Abstract
l-DOPA (3,4-dihydroxyphenyl-l-alanine) has been widely used as a drug for Parkinson’s disease caused by deficiency of the neurotransmitter dopamine. Since Monsanto developed the commercial process for l-DOPA synthesis for the first time, most of currently supplied l-DOPA has been produced by the asymmetric method, especially asymmetric hydrogenation. However, the asymmetric synthesis shows critical limitations such as a poor conversion rate and a low enantioselectivity. Accordingly, alternative biotechnological approaches have been researched for overcoming the shortcomings: microbial fermentation using microorganisms with tyrosinase, tyrosine phenol-lyase, or p-hydroxyphenylacetate 3-hydroxylase activity and enzymatic conversion by immobilized tyrosinase. Actually, Ajinomoto Co. Ltd commercialized Erwinia herbicola fermentation to produce l-DOPA from catechol. In addition, the electroenzymatic conversion system was recently introduced as a newly emerging scheme. In this review, we aim to not only overview the biotechnological l-DOPA production methods, but also to briefly compare and analyze their advantages and drawbacks. Furthermore, we suggest the future potential of biotechnological l-DOPA production as an industrial process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
l-DOPA (3,4-dihydroxyphenyl-l-alanine) is an amino acid analog from l-tyrosine in the human body. Since the 1960s, l-DOPA has been used as a drug for Parkinson’s disease, which is a degenerative disorder of the central nervous system and is usually caused by deficiency of the neurotransmitter dopamine in the brain. Because l-DOPA is a precursor of dopamine and can cross the blood–brain barrier whereas dopamine itself cannot, it is possible to increase the dopamine level for the treatment of Parkinson’s disease (Nagatsu and Sawada 2009; Min et al. 2010).
Nowadays, so many elderly people suffer from the symptoms of Parkinson’s disease such as rigidity, akinesia, and rest tumor. Accordingly, the market size for l-DOPA is substantial; the world market of l-DOPA is about 250 ton per year and the total market volume is about 101 billion per year (Koyanagi et al. 2005). Since Monsanto developed and introduced the commercial process for l-DOPA production by asymmetric hydrogenation for the first time, most l-DOPA has been supplied by chemical synthesis, mainly asymmetric synthesis (Knowles 2004). However, the chemical synthesis for l-DOPA has critical limitations such as a poor conversion rate and a low enantioselectivity: the chemical synthesis usually adapts a complicated reaction procedure and requires expensive metal catalysts (e.g., Rb-complex) that work under harsh operational conditions with a low substrate specificity (Sayyed and Sudalai 2004; Valdes et al. 2004). Thus, the biotechnological approaches utilizing microorganisms and/or enzymes have been explored as attractive alternatives not only for improving the conversion rate and the enantioselectivity but also for economizing the process. Actually, Ajinomoto Co. Ltd has biotechnologically produced l-DOPA by Erwinia herbicola, the most favorable strain for l-DOPA production with high tyrosine phenol-lyase (Tpl) activity since 1993 (Iizumi et al. 1991).
In this review, we introduce the biotechnological approaches for l-DOPA production, including microbial fermentation using microorganisms with tyrosinase, Tpl, or p-hydroxyphenylacetate 3-hydroxylase (PHAH) activity, biocatalytic synthesis by the immobilized tyrosinase, and the electroenzymatic system as a newly emerging scheme. A comparison of the chemical synthesis and the advantages and drawbacks of the biotechnological approaches is briefly discussed. In addition, we suggest that the future potential of the biotechnological approach, such as the electroenzymatic system as the solution for overcoming the limitations in the existing commercial l-DOPA process.
Microbial fermentation for l-DOPA production
Microbial fermentation has been researched as one of alternatives for chemically synthesizing l-DOPA, and microorganisms having tyrosinase, Tpl, and PAHA activity have been researched. Compared to chemical synthesis, microbial fermentation usually exhibits outstanding enantioselectivity, but (1) it is a time-consuming process that takes over 10 days including the cell-culture period and (2) it is not easy to purify l-DOPA from culture media including analogous substrates such as l-tyrosine. In this section, we introduce the microbial l-DOPA production based on tyrosinase, Tpl, and PAHA activity in detail.
Based on tyrosinase activity
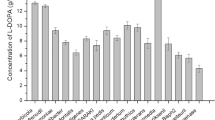
Tyrosinase (E.C. 1.14.18.1) is a kind of copper-containing oxidoreductase and has two catalytic activities. As shown in Scheme 1, tyrosinase catalyzes ortho-hydroxylation of monophenol to diphenol by cresolase activity. It also consecutively oxidizes diphenol to quinone by catecholase activity (Min and Yoo 2009). Accordingly, l-DOPA can be produced by the o-hydroxylation of l-tyrosine by the cresolase activity in tyrosinase (Ates et al. 2007; Min et al. 2010; Pialis et al. 1996; Seetharam and Saville 2002; Xu et al. 2012). As well as in biocatalytic conversion, microorganisms with tyrosinase activity have been widely used in microbial fermentation for l-DOPA production using l-tyrosine as the feedstock. Krishnaveni et al. screened Acremonium rutilum as a novel fungal tyrosinase-producer. They isolated seven tyrosinase-producing fungi by collecting samples rich in phenolic substances, such as tree bark, decomposed banana stud, and garden soil samples. Among the seven isolates, A. rutilum exhibited the highest tyrosinase activity, thereby showing the highest l-DOPA production. To maximize the l-DOPA production level, they optimized parameters for liquid fermentation of A. rutilum: a temperature of 25 °C, a pH of 5.5, an inoculum size of 2.5 mL, and an incubation time of 72–120 h, with 5 g L−1 of l-tyrosine. Under the optimum conditions, A. rutilum produced 0.89 g L−1 of l-DOPA with 1095 U mg−1 of tyrosinase activity (Krishnaveni et al. 2009).
Ikram-ul and Ali focused on Aspergillus oryzae as l-DOPA producing microorganism. To increase l-DOPA production, A. oryzae strain GCB-6 was mutated by ultraviolet irradiation and the mutant strain UV-7 yielded 3.72-fold l-DOPA than the parental strain (Ikram-ul et al. 2002). They further improved the mutant strain UV-7 by chemical mutation using n-methyl-l-3-nitro-n-nitrosoguanidine as the mutagen and consequently the double mutant maximally produced 444 mg of l-DOPA/g of cells. In addition, thermodynamic parameters such as activation enthalpy and entropy by the Arrhenius model revealed that the double mutant was thermodynamically stable against thermal denaturation during the product-formation process (Ali et al. 2005). They also aimed to utilize Yarrowia lipolytica NRRL-I43, a kind of non-conventional yeast producing significant amounts of intra- or extra-cellular metabolites, for microbiological transformation of l-tyrosine to l-DOPA. They used diatomite (2:1 clay mineral) as the enhancer to increase the substrate uptake and enzyme production rate with concomitant with l-DOPA production. As a result, a maximum of 2.96 g L−1 of l-DOPA was obtained from 2.68 g L−1 of l-tyrosine when 2.0 g L−1 of diatomite was added 15 min after the initiation of the reaction: The reaction media included 2.5 g L−1 of yeast cells, and 5.0 g L−1 of l-ascorbic acid. Compared to A. oryzae, a yeast such as Y. lipolytica is easy to handle and exhibits a rapid growth rate, so it is attractive for fermentation (Ali et al. 2007).
Surwase et al. for the first time reported that a bacterial strain was able to utilize l-tyrosine for producing l-DOPA. They isolated Bacillus sp. JPJ (NCBI accession no. FJ545652.1) having the ability to convert l-tyrosine to l-DOPA from soil samples from Kolhapur. Bacillus sp. JPJ had a 99.4 % conversion rate from 0.5 g L−1 of l-tyrosine in a phosphate buffer (pH 8) including 1 g L−1 of cell mass at 40 °C for 60 min. In addition, the inducing effect was investigated with 0.06 g L−1 of CuSO4 and 0.04 g L−1 of ascorbic acid cooperatively, because tyrosinase essentially requires copper ion in the active center and ascorbic acid is possible to prevent to oxidize l-DOPA to DOPAquinone. For maximizing bioconversion of l-tyrosine to l-DOPA, 2 g L−1 of activated charcoal was essential. The bacterial strain is more favorable for industrial fermentation than plant, fungi, and yeast, thereby concluding that Bacillus sp. JPJ was an efficient alternative for industrial scale production of l-DOPA (Surwase and Jadhav 2011). Surwase et al. also reported another bacterial strain for l-DOPA synthesis. Brevundimonas sp. SGJ (NCBI accession no. HM998899) was isolated from a garden soil sample collected from India and indicated an ability to produce l-DOPA. The optimal conditions for l-DOPA production were determined; pH of 8.0, temperature of 40 °C, 2 g L−1 of initial cell inoculation, 2 g L−1 of l-tyrosine as the substrate, 0.04 g L−1 of CuSO4, and 0.02 g L−1 of ascorbic acid. In addition, the effect of the enhancer was investigated. Carrageenan, diatomaceous earth, and activated charcoal were tested as the enhancer for l-DOPA production, and 0.5 g L−1 of carrageenan was selected as the most effective enhancer. As a result, 3.81 g L−1 of l-DOPA was maximally produced with 9201 U mg−1 of tyrosinase activity (Surwase et al. 2012a). To optimize l-DOPA production by Brevundimonas sp. SGJ, they conducted the response surface methodology (RSM) and determined the nutritional parameters influencing l-DOPA production: pH of 5.2, 1.55 g L−1 of tryptone, 4.21 g L−1 of l-tyrosine, and 0.037 g L−1 of CuSO4 resulted in the highest l-DOPA production, 3.36 g L−1. As a result, the optimized reaction media by RSM exhibited 8.36-fold increase in l-DOPA production and the highest tyrosinase activity was observed with 0.71 g L−1 of dry cell weight (Surwase et al. 2012b).
Based on tyrosine phenol-lyase (Tpl) activity
In addition to tyrosinase, microorganisms with Tpl (E.C. 4.1.99.2) activity have been used in microbial fermentation for l-DOPA production. Tpl usually degrades l-tyrosine to pyruvate, ammonia, and phenol. The reaction is reversible and accordingly l-DOPA can be produced if phenol is substituted by catechol as shown in Scheme 2. Since Tpl is a l-tyrosine inducible biocatalyst, the addition of l-tyrosine to the culture medium is inevitable, but severely hiders the purification of l-DOPA from the medium (Foor et al. 1993; Katayama et al. 2000).
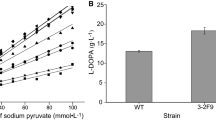
Kumagai group focused on the microbial production of l-DOPA using E. herbicola, which had extremely high Tpl activity. For high level expression of Tpl, they cloned the tyrR gene of E. herbicola and randomly mutagenized it. The mutant TyrR protein exhibited enhanced ability to activate tpl without addition of l-tyrosine to the culture medium. They also circumvented the problem by using recombinant E. herbicola with a mutant transcriptional regulator TyrR, which activated the tpl promoter in the absence of l-tyrosine (Katayama et al. 2000; Koyanagi et al. 2005). Using the reaction media (pH 8.0 and 15 °C) including 16 g L−1 of sodium pyruvate, 10 g L−1 of catechol, 47 g L−1 of ammonium chloride, 1.3 g L−1 of ammonium nitrate, 2.7 g L−1 of sodium sulfite, and 4 g L−1 of disodium ethylenediaminetetraacetic acid, the recombinant E. herbicola showed significantly enhanced productivity up to 11.1 g L−1 h−1, whereas the productivity of l-tyrosine-induced wild type E. herbicola was only 0.375 g L−1 h−1. The system using the recombinant E. herbicola greatly contributed to economizing the production cost due to the highly improved productivity and the low reaction temperature (15 °C) (Koyanagi et al. 2005). Accordingly, the recombinant E. herbicola was a potential candidate for industrial application and actually Ajinomoto Co. Ltd has produced l-DOPA by E. herbicola culture since 1993.
In addition to the Kumagai group, many researchers have attempted to produce l-DOPA by the microbial fermentation based on Tpl activity. For example, Foor et al. cloned the gene encoding Tpl from E. herbicola into Escherichia coli. After lag phase of about 6 h, l-DOPA was rapidly accumulated in the culture media by the recombinant E. coli. When using 330 mM of pyruvate, 230 mM of catechol, and 12 mM of ammonium acetate as the substrate, the recombinant E. coli produced 105 mM of l-DOPA in the batch fermentor (Foor et al. 1993). Bielecki and Bolek immobilized recombinant E. coli cells with the gene coding for Tpl from Citrobacter freundii in carrageenan, calcium alginate, polyvinyl alcohol, and polyacrylamide to produce l-DOPA. The immobilized E. coli exhibited maximal l-DOPA production at pH 8.5 and 37 °C, resulting in 4.53 mg mL−1 of l-DOPA production with 60.4 % of conversion rate (Bielecki and Bolek 1996). Lee and coworkers cloned a thermostable Tpl gene from thermophilic Symbiobacterium species and overexpressed it in E. coli in order to produce l-DOPA using pyrocatechol, sodium pyruvate, and ammonium chloride as substrates. The optimum condition was determined at pH 8.3 and 37 °C. Tpl activity was dependent on the concentration of ammonium chloride and ethanol played a role as the activator for Tpl activity. In addition, Tpl activity was inactivated by pyrocatechol at higher than 0.1 M. They conducted the fed-batch fermentation and finally obtained 29.8 g L−1 of l-DOPA after 6 h (Lee et al. 1996).
Based on the Tpl activity, Park et al. designed a hybrid pathway using toluene dioxygenase, toluene cis-glycerol dehydrogenase, and Tpl for utilizing benzene as the substrate. As shown in Scheme 3, dioxygenase and toluene cis-glycerol dehydrogenase converted benzene to catechol. Then, l-DOPA was synthesized from the resulting catechol by Tpl in the copresence of pyruvate and ammonia. The hybrid pathway was constructed in the E. coli at first, but l-DOPA production barely reached 3 mM in 4 h due to the toxic effect of benzene on the cells. Instead of E. coli, Pseudomonas aeruginosa was chosen from various Pseudomonas species tested for benzene-resistance, because some Pseudomonas species have been reported to be resistant to organic solvent. As a result, 14 mM of l-DOPA was produced in 9 h when using P. aeruginosa (Park et al. 1998). Because benzene is a cheaper raw material than catechol, the hybrid pathway can contribute to economize the l-DOPA production cost.
The microbial fermentation based on Tpl activity usually showed higher productivity than the microbial fermentation based on tyrosinase activity, because the solubility of the main substrate catechol in an aqueous phase is much greater than that of l-tyrosine. However, the microbial fermentation based on Tpl activity requires pyruvate and ammonia as the co-substrate and thus it is not easy to separate and purify l-DOPA from a culture media including various substrates.
Based on p-hydroxyphenylacetate 3-hydroxylase (PHAH) activity
Tyrosinase is usually used for producing l-DOPA from l-tyrosine, but Lee and Xun reported a novel process for l-DOPA production from l-tyrosine based on p-hydroxyphenylacetate 3-hydroxylase (PHAH, E.C. 1.14.14.9). PHAH catalyzes p-hydroxyphenylacetate to 3,4-dihydroxyphenylacetate and essentially requires NADH and O2. It shows a broad substrate spectrum and utilizes 3-hydroxyphenylacetate, phenol, p-cresol, and hydroquinone. Lee and Xun expected that PHAH might convert l-tyrosine to l-DOPA as shown in Scheme 4 and used the E. coli strain W ATCC11105 having PHAH activity to produce l-DOPA from l-tyrosine. Because PHAH required NADH, 5 % (v/v) glycerol was added to maintain the reducing power inside the cell for NADH regeneration. As a result, the E. coli strain W ATCC11105 successfully produced 8.2 and 48 mM of l-DOPA in the batch and the fed-batch reactor, respectively (Lee and Xun 1998).
Muñoz et al. metabolically engineered the E. coli strain to increase the carbon flux from glucose to l-tyrosine by eliminating TyrR (regulatory protein) repression. As a result, an 8.6-fold higher l-tyrosine yield from glucose was achieved in the eliminated strain. In addition, the PHAH gene from E. coli W was expressed for synthesizing l-DOPA from l-tyrosine. They conducted a batch culture to evaluate the performance of the engineered strain using an LB medium supplemented with 50 g L−1 of glucose. As a result, the engineered E. coli strain exhibited 0.31 h of specific growth rate and 6.75 g L−1 of maximum biomass concentration. When entering the stationary phase, l-DOPA accumulated and then 1.51 g L−1 of l-DOPA was obtained (Muñoz et al. 2011).
In comparison with tyrosinase, PHAH utilized the same substrate l-tyrosine and then produced l-DOPA, but it inevitably required NADH as the cofactor. NADH is usually too expensive to be used in stoichiometric amounts and the high cost of NADH is one of the bottlenecks for industrial application. Therefore, an efficient cofactor regeneration system should be simultaneously considered for the l-DOPA synthetic process by the microbial fermentation based on PHAH activity.
Enzymatic conversion: Hydroxylation of l- tyrosine to l-DOPA using the immobilized tyrosinase
Even though the microbial fermentation for l-DOPA usually exhibits better enantioselectivity than the chemical synthesis, the separation and purification process is complex due to culture media including other substrates and proteins. In addition, the microbial fermentation is a time-consuming process of over 10 days, including cell culture, and thus overall productivity is not good. Accordingly, biocatalytic production using immobilized enzyme has been attempted as an alternative, and tyrosinase has been most widely researched (Min et al. 2010).
As mentioned in the section based on tyrosinase activity, tyrosinase is able to produce l-DOPA from l-tyrosine by the cresolase activity. Comparing the chemical synthesis and the microbial fermentation, the enzymatic l-DOPA synthesis by tyrosinase is simple including only one reaction step and an environmental-friendly process under mild operational conditions, usually a neutral pH and around room temperature. In addition, the enzymatic l-DOPA synthesis exhibits outstanding enantioselectivity. Nevertheless, its application for the industrial scale has been hampered by low productivity, low conversion rate, and denaturation of the biocatalyst. In order to overcome these shortcomings, enzyme immobilization is noted as the solution because immobilization makes it easy to separate the enzyme from the reaction media and often increase stability and reusability. Also, confinement of the enzyme by the immobilization often prevents its denaturation (Min et al. 2012). Accordingly, various types of materials as immobilization support and reactors have been researched for improving the productivity and the conversion rate of the enzymatic l-DOPA synthesis as summarized in Table 1.
Ates et al. produced l-DOPA with entrapped tyrosinase in a copper-alginate gel and designed both a batch and a packed-bed reactor. In the batch reactor, 4.5 mg L−1 of l-DOPA was obtained with a productivity of 4.5 mg L−1 h−1. In addition, they investigated the effect of air on l-DOPA production in the packed-bed reactor. With aeration, the l-DOPA concentration increased from 1.2 to 7.7 mg L−1. The productivity was also enhanced up to 110 mg L−1 h−1 in the packed-bed reactor (Ates et al. 2007). Pialis and coworkers produced l-DOPA in the batch reactor using tyrosinase immobilized on chemically modified nylon 6,6 membrane. The membrane pore size and glutaraldehyde concentration affected the enzyme loading density and l-DOPA production. As a result, 0.143 g of l-DOPA was optimally produced with a productivity of 1.70 mg L−1 h−1 during 170 h in a 500 mL batch reactor using a membrane with a pore size of 0.20 μm and 3 % of glutaraldehyde as the cross linker. In addition, the immobilized tyrosinase retained 80 % of its initial activity over 14 days and thus the tyrosinase-immobilized membrane reactor might be suitable for long-term operation (Pialis et al. 1996). Catecholase activity in tyrosinase often hinders the enzymatic l-DOPA synthesis because l-DOPA rapidly oxidizes to the by-product DOPAquinone by the catecholase activity. In order to prevent formation of the by-product, thereby increasing the conversion rate and the productivity, Algieri et al. studied the continuous membrane bioreactor for enhancing antioxidant action of ascorbic acid. The continuous membrane bioreactor was prepared by the immobilized tyrosinase in an asymmetric tubular membrane made of polyamide. When the reactor was operated by the recycle mode, the conversion rate and the productivity were of 6.4 % and 1.2 mg L−1 h−1, respectively. The continuous mode was able to immediately remove l-DOPA from the reaction media, thus preventing sequential oxidation of l-DOPA to the by-product DOPAquinone. As a result, they achieved improved conversion rate and productivity up to 10.2 % and 23.7 mg L−1 h−1, respectively (Algieri et al. 2012). Seetharam and Saville covalently immobilized tyrosinase on sodium aluminosilicate (NaA) and calcium aluminosilicate (CaA) with immobilization efficiency of 85 and 82 %, respectively. Using the immobilized tyrosinase on NaA or CaA, the average productivity over 7 h was 34 mg L−1 h−1, producing 111–135 mg of l-DOPA in the 500 ml batch reactor. Furthermore, activity loss of the immobilized tyrosinase was not observed during 40–48 h of repeated batch operations. Accordingly, NaA and CaA might be a promising support for developing stable immobilized tyrosinase and thus might contribute to the design of an l-DOPA synthetic process (Seetharam and Saville 2002). Vilanova et al. immobilized tyrosinase from frog epidermis on Enzaryl-AA (a polyacrylamide-based support) and CPGzirclad-Arylamine (controlled pore glass) to stabilize cresolase activity for producing l-DOPA from l-tyrosine. As a result, the immobilized tyrosinase retained over 86 % activity of the free counterpart and immobilization efficiency was reached up to 90 %. The immobilized tyrosinase not only did not lose the initial activity during 120 days at storage conditions but also retained 50 % of its initial activity for 20 h of operation in the column reactor. Consequently, the immobilized tyrosinase showed improved stability and thus it was a potential candidate for industrial application (Vilanova et al. 1984). Xu and coworkers developed tyrosinase as cross-linked enzyme aggregates (CLEAs) for efficient l-DOPA production. Tyrosinase CLEAs were prepared by protein precipitation followed by cross-linking with glutaraldehyde. They investigated l-DOPA production using tyrosinase CLEAs in different reactors such as a batch reactor, a continuous stirred-tank reactor (STR), and a packed-bed reactor. As a result, a conversion rate of 53.0 % was achieved during 2 h with a productivity of 209 mg L−1 h−1 in the batch reactor. Compared to other results shown in Table 1, outstanding conversion rate and productivity were achieved in the batch reactor due to the high substrate concentration (4.0 vs. 2.5 mM commonly used in other studies) and a highly concentrated reducing reagent (30 vs. 2.5 mM ascorbic acid widely used). In the continuous process, the productivity was of 103.0 and 48.9 mg L−1 h−1 in the STR and packed-bed reactor, respectively. However, tyrosinase CLEAs were not stable in operational conditions and thus they doubly-entrapped tyrosinase CLEAs into a calcium alginate gel. CLEA/alginate beads retained 78.2 % of their initial activity after ten reaction cycles, whereas tyrosinase CLEAs lost 39.2 % of the initial activity after only five reaction cycles (Xu et al. 2012).
Even though various immobilization methods and reactors have been attempted, the biocatalytic l-DOPA synthesis is still limited for the industrial process owing to the by-product DOPAquinone. Kinetic studies revealed that catecholase activity was usually more efficient than cresolase activity (Min et al. 2013). Thus, l-DOPA is sequentially oxidized by the catecholase activity and the by-product DOPAquinone is unavoidably formed, resulting in a low conversion rate. To reduce the by-product and improve the conversion rate, a reducing reagent has been used, such as ascorbic acid, NH3OH, and NADH (Scheme 5). Nevertheless, the conversion rate and the productivity were not drastically enhanced because the reducing reagent was continually consumed during the biocatalysis and thus not sufficient in the batch reactor as time passed. Moreover, concentrated ascorbic acid often inhibits cresolase activity and irreversibly inactivates tyrosinase. Accordingly, countermeasures should be taken in order to reduce the by-product.
Electroenzymatic system as a newly emerging scheme for l-DOPA production
Recently, the electroenzymatic system as an innovative strategy for l-DOPA synthesis was researched, an outstanding conversion rate in the batch reactor was achieved. Min et al. for the first time designed an electroenzymatic system by utilizing electrical reducing power instead of a reducing reagent such as ascorbic acid in order to prevent the by-product accumulation in the batch reactor. They non-covalently functionalized single-walled carbon nanotubes (SWNTs) by 1-pyrenebutyric acid to retain their electrical properties. Then, tyrosinase covalently attached the functionalized SWNTs, and a tyrosinase-immobilized 3-dimensional cathode was fabricated using a conducting polymer polypyrrole (Min and Yoo 2009). In the biocathode, SWNTs were used as not only support for the immobilization but also as the electron carrier, because the SWNTs had a large surface area for higher loading density for the immobilization and exhibited good electrical conductivity. Thus, SWNTs have been widely used for preparing the bioelectrode. The electroenzymatic system was constructed by the tyrosinase-immobilized 3-dimensional cathode, coiled Pt wire, and Ag/AgCl electrode as working, counter, and reference electrode, respectively. As shown in Scheme 6, the by-product DOPAquinone produced by the catecholase activity was immediately reduced to l-DOPA again by electrons continuously supplied from the cathode in the electroenzymatic system. Consequently, the by-product was not accumulated in the batch reactor and thus an innovative conversion rate was achieved up to 95.9 % (Min et al. 2010). Comparing the conversion rate previously reported (Table 1), the electroenzymatic system exhibited an unbelievable conversion rate in the batch reactor. Furthermore, Min et al. elucidated the reason for the amazing conversion rate in the electroenzymatic system by determining the kinetic parameters. They found that the electrical reduction of the by-product to l-DOPA (k e) was dependent on the l-DOPA concentration and k e was faster than the oxidation of l-DOPA to the by-product by the catecholase activity (k 2) under experimental conditions (below 1.0 mM of l-DOPA). In the electroenzymatic system, undesirable loss of l-DOPA to the by-product was prevented by the rapid and continuous supplement of electrons from the cathode. Accordingly, the by-product was not accumulated in the batch reactor and the conversion rate was drastically enhanced (Min et al. 2013).
Electroenzymatic system for l-DOPA synthesis by tyrosinase-immobilized biocathode (Min et al. 2010)
Although theelectroenzymatic system achieved an almost complete conversion rate, the productivity was not good due to the low solubility of the substrate in the aqueous phase; solubility of l-tyrosine is usually below 4.0 mM in the aqueous phase. To prepare a concentrated substrate to improve the productivity, Min et al. attempted to disperse l-tyrosine by emulsification that was often efficient for insoluble or poorly soluble chemicals in the reaction phase (Min et al. 2013). Well-dispersed l-tyrosine was prepared by a wet milling method referred to in the US patent 5145684, thereby concentrating up to 500 mM in aqueous phase. They gradually increased the concentration of the well-dispersed l-tyrosine up to 500 mM by suitably increasing the electrode size, but mass transfer was restricted to the tyrosinase-immobilized cathode and thus the conversion rate was reduced. They conducted the electroenzymatic l-DOPA synthesis with 500 mM of well-dispersed l-tyrosine as the substrate and a 3-dimensional tyrosinase-immobilized biocathode (4 cm × 3 cm × 2 cm). After 5 h reaction, the conversion rate and the productivity reached up to 77.7 % and 15.3 g L−1 h−1, respectively. Finally, 76.6 g L−1 of l-DOPA was obtained from 500 mM of well-dispersed l-tyrosine. The productivity previously reported did not exceed 100 mg L−1 h−1 as shown in Table 1 (Min et al. 2013). The electroenzymatic system using well-dispersed l-tyrosine exhibited not only an outstanding conversion rate, but also amazing productivity and thus was noted as an innovative strategy.
Fauziyah et al. attempted an electroenzymatic system using free tyrosinase as the biocatalyst. They conducted an electroenzymatic reaction with a glassy carbon electrode, Pt wire, and an Ag/AgCl electrode as the working, the counter, and the reference electrodes, respectively. Without the immobilization step, they determined the optimum pH, temperature, enzyme amount, and kinetic parameters. Their results also confirmed that the electrochemical method was an alternative way to produce l-DOPA without a reducing reagent, but the conversion rate was just about 40 % (Fauziyah et al. 2012). Accordingly, the rapid and continuous supply of electrons from the cathode to the immobilized tyrosinase was crucial for an outstanding performance in the electroenzymatic system.
In this review, we introduced the electroenzymatic system presenting an amazingly improved conversion rate and productivity as an innovative strategy. The electroenzymatic system provides a promising insight for developing an efficient l-DOPA production strategy and opens up a potential opportunity for industrial application.
Summary and outlook
Since Monsanto developed the first commercial process based on asymmetric hydrogenation, l-DOPA has been widely used as a drug for Parkinson’s disease and the world market for l-DOPA is substantial. Ajinomoto Co. Ltd has commercialized an E. herbicolar culture for biotechnological l-DOPA production since 1993. In this review, we examined and compared the biotechnological approaches for l-DOPA production with chemical process (Table 2). We expect the biotechnological approaches presented in this study not only provides insights for developing a novel commercial process for l-DOPA production but also contributes further understanding of the microbiological and enzyme technology for practical and industrial applications.
References
Algieri C, Donato L, Bonacci P, Giorno L (2012) Tyrosinase immobilised on polyamide tubular membrane for the l-DOPA production: Total recycle and continuous reactor study. Biochem Eng J 66:14–19
Ali S, Haq IU, Qadeer MA, Rajoka MI (2005) Double mutant of Aspergillus oryzae for improved production of L-dopa (3,4-dihydroxyphenyl-L-alanine) from L-tyrosine. Biotechnol Appl Biochem 42:143–149
Ali S, Shultz J, Ikram-ul-Haq (2007) High performance microbiological transformation of L-tyrosine to L-dopa by Yarrowia lipolytica NRRL-143. BMC Biotech 7(1):50
Ates S, Cortenlioglu E, Bayraktar E, Mehmetoglu U (2007) Production of l-DOPA using Cu-alginate gel immobilized tyrosinase in a batch and packed bed reactor. Enzyme Microb Technol 40(4):683–687
Bielecki S, Bolek R (1996) Immobilization of recombinant E. coli cells with phenol-lyase activity. In: Wijffels RH, Buitelaar RM, Bucke C, Tramper J (eds) Progress in Biotechnology, vol 11. Elsevier, Amsterdam, pp 472–478
Fauziyah R, Gobikrishnan S, Indrawan N, Park S, Park J-H, Min K, Yoo YJ, Park D-H (2012) A study on electrochemical synthesis of L-DOPA using oxidoreductase enzymes: optimization of an electrochemical process. J Microbiol Biotechnol 22(10):1446–1451
Foor F, Morin N, Bostian KA (1993) Production of L-dihydroxyphenylalanine in Escherichia coli with the tyrosine phenol-lyase gene cloned from Erwinia herbicola. Appl Environ Microbiol 59(9):3070–3075
Iizumi K, Kotani T, Nishimoto Y, Tsuchida T (1991) Production of L-3,4-dihydroxyphenylalanine. Japan Patent JP5123177A
Ikram-ul H, Ali S, Qadeer MA (2002) Biosynthesis of l-DOPA by Aspergillus oryzae. Bioresour Technol 85(1):25–29
Katayama T, Suzuki H, Koyanagi T, Kumagai H (2000) Cloning and random mutagenesis of the Erwinia herbicola tyrR Gene for high-level expression of tyrosine phenol-lyase. Appl Environ Microbiol 66(11):4764–4771
Knowles WS (2004) Asymmetric Hydrogenations – The Monsanto L-Dopa Process. In: Blaser H-U, Schmidt E (eds) Asymmetric Catalysis on Industrial Scale. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, p 21–38
Koyanagi T, Katayama T, Suzuki H, Nakazawa H, Yokozeki K, Kumagai H (2005) Effective production of 3,4-dihydroxyphenyl-l-alanine (l-DOPA) with Erwinia herbicola cells carrying a mutant transcriptional regulator TyrR. J Biotechnol 115(3):303–306
Krishnaveni R, Rathod V, Thakur MS, Neelgund YF (2009) Transformation of L-Tyrosine to L-Dopa by a Novel Fungus, Acremonium rutilum, under submerged fermentation. Curr Microbiol 58(2):122–128
Lee J-Y, Xun L (1998) Novel biological process for L-DOPA Production from L-Tyrosine by P-Hydroxyphenylacetate 3-Hydroxylase. Biotechnol Lett 20(5):479–482
Lee S-G, Ro H-S, Hong S-P, Kim E-H, Sung M-H (1996) Production of L-DOPA by thermostable tyrosine phenol-lyase of a Themophilic Symbiobacterium species overexpressed in recombinant Escherichia coli. J Microbiol Biotechnol 6(2):98–102
Min K, Yoo YJ (2009) Amperometric detection of dopamine based on tyrosinase–SWNTs–Ppy composite electrode. Talanta 80(2):1007–1011
Min K, Park D-H, Yoo YJ (2010) Electroenzymatic synthesis of l-DOPA. J Biotechnol 146(1–2):40–44
Min K, Kim J, Park K, Yoo YJ (2012) Enzyme immobilization on carbon nanomaterials: Loading density investigation and zeta potential analysis. J Mol Catal B Enzym 83:87–93
Min K, Kathavarayan T, Park K, Yoo YJ (2013) Novel strategy for enhancing productivity in l-DOPA synthesis: the electroenzymatic approach using well-dispersed l-tyrosine. J Mol Catal B Enzym 90:87–90
Muñoz A, Hernández-Chávez G, Anda R, Martínez A, Bolívar F, Gosset G (2011) Metabolic engineering of Escherichia coli for improving l-3,4-dihydroxyphenylalanine (l-DOPA) synthesis from glucose. J Ind Microbiol Biotechnol 38(11):1845–1852
Nagatsu T, Sawada M (2009) L-dopa therapy for Parkinson's disease: Past, present, and future. Parkinsonism & Related Disorders 15. Suppl 1:S3–S8
Park H-S, Lee J-Y, Kim H-S (1998) Production of L-DOPA(3,4-dihydroxyphenyl-L-alanine) from benzene by using a hybrid pathway. Biotechnol Bioeng 58(2–3):339–343
Pialis P, Jimenez Hamann MC, Saville BA (1996) L-DOPA production from tyrosinase immobilized on nylon 6,6. Biotechnol Bioeng 51(2):141–147
Sayyed IA, Sudalai A (2004) Asymmetric synthesis of L-DOPA and (R)-selegiline via OsO4-catalyzed asymmetric dihydroxylation. Tetrahedron Asymmetry 15(19):3111–3116
Seetharam G, Saville BA (2002) l-DOPA production from tyrosinase immobilized on zeolite. Enzyme Microb Technol 31(6):747–753
Surwase S, Jadhav J (2011) Bioconversion of l-tyrosine to l-DOPA by a novel bacterium Bacillus sp. JPJ Amino Acids 41(2):495–506
Surwase S, Patil S, Apine O, Jadhav J (2012a) Efficient microbial conversion of l-Tyrosine to l-DOPA by Brevundimonas sp. SGJ. Appl Biochem Biotechnol 167(5):1015–1028
Surwase S, Patil S, Jadhav S, Jadhav J (2012b) Optimization of l-DOPA production by Brevundimonas sp. SGJ using response surface methodology. Microb Biotechnol 5(6):731–737
Valdes R, Puzer L, Gomes M, Marques C, Aranda D, Bastos M, Gernal A, Antunes O (2004) Production of L-DOPA under heterogeneous asymmetric catalysis. Catal Commun 5(10):631–634
Vilanova E, Manjon A, Iborra JL (1984) Tyrosine hydroxylase activity of immobilized tyrosinase on enzacryl-AA and CPG-AA supports: Stabilization and properties. Biotechnol Bioeng 26(11):1306–1312
Xu D-Y, Chen J-Y, Yang Z (2012) Use of cross-linked tyrosinase aggregates as catalyst for synthesis of l-DOPA. Biochem Eng J 63:88–94
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Min, K., Park, K., Park, DH. et al. Overview on the biotechnological production of l-DOPA. Appl Microbiol Biotechnol 99, 575–584 (2015). https://doi.org/10.1007/s00253-014-6215-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6215-4