1 %, indicating that compounds in the balance were identified and accurately measured. The model-derived amounts of lactate, formate, and acetate describe the measured HPLC data within an error range of less than 3 %.
Net production rates of carbon dioxide, hydrogen, and methane during the batch experiment on 20 mM lactate and 20 mM formate using a coculture of Desulfovibrio sp. G11 and Methanobrevibacter arboriphilus DH1. The obtained data set was subdivided into three phases: (1) lactate and formate conversion by Desulfovibrio sp. G11, (2) increase of methane production, and (3) syntrophic lactate conversion after formate depletion
Amount of lactate (Lac), formate (Form), and acetate (Ac) in course of time, for the batch experiment shown in Fig. 2, as obtained from the HPLC measurement (a, symbols) and derived from off-gas measurements using reaction stoichiometry (a, lines). Biomass amounts of Desulfovibrio sp. G11 (X G11 ) and Methanobrevibacter arboriphilus DH1 (X Ma ) were derived from off-gas measurements using reaction stoichiometry (b)
Based on the net hydrogen production, the experiment can be subdivided into three major phases (see Fig. 2). A steep increase of the hydrogen partial pressure was observed during the initial 20 h. At the same time, gas stripping of residual carbon dioxide from the inoculum occurred. The first phase (20–80 h) is marked by a relatively high and constant hydrogen partial pressure (1,204 ± 31 ppm) resulting from both lactate and formate degradation. However, methane production remained low during this phase demonstrating that Desulfovibrio sp. G11 was the main actor. The second phase (80–160 h) shows a strong increase in R CH4 concomitant with a steep decrease of the hydrogen net production rate. This observation, together with the increased consumption rate of lactate and formate, indicates the occurrence of both, syntrophic lactate and syntrophic formate conversion. At the end of the second phase, formate was depleted. During the third phase (160–220 h), solely syntrophic lactate degradation occurred as reflected in a CH4/CO2 ratio equal to one. The hydrogen net production rate was constant and the hydrogen partial pressure amounted to 106 ± 12 ppm. Lactate depletion marks the end of the experiment.
Phase 1 of the batch experiment
In the first phase, the hydrogen partial pressure was constant at 1,204 ± 31 ppm. Carbon dioxide and methane partial pressure were low. The rates of lactate and formate consumption (R Lac and R Form) remained nearly constant and amounted to 0.078 ± 0.007 mmol-Lac/h and 0.112 ± 0.011 mmol-Form/h. According to the yield-based calculation, a higher biomass increase of Desulfovibrio sp. G11 (26.8 %) was obtained compared to Methanobrevibacter arboriphilus DH1 (14.1 %). Figure 4a shows q Lac and the biomass-specific formate consumption rate (q Form) in course of time. During the first phase, both biomass-specific consumption rates were constant and amounted to 0.023 ± 0.001 mol-Lac/mol-XG11/h and 0.033 ± 0.002 mol-Form/mol-XG11/h.
Biomass-specific lactate and formate consumption rates (qLac and qForm) as a function of time (a), for the batch experiment shown in Fig. 2. The biomass-specific growth rate of Desulfovibrio sp. G11 (μG11) and Methanobrevibacter arboriphilus DH1 (μMa) are shown in the bottom panel (b)
Figure 5 shows the interspecies hydrogen transfer efficiency expressed as the amount of hydrogen consumed by Methanobrevibacter arboriphilus DH1 of the total hydrogen produced. It is seen that the interspecies hydrogen transfer efficiency increased in course of the experiment reaching its maximum during the third phase in which solely syntrophic lactate degradation occurred. During the first phase, only 36 % of the total hydrogen produced was consumed by the methanogen. Hydrogen leakage amounted to 64 % of the produced amount, demonstrating that Desulfovibrio sp. G11 was the main actor during the first phase.
Interspecies hydrogen transfer efficiency expressed as the percentage of hydrogen consumed by Methanobrevibacter arboriphilus DH1 of the total hydrogen produced, for the batch experiment shown in Fig. 2
Phase 2 of the batch experiment
In the second phase, the hydrogen partial pressure decreased from 1,204 ± 31 to 106 ± 12 ppm. At the same time, a strong increase of the methane and carbon dioxide partial pressure were observed in the off-gas. Both, methane (3,312 ppm) and carbon dioxide (7,040 ppm) peaked after 157 h followed by a decrease to partial pressures of 1,579 ppm (methane, 160 h) and 1,899 ppm (carbon dioxide, 175 h). Due to the stripping effect, a delay of about 20 h in the decrease of the carbon dioxide partial pressure was observed. At the end of the second phase (160 h), formate was depleted.
Figure 6 shows the dependence of q Lac and q Form on the hydrogen partial pressure. A clear increase of q Lac and q Form was observed with decreasing hydrogen partial pressures ranging from 1,200 to 250 ppm. At the end of the second phase, R CH4 was limited by the decreasing hydrogen partial pressure. Figure 7 shows the dependence of the biomass-specific methane production rate (q CH4) on the dissolved hydrogen concentration in the hydrogen-limiting regime. The K S,H2 of 0.601 ± 0.022 μM dissolved hydrogen (single standard deviation) was obtained by fitting the Michaelis-Menten equation to these data. This corresponds to a hydrogen partial pressure of 721 ± 26 ppm in the gas phase.
Biomass-specific lactate consumption rate (qLac) and biomass-specific formate consumption rate (qForm) as a function of the hydrogen partial pressure during the second phase of the batch experiment shown in Fig. 2
Dependence of q CH4 on the dissolved hydrogen concentration. The K S,H2 of 0.601 ± 0.022 μM dissolved hydrogen (single standard deviation) was obtained by fitting the Michaelis-Menten equation to these data. This corresponds to a hydrogen partial pressure of 721 ± 26 ppm in the gas phase. The upper and lower bound of the 95 % confidence interval represent K S,H2 values of 641 and 561 μM, respectively
The estimated biomass amount of Methanobrevibacter arboriphilus DH1 doubled in course of the second phase, while the biomass amount of Desulfovibrio sp. G11 increased by 66.5 % (Fig. 3b). The biomass-specific growth rate of Desulfovibrio sp. G11 (μ G11) and the biomass-specific growth rate of Methanobrevibacter arboriphilus DH1 (μ Ma) are shown in Fig. 4b. The model-derived apparent μ Ma,max (maximum μ Ma) amounts to 0.021 ± 0.001 1/h. Considering the obtained K S,H2, the actual μ Ma,max equals 0.055 ± 0.005 1/h under hydrogen non-limiting conditions. During the second phase, 83 % of the total hydrogen produced was consumed by the methanogen, i.e., only 17 % hydrogen leakage was observed (Fig. 5).
Phase 3 of the batch experiment
During the third phase of the batch experiment, solely syntrophic lactate degradation occurred which is reflected in the CH4/CO2 ratio equal to one. This phase is marked by a constant hydrogen partial pressure of 106 ± 12 ppm and a constant increase of the methane and carbon dioxide partial pressures. The R Lac and R CH4 increased by 64.1 and 65.5 %, respectively. The biomass amount of Desulfovibrio sp. G11 increased by 64.1 %, whereas the biomass amount of Methanobrevibacter arboriphilus DH1 increased by 59.7 % (Fig. 3b). At the end of the third phase, lactate was depleted.
The interspecies hydrogen transfer efficiency was above 99 % during the last phase of the batch experiment meaning that almost all the hydrogen produced was consumed by the methanogen (Fig. 5). Both biomass-specific growth rates remained constant during the third phase at 0.0098 ± 0.0003 1/h (Desulfovibrio sp. G11) and 0.0092 ± 0.0003 1/h (Methanobrevibacter arboriphilus DH1), respectively (Fig. 4b).
As shown in Fig. 4a, q Lac was constant during the third phase of the batch experiment amounting to 0.069 ± 0.003 mol-Lac/mol-XG11/h. The q Lac of the third phase was equal to the apparent q Lac,max as determined during precultivation (0.066 ± 0.007 mol-Lac/mol-XG11/h). The approximately three times lower q Lac of the first phase indicates an inhibition of lactate degradation during that phase. A 50 % inhibition constant of hydrogen on lactate degradation was determined, yielding a K iH2,Lacox of 0.692 ± 0.064 μM dissolved hydrogen (831 ± 77 ppm in the gas phase). Considering the obtained K iH2,Lacox, the actual q Lac,max is 0.083 ± 0.006 mol-Lac/mol-XG11/h.
Discussion
System description
The high information density of online off-gas composition measurements combined with liquid measurements allowed for a detailed analysis of metabolic fluxes and the thermodynamic system state in the investigated methanogenic consortium. The yield-based model applied in this study described the system accurately. Stoichiometric yields derived by means of the Gibbs energy dissipation method agreed well with reported values. The estimated total biomass yield on lactate (0.198 ± 0.006 mol-X/mol-Lac) is in accordance with the measured total biomass yield on lactate (0.188 ± 0.010 mol-X/mol-Lac). The estimated biomass yield of Methanobrevibacter arboriphilus DH1 on methane amounted to 0.068 ± 0.002 mol-XMa/mol-CH4, which is very close to the values reported for its closest relatives Methanobrevibacter cuticularis RFM1 (0.063 mol-XMa/mol-CH4, 96.9 % gene similarity) and Methanobrevibacter curvatus RFM2 (0.052 mol-XMa/mol-CH4, 95.3 % gene similarity) (Leadbetter and Breznak 1996). The estimated biomass yield of Desulfovibrio sp. G11 on lactate amounted to 0.142 ± 0.004 mol-XG11/mol-Lac. Literature values for the growth yield of Desulfovibrio sp. G11 in a coculture with Methanobrevibacter arboriphilus DH1 on lactate have not been reported. Traore et al. (1983) obtained a growth yield of D. vulgaris strain Hildenborough (0.215 ± 0.037 mol-X/mol-Lac, 91 % gene similarity) in a coculture with Methanosarcina barkeri on lactate. A biomass yield on lactate of 0.171 ± 0.004 mol-X/mol-Lac was reported for D. vulgaris strain Hildenborough in a coculture with a different methanogen, Methanococcus maripaludis S2 (Walker et al. 2009). The model-derived biomass yield of Desulfovibrio sp. G11 on formate amounted to 0.023 ± 0.001 mol-XG11/mol-Form and the estimated total biomass yield of the coculture on formate was 0.038 ± 0.002 mol-X/mol-Form. Dolfing et al. (2008) reported a total biomass yield on formate of 0.021 ± 0.005 mol-X/mol-Form for the coculture of Desulfovibrio sp. G11 and Methanobrevibacter arboriphilus strain AZ, which is in the same order of magnitude.
The q Lac,max of Desulfovibrio sp. G11 determined in this study is the first reported value so far and amounts to 0.083 ± 0.006 mol-Lac/mol-XG11/h. The model-derived q Lac,max was identical to the q Lac,max obtained during precultivation. The q CH4,max of Methanobrevibacter arboriphilus DH1 is 0.818 ± 0.099 mol-CH4/mol-XMa/h and lies within the previously reported range of 0.574–1.015 mol-CH4/mol-XMa/h when using the estimated biomass yield per methane (Zeikus and Henning 1975).
Dependence of R Lac on the hydrogen partial pressure
During the initial 80 h of the batch experiment, a low R CH4 was observed. Hydrogen consumption by hydrogenotrophic methanogens is likewise low and practically negligible. In this case, the measured net rate of hydrogen production corresponds to the total hydrogen production rate.
In the absence of limitation, the rate of lactate and formate degradation would normally increase over time, resulting in an increasing hydrogen production rate due to biomass growth. During the first phase of the batch experiment, however, the hydrogen net production rate remained constant. The hydrogen partial pressure amounted to 1,204 ± 31 ppm. Moreover, in the first phase, q Lac was only one third of q Lac in the third phase where the hydrogen partial pressure is more than 11 times lower. These results imply a limitation of lactate and formate conversion due to elevated hydrogen partial pressures during the first phase of the batch experiment. Dolfing et al. (2008) reported a similar inhibitory effect of the hydrogen partial pressure during formate conversion in a pure culture of Desulfovibrio sp. G11, resulting in complete inhibition at about 1,000 ppm hydrogen in the gas phase. The observed inhibitory effect of the hydrogen partial pressure on lactate and formate degradation in this study cannot be explained by means of the thermodynamics of the partial reactions (Table 1) since all reactions were strongly energetically favorable under the prevailing conditions (Fig. 8).
Gibbs energy change of the three partial reactions shown in Table 1 in course of the batch experiment shown in Fig. 2. Reaction 1, lactate conversion; Reaction 2, formate conversion; and Reaction 3, hydrogenotrophic methanogenesis. For the calculations of the Gibbs energy change, a temperature of 37 °C and a pH of 7.0 were used
A possible explanation is found by considering the intermediate steps of lactate and formate degradation. As described previously by Pankhania et al. (1988), the first step of lactate conversion, namely the oxidation of lactate to pyruvate and hydrogen, is an endergonic reaction under standard conditions (∆G 01 = +43 kJ/mol lactate), whereas subsequent pyruvate oxidation to acetate, carbon dioxide, and hydrogen is exergonic (∆G 01 = −52 kJ/mol pyruvate). Considering the reactor conditions during the first two phases of the batch experiment (1,204 ppm H2 before 1.8-fold oversaturation correction, 23 mM lactate, pH 7.0, and 310.15 K), lactate oxidation would become thermodynamically favorable only when assuming nanomolar pyruvate concentrations. Given the important role of pyruvate as intermediate in bacterial metabolism, such low concentrations appear very unlikely (Pankhania et al. 1988). Therefore, in line with previous studies (Fitz and Cypionka 1991; Pankhania et al. 1988; Sieber et al. 2012), our findings support the theory that lactate oxidation to pyruvate and hydrogen is an energy-driven process. As a consequence, lactate oxidation to pyruvate could become more costly in a reduced system state marked by increased hydrogen partial pressures.
Formate conversion to hydrogen and carbon dioxide is an endergonic reaction under standard conditions (∆G 01 = +1.3 kJ/mol formate); however, under reactor conditions, this reaction was thermodynamically favorable throughout the experiment (Fig. 8). A limitation to formate conversion might however be the regeneration of the electron carrier pool, offering to explain why both formate and lactate conversion were equally affected at increased hydrogen partial pressures.
Several studies demonstrated that lactate and formate oxidation are coupled to ferredoxin, NAD+, and other electron carriers. For different microbial strains, it was shown that ferredoxin is involved in the oxidation of formate to bicarbonate, and in the oxidation of pyruvate to acetyl-CoA (Brill et al. 1964; Pieulle et al. 1995; Sieber et al. 2012; Uyeda and Rabinowitz 1971). Weghoff et al. (2014) reported on a bifurcation process in Acetobacterium woodii that involves both NAD+ and ferredoxin during endergonic lactate oxidation to pyruvate. Although different metabolic pathways have been suggested for Desulfovibrio species (Li et al. 2011; Meyer et al. 2013; Noguera et al. 1998; Sieber et al. 2012; Walker et al. 2009), experimental evidence for the metabolic pathways and the electron carriers involved in the different steps of lactate and formate conversion of Desulfovibrio sp. G11 is still lacking. For this reason, the proposed regulatory mechanism of the hydrogen partial pressure was analyzed using NADH-dependent hydrogen formation as an example. At the experimental conditions found during the first two phases of the batch experiment, a NADH/NAD+ ratio of at least three is required for NADH-dependent hydrogen formation to be thermodynamically feasible. Reported in vivo NADH/NAD+ ratios range from about 1 for anaerobic chemostat cultures of Enterococcus faecalis on glucose to 57 for Clostridium cellulolyticum grown on cellulose (Payot et al. 1998; Snoep et al. 1991). Reoxidation of reduced electron carriers could become a limiting factor for the catabolic pathway of lactate and formate conversion.
Pankhania et al. (1988) reported a K iH2,Lacox of 2 % in a pure culture of D. vulgaris strain Marburg at comparable growth conditions (37 °C, pH 7.2). The formation of hydrogen from lactate was studied as a function of externally supplied hydrogen in the gas phase at the beginning of the experiment. The external supply of hydrogen, however, suffers from mass transfer limitation when hydrogen is simultaneously consumed resulting in potential overestimation of K iH2,Lacox. In the present study, hydrogen and carbon dioxide resulted from the bioconversion of lactate and formate in the liquid, avoiding the problem of mass transfer limitation in the presence of the hydrogenotrophic methanogen, Methanobrevibacter arboriphilus DH1.
During the second phase of the batch experiment, both, q Lac and q Form, increased threefold with decreasing hydrogen partial pressure in a range from 1,200 to 250 ppm. The declining hydrogen partial pressure significantly reduces the inhibitory effect of hydrogen on lactate and formate degradation. The comparable increase of q Lac and q Form agrees with the proposed NADH dependence of hydrogen formation during lactate and formate degradation.
Hydrogen limitation of hydrogenotrophic methanogenesis
The K S,H2 of Methanobrevibacter arboriphilus DH1 identified in this study, 0.601 ± 0.022 μM dissolved hydrogen (721 ± 26 ppm hydrogen in the gas phase), is the first reported value for this strain. The obtained K S,H2 is about 10 times lower than the K S,H2 reported for Methanobrevibacter arboriphilus AZ (6 μM) (Kristjansson et al. 1982), Methanospirillum hungatei JF1 (5 μM) (Robinson and Tiedje 1984), and Methanobacterium formicicum JF1 (6 μM) (Schauer et al. 1982). Goodwin et al. (1991) reported a K S,H2 of 0.386 μM dissolved hydrogen for a methanogenic coculture with a lactate-degrading partner, which is close to the K S,H2 determined in this study. Hydrogen measurements in the off-gas can lead to inaccurate determination of K S,H2 especially at low hydrogen concentrations when hydrogen is supplied in the gas phase. Overestimation of K S,H2 is likely when hydrogen is externally supplied to the gas phase while hydrogen is at the same time consumed. In contrast, when hydrogen is produced in the liquid phase, K S,H2 may be underestimated due to oversaturation of dissolved hydrogen but the extent of oversaturation can be estimated well from mass transfer considerations. In the present study, hydrogen production resulted from the bioconversion of lactate and formate immediately in the liquid phase. The effect of 1.8-fold oversaturation of hydrogen in the liquid phase was appropriately considered in this study.
Bicarbonate limitation of hydrogenotrophic methanogenesis
During the first two phases of the batch experiment, R CH4 increased 22-fold (Fig. 2), while the estimated growth of Methanobrevibacter arboriphilus DH1 was approximately 2.2-fold. The remaining 10-fold increase might be explained by a temporary bicarbonate limitation upon transfer, followed by adaptation to low bicarbonate concentration during phases 1 and 2. The bicarbonate concentration in the batch experiment ranged between 0.3 and 2.5 % of the bicarbonate concentration during precultivation (60 mM). Inactivation of Methanobrevibacter arboriphilus DH1 upon inoculation can only describe the 22-fold increase of R CH4 when assuming 0.5 % of the theoretical biomass concentration of the methanogen and significant reactivation.
Control and regulation in syntrophic communities
A widely established view is that the biomass-specific growth rate of a syntrophic coculture is limited by the hydrogen-utilizing partner (Archer and Powell 1985; Warikoo et al. 1996). In this study, it was found that in a syntrophic consortium of Desulfovibrio sp. G11 and Methanobrevibacter arboriphilus DH1 on lactate and formate, the acetogenic bacterium determined the biomass-specific growth rate of the coculture.
During phase 3 of the batch experiment, the interspecies hydrogen transfer efficiency was above 99 % indicating the tight coupling of both syntrophic partners. As a result, the hydrogen partial pressure in the third phase was low (106 ± 12 ppm) reducing the inhibitory effect of hydrogen on lactate degradation. Despite the efficient syntrophy, the actual growth rate of the syntrophic culture as reflected in the increase of R CH4 was rather low, raising the question about the limiting conditions for methane production during the third phase. Firstly, with the depletion of formate at the end of the second phase, the additional source of hydrogen and carbon dioxide for Methanobrevibacter arboriphilus DH1 was exhausted. Secondly, the biomass increase of hydrogenotrophic methanogens during the first two phases must have led to an overcapacity and thus to hydrogen limitation during syntrophic lactate degradation in phase 3. In fact, the model-based q CH4 in the third phase was only 43 % of the model-based q CH4 observed at the end of the second phase and lactate degradation proceeded at q Lac,max during phase 3. Moreover, the hydrogen partial pressure in the gas phase during phase 3 was significantly lower than the K S,H2 of the methanogen and lower than the estimated K iH2,Lacox. This confirms hydrogen limitation of the methanogen and operation of Desulfovibrio sp. G11 at q Lac,max. Therefore, it can be concluded that the biomass concentration of Desulfovibrio sp. G11 determined the overall lactate conversion rate, and not the methanogen. Potential effects of acetate inhibition on lactate degradation or hydrogenotrophic methanogenesis during phase 3 can be excluded since Desulfovibrio sp. G11 operated at q Lac,max and Methanobrevibacter arboriphilus DH1 consumed almost all the hydrogen produced. Furthermore, the increase of the biomass concentration of Desulfovibrio sp. G11 in the third phase (64.1 %) corresponds to the increase of R CH4 (65.5 %). These findings provide strong support for the limitation of methane production by the biomass concentration of Desulfovibrio sp. G11 during syntrophic lactate conversion in the third part of the batch experiment.
References
Archer DB, Powell GE (1985) Dependence of the specific growth-rate of methanogenic mutualistic cocultures on the methanogen. Arch Microbiol 141(2):133–137. doi:10.1007/Bf00423273
Brill WJ, Wolin EA, Wolfe RS (1964) Anaerobic formate oxidation: a ferredoxin-dependent reaction. Science 144(3616):297–298. doi:10.1126/science.144.3616.297
Cussler EL (1997) Mass Transfer in Fluid Systems, 2nd edn. Cambridge University Press, New York
de Kok S, Meijer J, van Loosdrecht MCM, Kleerebezem R (2013) Impact of dissolved hydrogen partial pressure on mixed culture fermentations. Appl Microbiol Biot 97(6):2617–2625. doi:10.1007/s00253-012-4400-x
Dolfing J, Jiang B, Henstra AM, Stams AJ, Plugge CM (2008) Syntrophic growth on formate: a new microbial niche in anoxic environments. Appl Environ Microbiol 74(19):6126–6131. doi:10.1128/AEM. 01428-08
Fitz RM, Cypionka H (1991) Generation of a proton gradient in Desulfovibrio vulgaris. Arch Microbiol 155(5):444–448. doi:10.1007/bf00244959
Goodwin S, Giraldogomez E, Mobarry B, Switzenbaum MS (1991) Comparison of diffusion and reaction-rates in anaerobic microbial aggregates. Microbial Ecol 22(2):161–174. doi:10.1007/Bf02540221
Junicke H, Abbas B, Oentoro J, van Loosdrecht M, Kleerebezem R (2014) Absolute quantification of individual biomass concentrations in a methanogenic coculture. AMB Express 4:35. doi:10.1186/s13568-014-0035-x
Kleerebezem R, Stams AJM (2000) Kinetics of syntrophic cultures: a theoretical treatise on butyrate fermentation. Biotechnol Bioeng 67(5):529–543. doi:10.1002/(Sici)1097-0290(20000305)67:5<529::Aid-Bit4>3.0.Co;2-Q
Kleerebezem R, Van Loosdrecht MCM (2010) A generalized method for thermodynamic state analysis of environmental systems. Crit Rev Env Sci Tec 40(1):1–54. doi:10.1080/10643380802000974
Kristjansson JK, Schonheit P, Thauer RK (1982) Different Ks-values for hydrogen of methanogenic bacteria and sulfate reducing bacteria—an explanation for the apparent inhibition of methanogenesis by sulfate. Arch Microbiol 131(3):278–282. doi:10.1007/Bf00405893
Leadbetter JR, Breznak JA (1996) Physiological ecology of Methanobrevibacter cuticularis sp. nov. and Methanobrevibacter curvatus sp. nov., isolated from the hindgut of the termite Reticulitermes flavipes. Appl Environ Microbiol 62(10):3620–3631
Li X, McInerney MJ, Stahl DA, Krumholz LR (2011) Metabolism of H2 by Desulfovibrio alaskensis G20 during syntrophic growth on lactate. Microbiology 157(10):2912–2921. doi:10.1099/mic. 0.051284-0
McCarty PL, Bae J (2011) Model to couple anaerobic process kinetics with biological growth equilibrium thermodynamics. Environ Sci Technol 45(16):6838–6844. doi:10.1021/Es2009055
Meyer B, Kuehl J, Deutschbauer AM, Price MN, Arkin AP, Stahl DA (2013) Variation among Desulfovibrio species in electron transfer systems used for syntrophic growth. J Bacteriol 195(5):990–1004. doi:10.1128/JB.01959-12
Noguera DR, Brusseau GA, Rittmann BE, Stahl DA (1998) A unified model describing the role of hydrogen in the growth of Desulfovibrio vulgaris under different environmental conditions. Biotechnol Bioeng 59(6):732–746
Pankhania IP, Spormann AM, Hamilton WA, Thauer RK (1988) Lactate conversion to acetate, Co2 and H-2 in cell-suspensions of Desulfovibrio vulgaris (Marburg)—indications for the involvement of an energy driven reaction. Arch Microbiol 150(1):26–31. doi:10.1007/Bf00409713
Payot S, Guedon E, Cailliez C, Gelhaye E, Petitdemange H (1998) Metabolism of cellobiose by Clostridium cellulolyticum growing in continuous culture: evidence for decreased NADH reoxidation as a factor limiting growth. Microbiol-Uk 144:375–384
Pieulle L, Guigliarelli B, Asso M, Dole F, Bernadac A, Hatchikian EC (1995) Isolation and characterization of the pyruvate-ferredoxin oxidoreductase from the sulfate-reducing bacterium Desulfovibrio africanus. BBA-Protein Struct M 1250(1):49–59. doi:10.1016/0167-4838(95)00029-t
Plugge CM (2005) Anoxic media design, preparation, and considerations. Methods Enzymol 397:3–16. doi:10.1016/S0076-6879(05)97001-8
Robinson JA, Tiedje JM (1984) Competition between sulfate-reducing and methanogenic bacteria for H-2 under resting and growing conditions. Arch Microbiol 137(1):26–32. doi:10.1007/Bf00425803
Rodriguez J, Kleerebezem R, Lema JM, van Loosdrecht MCM (2006) Modeling product formation in anaerobic mixed culture fermentations. Biotechnol Bioeng 93(3):592–606. doi:10.1002/Bit.20765
Schauer NL, Brown DP, Ferry JG (1982) Kinetics of formate metabolism in Methanobacterium formicicum and Methanospirillum hungatei. Appl Environ Microbiol 44(3):549–554
Schink B (1997) Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Mol Biol R 61(2):262
Sieber JR, McInerney MJ, Gunsalus RP (2012) Genomic insights into syntrophy: the paradigm for anaerobic metabolic cooperation. Annu Rev Microbiol 66:429–452. doi:10.1146/annurev-micro-090110-102844
Snoep JL, Joost M, Demattos T, Neijssel OM (1991) Effect of the energy-source on the NADH/NAD ratio and on pyruvate catabolism in anaerobic chemostat cultures of Enterococcus-faecalis NCTC-775. Fems Microbiol Lett 81(1):63–66
Traore AS, Fardeau ML, Hatchikian CE, Legall J, Belaich JP (1983) Energetics of growth of a defined mixed culture of Desulfovibrio vulgaris and Methanosarcina barkeri—interspecies hydrogen transfer in batch and continuous cultures. Appl Environ Microbiol 46(5):1152–1156
Uyeda K, Rabinowitz JC (1971) Pyruvate-ferredoxin oxidoreductase. 3. Purification and properties of the enzyme. J Biol Chem 246(10):3111–3119
Walker CB, He Z, Yang ZK, Ringbauer JA Jr, He Q, Zhou J, Voordouw G, Wall JD, Arkin AP, Hazen TC, Stolyar S, Stahl DA (2009) The electron transfer system of syntrophically grown Desulfovibrio vulgaris. J Bacteriol 191(18):5793–5801. doi:10.1128/JB.00356-09
Warikoo V, McInerney MJ, Robinson JA, Suflita JM (1996) Interspecies acetate transfer influences the extent of anaerobic benzoate degradation by syntrophic consortia. Appl Environ Microbiol 62(1):26–32
Weghoff MC, Bertsch J, Muller V (2014) A novel mode of lactate metabolism in strictly anaerobic bacteria. Environ Microbiol. doi:10.1111/1462-2920.12493
Willquist K, Nkemka VN, Svensson H, Pawar S, Ljunggren M, Karlsson H, Murto M, Hulteberg C, van Niel EWJ, Liden G (2012) Design of a novel biohythane process with high H2 and CH4 production rates. Int J Hydrogen Energy 37(23):17749–17762. doi:10.1016/j.ijhydene.2012.08.092
Zeikus JG, Henning DL (1975) Methanobacterium arbophilicum sp. nov—obligate anaerobe isolated from wetwood of living trees. A Van Leeuw J Microb 41(4):543–552. doi:10.1007/Bf02565096
Acknowledgments
The authors wish to thank Prof. Dr. AJM Stams and the Laboratory of Microbiology at Wageningen University for the supply of microbial cultures. The financial support of the Stichting voor de Technische Wetenschappen (STW, project number 11603) and Veolia Water is gratefully acknowledged.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Junicke, H., Feldman, H., van Loosdrecht, M.C.M. et al. Impact of the hydrogen partial pressure on lactate degradation in a coculture of Desulfovibrio sp. G11 and Methanobrevibacter arboriphilus DH1. Appl Microbiol Biotechnol 99, 3599–3608 (2015). https://doi.org/10.1007/s00253-014-6241-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6241-2