Abstract
Microalgae harbor a not fully exploited industrial and environmental potential due to their high metabolic plasticity. In this context, a better understanding of the metabolism of microalgae and microalgal-bacterial consortia under stress conditions is essential to optimize any waste-to-value approach for their mass cultivation. This work constitutes a fundamental study of the mixotrophic metabolism under stress conditions of an axenic culture of Chlorella sorokiniana and a microalgal-bacterial consortium using carbon, nitrogen, and phosphorous mass balances. The hydrolysis of glucose into volatile fatty acids (VFA) during dark periods occurred only in microalgal-bacterial cultures and resulted in organic carbon removals in the subsequent illuminated periods higher than in C. sorokiniana cultures, which highlighted the symbiotic role of bacterial metabolism. Acetic acid was preferentially assimilated over glucose and inorganic carbon by C. sorokiniana and by the microalgal-bacterial consortium during light periods. N-NH4 + and P-PO4 −3 removals in the light stages decreased at decreasing duration of the dark stages, which suggested that N and P assimilation in microalgal-bacterial cultures was proportional to the carbon available as VFA to produce new biomass. Unlike microalgal-bacterial cultures, C. sorokiniana released P-PO4 −3 under anaerobic conditions, but this excretion was not related to polyhydroxybutyrate accumulation. Finally, while no changes were observed in the carbohydrate, lipid and protein content during repeated extended dark-light periods, nutrient deprivation boosted both C-acetate and C-glucose assimilation and resulted in significantly high biomass productivities and carbohydrate contents in both C. sorokiniana and the microalgal-bacterial cultures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae can play a key role in the treatment of water pollution and support a green bioeconomy in this XXI century due to their high metabolic versatility and productivity rates. Microalgae are able to grow simultaneously under photoautotrophic and heterotrophic conditions (Pérez-García et al. 2010), and even nitrifying activity has been detected in Chlorella in the presence of ammonium (Kessler and Oesterheld 1970). Guieysse et al. (2013) confirmed the presence of the enzyme nitrate reductase (an enzyme involved in the bacterial denitrification pathway) in axenic cultures of Chlorella vulgaris. In addition, luxury P uptake in microalgae can result into structural P contents of up to 3 %, which could support a microalgae-based enhanced biological P removal (EBPR) and allow for nutrient recycling via fertilization with microalgae biomass (Powell et al. 2008, 2009; Arbib et al. 2014). In this context, microalgae cultivation as a platform technology for secondary or tertiary wastewater treatment can support a simultaneous C, N, and P removal via mixotrophic fixation and the heterotrophic degradation of persistent organic pollutant (Muñoz and Guieysse 2006; Abreu et al. 2012). Microalgae-based wastewater treatment results in a large production of residual biomass from which high-added-value products such as lipids, carbohydrates, amino acids, and polyunsaturated fatty acids or pigments could be extracted (Pérez-García et al. 2011a; Cea-Barcía et al. 2014).
The potential of microalgae to support a sustainable and economically profitable bioeconomy is based on the ability of microalgae to accumulate or biotransform biogenic and xenobiotic compounds when exposed to stress growth conditions using mechanisms similar to those found in bacterial metabolic routes (Prajapati et al. 2013; Markou and Nerantzis 2013). For instance, lipid accumulation occurs under nutrient deprivation conditions (Chu et al. 2013), salinity (Takagi et al. 2006), or heavy metal stress (Liu et al. 2008). Likewise, nutrient stress cultivation is used to enhance microalgal carbohydrate content (Kim et al. 2014). Recent studies have shown that certain cyanobacteria are capable of producing polyhydroxyalkanoates under extended dark-light periods or nutrient deprivation conditions similarly to polyphosphate accumulating bacteria (PAO) (Panda and Mallick 2007). In this context, Sharma and Mallick (2005) reported polyhydroxybutyrate (PHB) accumulation in Nostoc muscorum of 14, 20, and 35 % (w/w) when supplemented with 0.2 % acetate (585 g/m3 C-acetate) and incubated under dark conditions for 3, 5, and 7 days, respectively. All these findings suggest that bacterial and algal metabolic pathways potentially involve similar precursors and enzymes (Subashchandrabose et al. 2011), which indicates that microalgal biotechnology harbors an industrial and environmental potential higher than that currently exploited. Thus, the elucidation of the influence of stress cultivation conditions on carbon and nutrient uptake, and biomass composition of microalgae and microalgae-bacteria consortia, is of key relevance in the optimization of any waste-to-value approach for microalgae mass cultivation and constitutes one of the most relevant knowledge gaps in this field. However, the lack of systematic empirical studies quantitatively evaluating the metabolism of microalgae and microalgae-bacteria consortia under stress conditions still hampers the exploitation of the full potential of these microorganisms. Therefore, the synergistic effects derived from the symbiosis between microalgae and bacteria in terms of metabolic plasticity and robustness represents a key niche for research in microalgal biotechnology.
This work constitutes a fundamental study using a carbon, nitrogen, and phosphorous mass balance approach of the mixotrophic metabolism under stress conditions of an axenic culture of Chlorella sorokiniana and a microalgal-bacterial consortium. Hence, the influence of repeated extended dark-light periods and nutrient deprivation on microbial growth, PHB and phosphorous accumulation, macroscopic biomass composition, and removal efficiencies of organic and inorganic carbon, nitrogen, and phosphorus was assessed.
Materials and methods
Microorganisms and inoculum cultivation conditions
The microalga C. sorokiniana 211/8k was obtained from the Culture Collection of Algae (SAG) of Göttingen University (Germany). The algal-bacterial consortium was obtained from a high-rate algal pond (HRAP) treating diluted centrates at the Department of Chemical Engineering and Environmental Technology of Valladolid University (Spain). This consortium was harvested from the HRAP broth by centrifugation for 10 min at 15,317×g (Sorvall, LEGEND RT+ centrifuge, Thermo Scientific) and resuspended in Sorokin-Krauss mineral salt medium (SK MSM). The microalgae/cyanobacteria population (from now on referred to as microalgae) was composed of (percentage of cells) Limnothrix mirabilis (Böcher) Anagnostidis (57.1 %), Woronichinia sp. (15.9 %), Synechocystis aquatilis Sauvageau (12.7 %), Geitlerinema sp. (11.4 %) and Cyanosarcina sp. (2.9 %). The composition of the bacterial population was not analyzed quantitatively by molecular tools but qualitatively assessed by microscopic observations, which confirmed the presence of bacteria in the algal-bacterial consortium used. C. sorokiniana and the microalgal-bacterial inocula were incubated in enriched SK MSM at 30 °C under magnetic agitation at 300 rpm and a photosynthetic active radiation (PAR) of 100 ± 11 μE/m2 · s for 5 days. The SK medium was prepared according to Alcántara et al. (2013) and enriched with sterile solutions of glucose, CH3COONa, peptone, and yeast extract to a final concentration of 1.25, 1.71, 0.0625, and 0.0625 g/dm3, respectively.
Mixotrophic cultivation under extended dark-light periods
The first series of experiments consisted of cycles of extended dark stages (DS) under anaerobic conditions followed by illuminated stages (LS) at 100 ± 11 μE/m2 · s. The duration of the dark stages in C. sorokiniana cultures was fixed at 7 days, while the illuminated stages lasted for 8, 14, and 30 days. On the other hand, the dark stages lasted for 7, 5, and 2.5 days in the tests conducted with the algal-bacterial consortium, while the duration of the subsequent illuminated stages was set at 14 days (Table S1 and Table S2 in “Supplementary Material”). The experiments were performed batchwise in 2.1 dm3 glass bottles (five bottles for C. sorokiniana under sterile conditions and five bottles for the algal-bacterial consortium) containing 1.9 dm3 of sterile modified BG-11 mineral salt medium (BG-11 MSM). This medium was composed of (per dm3 of distilled water) the following: 0.1909 g NH4Cl, 0.04 g K2HPO4, 0.075 g MgSO4 · 7H2O, 0.036 g CaCl2 · 2H2O, 0.006 g citric acid, 0.006 g ferric ammonium citrate, 0.001 g Na2EDTA, 0.02 g Na2CO3, and 1 cm3 of a trace element solution containing (per dm3 of distilled water) 2.86 g H3BO3, 1.81 g MnCl2 · 4H2O, 0.22 g ZnSO4 · 7H2O, 0.08 g CuSO4 · 5H2O, 0.39 g Na2MoO4 · 2H2O, and 0.0404 g CoCl2 · 6H2O. The final pH of the medium was ≈7.2. The BG-11 MSM was supplemented with 0.375 g glucose/dm3 and 0.513 g CH3COONa/dm3 as organic carbon source at the beginning of each dark period, which resulted in an initial total organic carbon (TOC) concentration of 300 g/m3 (150 g/m3 as C-CH3COONa and 150 g/m3 as C-glucose) (typical TOC concentrations in domestic wastewaters according to Asano et al. 2002). The initial nitrogen and phosphorus concentrations (50 g N-NH4 +/m3 and 7 g P-PO4 −3/m3, respectively) also mimicked the typical N and P concentrations in medium-strength domestic wastewater (Asano et al. 2002). In addition, C. sorokiniana and the algal-bacterial cultures were supplemented at the beginning of every dark stage with a sterile buffer solution of NaHCO3 and NaCO3 in order to increase the pH up to 9.0 ± 0.3 to prevent acidification during the dark stage. The different buffer capacity observed in C. sorokiniana and the algal-bacterial broths resulted in average inorganic carbon (IC) concentrations of 105 ± 2 and 147 ± 17 g/m3 at the beginning of each dark stage, respectively. These IC concentrations also corresponded to typical IC concentrations in domestic wastewater (≈100–150 g/m3) (Asano et al. 2002). The term TIC represents here the total inorganic carbon in the system (gas C-CO2 + dissolved IC). The bottles were always flushed with sterile nitrogen (N2) for 15 min at the beginning of every cycle and allowed to equilibrate for 2 h prior to sampling (the renewal of the bottle’s headspace was performed in a sterile bench by filtering the N2 through 0.20-μm nylon filters previously autoclaved to maintain the sterility in C. sorokiniana cultures). The cultures were incubated at 30 °C under continuous magnetic agitation at 300 rpm.
Gas samples of 100 μL were drawn from the headspace of the bottles to measure the concentrations of CO2, O2, N2, and CH4 by GC-TCD. Liquid samples of 200 cm3 were also drawn (under sterile conditions using sterile plastic syringes in an sterile bench in C. sorokiniana cultures) at the beginning and end of each dark and light stage in order to determine the concentrations of dissolved TOC, dissolved IC, dissolved N species (TN, N-NH4 +, N-NO2 −, N-NO3 −, and Norganic), dissolved P (P-PO4 −3), and biomass concentration as total (TSS) and volatile (VSS) suspended solid concentration. The concentration of acetic, propionic, isobutyric, butyric, isovaleric, valeric, isocaproic, hexanoic, and heptanoic acids were quantified by GC-FID. The term VFA* stands here for the total carbon concentration of volatile fatty acids (C-VFA) except acetic acid. The liquid volume extracted was replaced by fresh BG-11 MSM (previously autoclaved for 20 min at 120 °C in C. sorokiniana cultures) before the beginning of each DS, in order to maintain the initial cultivation volume (1.9 dm3). Likewise, the pH and TOC, N, and P concentrations were also adjusted at the beginning of each DS at a pH of 9, 300 g/m3 of TOC, 50 g/m3 of N-NH4 +, and 7 g/m3 of P-PO4 −3. The C, N, and P contents of the algal and algal-bacterial biomass formed were also experimentally determined along with the PHB and lipid content. The protein and carbohydrate contents in the biomass were also determined.
Mixotrophic growth under nutrient deprivation conditions
A second series of experiments using C. sorokiniana and the microalgal-bacterial consortium was conducted in the presence of continuous irradiation (PAR of 100 ± 11 μE/m2 · s) and initial C-CH3COONa and C-glucose concentrations of 150 g C/m3 (initial TOC concentration of 300 g/m3) under N and P deprivation, where K2HPO4 was replaced by an equimolar concentration of KCl in the BG-11 MSM. The bottles were also initially flushed with sterile N2 for 15 min to remove the O2 from the headspace and allowed to equilibrate for 2 h prior to sampling. The test was monitored for 12 days as above-described sampling every 3 days until the N and P contents in the biomass remained constant (Table S3 in “Supplementary Material”).
Mass balance calculation
A mass balance calculation was conducted for C, N, and P considering all their chemical species at the beginning and end of each dark and light stage. The validity of the experimentation carried out was assessed by means of recovery factors defined as follows (Alcántara et al. 2013):
where C-CO2 is the carbon concentration as gas CO2 in the bottles’ headspace, TOC is the total dissolved organic carbon concentration in the aqueous phase (C-CH3COONa + C-glucose + Corganic), IC is the dissolved inorganic carbon concentration in the aqueous phase in equilibrium with C-CO2, Cbiomass is the particulate carbon concentration in the form of microalgal or microalgal-bacterial biomass, Corganic is the dissolved organic carbon concentration from the particulate carbon hydrolyzed, and N-NH4 +, N-NO2 −, and N-NO3 − represent the concentration of ammonium, nitrite, and nitrate, respectively, while Nbiomass and Norganic account for the concentration of particulate organic nitrogen in the biomass and dissolved organic nitrogen from the particulate nitrogen hydrolyzed. P-PO4 −3 is the phosphorus concentration in the aqueous phase, and Pbiomass is the particulate phosphorus concentration in the form of biomass.
Analytical procedures
The irradiation was measured as PAR using a LI-250A light meter (LI-COR Biosciences, Germany). The pressure of the bottles’ headspace was measured using a PN 5007 pressure sensor (IFM, Germany). The gas concentrations of CO2, CH4, O2, and N2 were determined using a CP-3800 gas chromatograph (Varian, USA) coupled with a thermal conductivity detector and equipped with a CP-Molsieve 5A (15 m × 0.53 mm × 15 μm) and a CP-Pora BOND Q (25 m × 0.53 mm × 15 μm) columns. The injector, detector, and oven temperatures were maintained at 150, 175, and 40 °C, respectively. Helium was used as the carrier gas at 13.7 cm3/min. TOC, IC, and TN concentrations were determined using a TOC-V CSH analyzer equipped with a TNM-1 module (Shimadzu, Japan). N-NO3 −, N-NO2 −, and P-PO4 −3 were analyzed by HPLC-IC according to Alcántara et al. (2013). The soluble P concentration was also determined according to Eaton et al. (2005) using a spectrophotometer U-2000 (Hitachi, Japan). A Crison micropH 2002 (Crison instruments, Spain) was used for pH determination. Aliquots of 10 cm3 of cultivation broth were filtered through 0.22 μm and acidified with H2SO4 to pH 2 prior to volatile fatty acid analysis in an Agilent 7820A GC-FID equipped with a G4513A autosampler and a Chromosorb WAW packed column (2 m × 1/8″ × 2.1 mm SS) (10 % SP 1000, 1 % H3PO4, WAW 100/120) (Teknokroma, Spain). The injector, oven, and detector temperatures were 375, 130, and 350 °C, respectively. N2 was used as the carrier gas at 45 cm3/min. The concentration of C-glucose was determined as the difference between the TOC and the sum of C-VFA and Corganic. The determination of the TSS and VSS concentrations of microalgal and microalgal-bacterial biomass was performed according to Eaton et al. (2005). The analysis of Cbiomass and Nbiomass was conducted using a LECO CHNS-932, while P biomass was measured using a 725-ICP Optical Emission Spectrophotometer (Agilent, USA) at 213.62 nm. The concentration of Corganic and Norganic released into the liquid phase was determined based on the percentage of structural C and N in the biomass and the decrease in TSS concentration in the dark stages. The identification, quantification, and biometry measurements of microalgae were carried out by microscopic examination (OLYMPUS IX70, USA) of microalgal samples (fixed with lugol acid at 5 % and stored at 4 °C prior to analysis) according to Sournia (1978). The determination of PHB was carried according to Zúñiga et al. (2011) using chloroform as extraction solvent in an Agilent 6890N GC-MS equipped with a DB-WAX column (30 m × 0.250 mm × 0.25 μm) (J&W Scientific®, USA). The injector temperature was set at 250 °C. The oven temperature was initially maintained at 40 °C for 5 min, increased at 10 °C/min up to 200 °C and finally increased at 5 °C/min up to 240 °C (maintained for 2 min). Total lipid content in the biomass was quantified gravimetrically according to Gómez et al. (2013). Protein content in the biomass was estimated using a nitrogen to protein conversion factor of 4.44 (González et al. 2010). The carbohydrate content was estimated from the difference between the total biomass concentration and its content of lipids, proteins, and ashes.
Results
The results obtained, given as the average ± the error at 95 % confidence interval (n = 5), were summarized in Tables S1, S2, and S3. The C, N, and P mass balances during dark cultivation showed recovery factors of 99.9 ± 1.7, 100.2 ± 2.8, and 101.1 ± 4.3 % in C. sorokiniana cultures, respectively, and 100.0 ± 0.4, 99.8 ± 0.4, and 99.8 ± 19.6 % in the algal-bacterial cultures (Tables S1 and S2). Similarly, the percentages of recovery obtained during illuminated cultivation were 105.7 ± 12.5 % for C, 100.0 ± 3.0 % for N, and 103.8 ± 16.4 % for P in C. sorokiniana cultures and 100.3 ± 0.5 % for C, 101.2 ± 4.6 % for N, and 105.7 ± 34.1 % for P in the algal-bacterial cultures (Tables S1 and S2). Finally, the C mass balance under nutrient deprivation conditions showed recovery factors of 99.8 ± 5.3 and 99.6 ± 1.7 % in C. sorokiniana and algal-bacterial cultures, respectively (Table S3).
Mixotrophic cultivation under extended dark-light periods
C. sorokiniana underwent a partial hydrolysis during the DS with a decrease in biomass concentration (as TSS) of 7.6 ± 0.8, 15 ± 6 and 17 ± 9 % during DS I, DS II, and DS III, respectively. This hydrolysis entailed the built up of Corganic and Norganic in the liquid phase as a consequence of the solubilization of Cbiomass and Nbiomass (Fig. 1a and Table S1). However, biomass hydrolysis was negligible in algal-bacterial consortium and acidogenesis from glucose occur in all DS, which resulted in increasing VFA concentrations and therefore in the acidification of the cultivation broth to neutral pH (Fig. 1b and Table S2). The fraction of glucose hydrolyzed to VFAs remained constant following 7 and 5 days of dark cultivation (63 ± 2 % and a final pH of 7.2 ± 0.2), while glucose biotransformation decreased to 39 ± 6 % when the dark cultivation decreased to 2.5 days (final pH of 7.7 ± 0.3). This acidogenesis from glucose in algal-bacterial cultures increased propionic acid concentrations at decreasing durations of the dark stage, with a maximum share of 60 % of the total VFA following 2.5 days of DS (Fig. 2). Neither isocaproic, hexanoic, nor heptanoic acid was detected at the end of the different DS evaluated (Fig. 2). On the other hand, an increase in the soluble P-PO4 −3 concentration of 43 and 45 % was reported in C. sorokiniana cultures following DS I and DS II, respectively, while this value decreased to 20 % after DS III (Fig. 3a). Surprisingly, a negligible phosphorus release to the cultivation broth was recorded in the algal-bacterial consortium (Fig. 3b). The PHB (Fig. 3a), carbohydrate, protein, and lipid content in C. sorokiniana during dark cultivation remained constant at 0.1 ± 0.0, 45 ± 2, 43 ± 2, and 0.7 ± 0.1 %, along the three cycles tested. In the algal-bacterial cultures, an increase in the PHB concentration from 0.5 ± 0.0 to 3.3 ± 0.3 % occurred during the first 7 days of DS, but this value decreased during the first illuminated period and remained constant at 0.7 ± 0.2 % along the entire experiment (Fig. 3b). The carbohydrate, protein, and lipid contents of the microalgal-bacterial biomass remained constant at 50 ± 1, 32 ± 1, and 2.5 ± 0.1 %, respectively.
The biomass stoichiometric formulas experimentally determined following the LS I, II, and III for C. sorokiniana, and the algal-bacterial consortium were CH1.73O0.32N0.17S0.005P0.007 and CH1.74O0.53N0.15S0.007P0.009, respectively. C. sorokiniana presented biomass productivities of 70 g TSS/m3 · d, 19 g TSS/m3 · d, and 16 g TSS/m3 · d during the illuminated stages I, II, and III, respectively and a complete C-acetate assimilations (161 ± 1, 150 ± 3 and 160 ± 5 g C-acetate/m3, respectively) (Fig. 1a and Table S1). Surprisingly, C. sorokiniana only assimilated 26 ± 5 % of the C-glucose during LS I (40 ± 8 g C-glucose/m3), while no glucose assimilation was recorded during LS II and III (Fig. 1a and Table S1). In the algal-bacterial cultures, both the initial C-acetate and the VFA formed from glucose biotransformation during DS were completely removed in LS I and LS II, with biomass productivities of 43 g TSS/m3 · d and 50 g TSS/m3 · d, respectively. These C eliminations corresponded with an assimilation of 191 ± 17 g C-acetate/m3 (136 ± 25 g/m3 from the initial C-acetate and 55 ± 30 g/m3 from glucose acidogenesis) and 46 ± 5 g/m3 corresponding to C-VFA* during LS I and 198 ± 42 g C-acetate/m3 (150 ± 34 g/m3 from the initial C-acetate and 48 ± 55 g/m3 from glucose acidogenesis) and 45 ± 4 g/m3 of C-VFA* during LS II (Fig. 1b and Table S2). Biomass productivity decreased to 24 g TSS/m3 · d in the illuminated stage III, which corresponded with an assimilation of 194 ± 8 g C-acetate/m3 and 17 ± 9 g/m3 of C-VFA*. C-glucose removal accounted for 0.2 ± 0.0 % (3 ± 10 g C-glucose/m3 assimilated), 68 ± 7 % (37 ± 14 g C-glucose/m3 assimilated), and 13 ± 3 % (11 ± 16 g C-glucose/m3 assimilated) in LS I, LS II, and LS III, respectively (Fig. 1b and Table S2). TIC concentrations remained roughly constant during the illuminated stages, with an average TIC assimilation of 5.1 ± 1.4 % in C. sorokiniana cultures and a TIC generation of 7.2 ± 1.7 % (as a result of an intense respiratory release of CO2) in the microalgal-bacterial broths (Fig. 1). C. sorokiniana supported N-NH4 + removals of 97 ± 1, 59 ± 12, and 67 ± 13 % during the illuminated stages I, II, and III, respectively. Likewise, N-NH4 + removals of 97 ± 7, 100 ± 2, and 55 ± 8 % were recorded in the algal-bacterial cultures during LS I, LS II, and LS III. Neither NO2 − nor NO3 − was produced by microalgae or by the algal-bacterial cultures (Tables S1 and S2). On the other hand, P-PO4 −3 removal efficiencies of 81 ± 21, 32 ± 16, and 29 ± 18 % were recorded in C. sorokiniana cultures in LS I, LS II, and LS III. Likewise, the algal-bacterial consortia supported P-PO4 −3 removals of 84 ± 18, 79 ± 18, and 42 ± 23 % in LS I, LS II, and LS III, respectively (Fig. 3).
Mixotrophic growth under nutrient deprivation conditions
Surprisingly, C. sorokiniana presented an average biomass productivity of 134 g TSS/m3 · d after 3 days of mixotrophic cultivation in the absence of N and P, while this productivity decreased sharply to 14 g TSS/m3 · d by day 6 and to 9 g TSS/m3 · d by day 12. Likewise, an average biomass productivity of 185 g TSS/m3 · d was recorded in the algal-bacterial cultures after 3 days of cultivation, which gradually decreased to 8 g TSS/m3 · d by day 12. A total C-acetate assimilation of 86 ± 19 % (corresponding to 130 ± 21 g C-acetate/m3) was observed during C. sorokiniana cultivation, with removals of 43 ± 15, 4.7 ± 0.9, 19 ± 8, and 20 ± 8 % by day 3, 6, 9, and 12, respectively (Fig. 4a and Table S3). On the other hand, the algal-bacterial consortia presented a total C-acetate removal of 100 % (150 ± 6 g C/m3), with an assimilation of 91 ± 7 % within the first 3 days (Fig. 4b and Table S3). Microalgae cultures assimilated 83 ± 14 % of the C-glucose available (141 ± 19 g C/m3) within the first 3 days, and surprisingly glucose assimilation ceased afterwards (Fig. 4a and Table S3). Similarly, 93 ± 8 % of the initial C-glucose (146 ± 9 g C/m3) was assimilated by the algal-bacterial cultures, with a removal of 70 ± 9 % within the first 3 days of cultivation (Fig. 4b and Table S3). C. sorokiniana cultures underwent an increase in TIC from 7 ± 1 to 11 ± 1 % within the first 3 days likely associated with the aerobic biodegradation of C-acetate and C-glucose. Afterwards, TIC concentration remained roughly constant during the entire cultivation (Fig. 4a and Table S3). In contrast, the algal-bacterial cultures showed a gradual TIC assimilation from 13 ± 2 to 3 ± 0 % (Fig. 4b and Table S3).
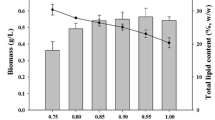
The carbohydrate content in C. sorokiniana increased from 48 ± 1 to 72 ± 1 %. This increase was concomitant with a decrease in the protein content from 40 ± 1 to 16 ± 1 %, while lipid and PHB contents remained constant at 0.8 ± 0.1 and 0.4 ± 0.0 %, respectively (Fig. 5a). Similarly, the carbohydrate content in the algal-bacterial consortium increased from 49 ± 3 to 65 ± 2 %, along with a severe decrease in the protein content from 33 ± 3 to 18 ± 2 %. The lipid content remained constant at 2.3 ± 0.3 %, while PHB concentration initially increased from 2.9 ± 0.2 to 3.9 ± 0.1 % within the first 3 days under nutrient limitation but gradually decreased to 1.1 ± 0.2 % (Fig. 5b).
Discussion
Carbon assimilation
The recovery factors of ≈100 % in all C mass balances validated both the analytical and instrumental methods used. C. sorokiniana clearly showed a higher affinity for acetate than for glucose or IC as a carbon source in both series of experiments, which suggest that C-acetate assimilation resulted more energetically favorable. It seems that as long as the level of acetate remains low (≤4000 g/m3), some microalgae can use it as their preferential carbon source. This is important because acetate is a readily available and inexpensive substrate derived from many industrial applications and its use does not entail limitations during microalgae cultivation (Pérez-García et al. 2011a, b). In addition, the high pH recorded at the end of every LS (9.5 ± 1.4 in C. sorokiniana and 10 ± 0.7 in algal-bacterial cultures) as a result of photosynthetic activity, mediated an IC distribution mainly shifted towards bicarbonate and carbonate, which constitute IC species not readily available for C. sorokiniana growth (De Godos et al. 2010; Alcántara et al. 2013). Glucose assimilation by C. sorokiniana occurred only during LS I and resulted in a 26 ± 5 % glucose removal, which was in agreement with the limited glucose assimilation (28 ± 2 %) reported by Alcántara et al. (2013) during the mixotrophic cultivation of C. sorokiniana in a minimum MSM at initial N-NH4 + and P-PO4 −3 concentrations of 95 ± 1 and 13 ± 1 g/m3, respectively. Although it is generally accepted that glucose can serve as a carbon source for the growth of certain microalgae, the effect of glucose on microalgae metabolism is species specific and influenced by the impinging irradiation (Kamiya and Kowallik 1987; Kamiya and Saitoh 2002). Interestingly, 83 ± 14 % of C-glucose was assimilated by C. sorokiniana during the first 3 days of mixotrophic cultivation under nutrient starvation, which suggests that nutrient deprivation boosted both C-acetate and C-glucose assimilations. This additional TOC assimilation resulted in an increase of the dissolved IC within the first 3 days due to combined effect of an intensive respiratory release of CO2 and a gradual increase in the pH of the cultivation broth (Fig. 4a and Table S3). These results confirm that microorganisms exhibiting a dual photoautotrophic and heterotrophic metabolism can shift their nutritional mode based on substrate and light availability (Abreu et al. 2012). Hence, when simple carbohydrates or organic acids are present in the medium, microalgae and cyanobacteria likely shift their metabolism from an autotrophic to a mixotrophic nutrition mode to save energy (Venkata Mohan et al. 2014). The hydrolysis experienced by C. sorokiniana during the DS resulted in a decrease of TSS and consequently in increases in the concentration of dissolved Corganic and Norganic in the cultivation broth that were not further assimilated into biomass during the subsequent LS (Fig. 1a and Table S1). Based on the negligible hydrolysis of the algal-bacterial culture and its higher biomass productivity during LS II and III, the results suggest that C. sorokiniana was more sensitive to the absence of energy supply during the extended dark stages.
The results obtained in the cultivation of the algal-bacterial consortium suggested that the hydrolysis of glucose into VFAs was mediated by bacterial metabolism. The fact that the propionate to acetate ratio increased at decreasing duration of the dark stage indicated that glucose was firstly converted into propionic acid and finally into acetic acid. Thus, the ability of microalgal-bacterial consortia to hydrolyze and biotransform complex carbohydrates into simple organic molecules confirm the potential of these symbiotic consortia for wastewater treatment (He et al. 2013). The algal-bacterial biomass productivities and the negligible IC assimilation recorded also suggest that the extent of acidogenesis from glucose boosted TOC removal and consequently the superior algal-bacterial growth during the illuminated stages (Fig. 1b and Table S2). Nevertheless, the assimilation of the glucose not hydrolyzed was negligible. Similarly to C. sorokiniana cultures, nutrient starvation triggered C-glucose assimilation by the algal-bacterial consortium, which assimilated 93 ± 8 % of the initial C-glucose (Fig. 4b and Table S3). Surprisingly, the 185 g TSS/m3 · d algal-bacterial productivity recorded during the first 3 days of cultivation in the absence of nutrients was higher than C. sorokiniana productivity (134 g TSS/m3 · d). This higher productivity was associated to the simultaneous occurrence of photoautotrophic microalgae growth, which entailed an assimilation of total IC of 78 ± 7 % (165 ± 14 mg of TIC consumed) during experiment II (Fig. 4b and Table S3).
Nitrogen and phosphorus removal
The recovery factors of ≈100 % in N and P mass balances also validated both the analytical and instrumental methods used during the evaluation of the fate of these nutrients. The absence of both N losses by stripping (closed bottles) and nitrification in both C. sorokiniana and algal-bacterial cultures confirm that the main mechanism for N-NH4 + removal was assimilation into biomass (as Nbiomass) (Table S1 and Table S2). N-NH4 + removals of 97 ± 1, 59 ± 12, and 67 ± 13 % and P-PO4 −3 removals of 81 ± 7, 32 ± 4, and 29 ± 6 % were recorded in C. sorokiniana cultures during the illuminated periods I, II, and III, respectively (Table S1). Based on the fact that the microalgal N content experimentally determined in C. sorokiniana remained constant at 9.7 ± 0.3 %, the decrease in N-NH4 + removal during LS II and LS III points out to a deterioration in C. sorokiniana metabolism mediated by the prolonged absence of light during DS II and III (Table S1). On the other hand, the release of P-PO4 −3 by C. sorokiniana during the dark stages together with the decrease in P-PO4 −3 assimilation during LS II and III induced a progressive decline in microalgal Pbiomass. Hence, the P content of C. sorokiniana decreased from 1.5 ± 0.2 % in LS I to 0.9 ± 0.0 and 0.6 ± 0.2 % by the end of LS II and LS III, respectively. These results confirmed that microalgae, similarly to PAOs (De-Bashan and Bashan 2004; Bajekal and Dharmadhikari 2008; Mesquita et al. 2013), can release P under anaerobic conditions in the absence of light, but P assimilation in the subsequent illuminated stages did not occur in a similar extent (e.g., structural P decreased from 1.9 to 0.8 % during DS I and increased again to 1.5 % at the end of LS I). Luxury P uptake in microalgae is influenced by the dissolved phosphate concentration, light intensity, and temperature during microalgae cultivation (Cade-Menun and Paytan 2010; Fanta et al. 2010) and might explain the highly variable phosphorus removal often reported in microalgae-based wastewater treatment. Indeed, the amount of Pbiomass is the result of the combined effects of a luxury uptake and a growth-associated P uptake (Powell et al. 2008, 2009). In this regard, the deterioration in the activity of C. sorokiniana caused by the cultivation under prolonged dark periods likely promote the preferential uptake of P-PO4 −3 for growth during LS periods.
N-NH4 + removals in the microalgal-bacterial cultures of 97 ± 7, 100 ± 2, and 55 ± 8 % and P-PO4 −3 removals of 84 ± 7, 79 ± 11, and 42 ± 6 % were recorded in the illuminated stages I, II, and III, respectively (Table S2). Despite similar N-NH4 + concentrations were initially present at the beginning of each cycle in both cultures, the algal-bacterial biomass exhibited lower Nbiomass contents than microalgae (7.3 ± 0.2 % regardless of the operational stage). The absence of phosphorus release to the cultivation medium during the dark period along with the steady P-PO4 −3 removal rates during the illuminated stages can explain the constant Pbiomass content in the algal-bacterial biomass (1.1 ± 0.1, 0.9 ± 0.2, and 0.9 ± 0.1 % at the end of LS I, LS II, and LS III, respectively). Finally, it must be stressed that both N-NH4 + and P-PO4 −3 removals surprisingly decreased at decreasing duration of the dark stages, which suggested that N and P assimilation in algal-bacterial cultures was influenced by the availability of VFAs, which itself was influenced by the duration of the dark stages.
Macroscopic biomass composition
While no changes were observed in the carbohydrate, lipid, and protein contents in the test series performed using extended dark-light cycles, nutrient starvation resulted in a steady increase in carbohydrate content in both C. sorokiniana and microalgal-bacterial cultures. In this context, phosphorus-starved microalgae and cyanobacteria can experience an increased intracellular storage of carbohydrates, the extent of this accumulation being strain specific (González-Fernández and Ballesteros 2012). In our particular case, the carbohydrate/protein ratio in C. sorokiniana under N and P starvation increased from 1.2 to 2.7 within the first 3 days and up to 4.5 by day 12, which corresponded with an initial and final carbohydrate content of 48 ± 1 and 72 ± 1 %, respectively (Fig. 5a). These results were in agreement with the increase from 0.15 to 3.7 reported by Dean et al. (2008) in the carbohydrate/protein ratio during Chlamydomonas reinhardtii cultivation under P deficient conditions, while a lower increase in the carbohydrate/protein ratio from 0.4 to 1.0 was observed by Sigee et al. (2007) in the cultivation of P-starved Scenedesmus subspicatus. A slightly lower carbohydrate accumulation from 49 ± 3 to 65 ± 2 % was obtained in the algal-bacterial cultures (Fig. 5b), where the carbohydrate/protein ratio increased from 1.5 to 2.7 during the first 3 days and up to 3.7 by day 12. A negligible lipid accumulation concomitant with a decrease in the protein content was observed in both C. sorokiniana and algal-bacterial cultures under N and P deprivation (Fig. 5). While the fact that nitrogen starvation induces an increase in the biomass lipid content has been consistently proven (Li et al. 2012; Simionato et al. 2013), the presence of P plays a key role on lipid productivity under nitrogen deficient conditions in both microalgae (Feng et al. 2012; Chu et al. 2013) and bacteria (Harold 1966; Kulaev et al. 1999). For instance, Chu et al. (2013) reported that the P assimilated under nitrogen deficiency was utilized by the algal cells to metabolically synthesize enzymes for the lipid synthesis. Thus, the absence of phosphorous under nitrogen starvation in our cultivation medium was likely a suppression factor for lipid accumulation and promoted instead carbohydrates accumulation (Chu et al. 2013).
Cultivation under extended dark-light periods did not boost PHB accumulation neither in C. sorokiniana nor in the algal-bacterial consortium. In our particular study, PHB accumulation in C. sorokiniana cultures was negligible despite the release of P during the dark stages (which was hypothesized to be associated with the supply of the microbial energy demand in the absence of light) (Fig. 3a). On the other hand, a maximum accumulation of PHB of 3.3 ± 0.3 % was observed at the end of the first 7 days of dark stage in the algal-bacterial cultures, which in fact was not associated with a P-PO4 −3 release to the cultivation medium (Fig. 3b). PHB content decreased to 0.9 ± 0.2 % during the first illuminated stage and remained roughly constant afterwards.
In the absence of N and P, C. sorokiniana possessed a constant PHB content of 0.4 ± 0.0 % along the 12 days of cultivation (Fig. 5a), which was in agreement with the 0.7 % PHB content reported by De Philippis et al. (1992) during photoautotrophic cultivation of Spirulina maxima under N starvation. On the other hand, the PHB concentration in the microalgal-bacterial biomass increased during the first 3 days from 2.9 ± 0.2 to 3.9 ± 0.1 % but gradually decreased to 1.1 ± 0.2 % afterwards (Fig. 5b). The results here obtained suggest that despite the ability of some cyanobacteria to accumulate significant amounts of PHB during mixotrophic cultivation under extended dark periods or nutrient deprivation (Sharma and Mallick 2005; Panda and Mallick 2007), PHB accumulation in C. sorokiniana and the algal-bacterial consortium here tested was not induced under N and P limiting conditions.
In brief, the ability of microalgal-bacterial consortia to hydrolyze and biotransform glucose into simple organic molecules under extended dark periods confirmed the potential of these symbiotic consortia for wastewater treatment. N and P assimilation in algal-bacterial cultures during the illuminated periods was influenced by the carbon available as VFAs, which itself was a function of the duration of the dark stages. C-VFA (initial C-acetate + C-VFA from glucose acidogenesis in the algal-bacterial cultures) was the preferred carbon source in C. sorokiniana and in the algal-bacterial consortium based on the low-glucose and inorganic carbon assimilations recorded. Hence, in the presence of simple carbohydrates or organic acids, microalgae and cyanobacteria can shift their metabolism from an autotrophic to a mixotrophic nutrition mode to save energy. Neither PHB nor lipid accumulation was induced in C. sorokiniana or in the algal-bacterial consortium under extended dark-light periods or N and P deprivation. Surprisingly, nutrient deprivation boosted an efficient C-acetate and C-glucose assimilation and resulted in a steady increase in carbohydrate content concomitant with a decrease in protein concentration in C. sorokiniana and microalgal-bacterial cultures. This work provided new insights on the potential of indigenous microalgae-bacteria symbiotic consortia as a platform technology to avoid the high cost and technical limitations associated with the axenic cultivation of microalgae in order to consolidate an industrial scale microalgae-to-biofuel technology based on wastewater treatment.
References
Abreu AP, Fernandes B, Vicente AA, Teixeira J, Dragone G (2012) Mixotrophic cultivation of Chlorella vulgaris using industrial dairy waste as organic carbon source. Bioresour Technol 118:61–66
Alcántara C, García-Encina PA, Muñoz R (2013) Evaluation of mass and energy balances in the integrated microalgae growth-anaerobic digestion process. Chem Eng J 221:238–246
Arbib Z, Ruiz J, Álvarez-Díaz P, Garrido-Pérez C, Perales JA (2014) Capability of different microalgae species for phytoremediation processes: wastewater tertiary treatment, CO2 bio-fixation and low cost biofuels production. Water Res 49:465–474
Asano T, Burton FL, Leverenz HL, Tsuchihashi R, Tchobanoglous G (2002) Metcalf and Eddy, wastewater engineering: treatment and reuse, 4th edn. McGraw-Hill, New York
Bajekal SS, Dharmadhikari NS (2008) Use of polyphosphate accumulating organisms (PAO) for treatment of phosphate sludge. The 12th World Lake Conference: 918–922
Cade-Menun BJ, Paytan A (2010) Nutrient temperature and light stress alter phosphorus and carbon forms in culture-grown algae. Mar Chem 121:27–36
Cea-Barcía G, Buitrón G, Moreno G, Kumar G (2014) A cost-effective strategy for the bio-prospecting of mixed microalgae with high carbohydrate content: diversity fluctuations in different growth media. Bioresour Technol 163:370–373
Chu FF, Chu PN, Cai PJ, Li WW, Lam PKS, Zeng RJ (2013) Phosphorus plays an important role in enhancing biodiesel productivity of Chlorella vulgaris under nitrogen deficiency. Bioresour Technol 134:341–346
De Godos I, Vargas VA, Blanco S, García-González MC, Soto R, García-Encina PA, Becares E, Muñoz R (2010) A comparative evaluation of microalgae for the degradation of piggery wastewater under photosynthetic oxygenation. Bioresour Technol 101:5150–5158
De Philippis R, Ena A, Guastiini M, Sili C, Vincenzini M (1992) Factors affecting poly-β-hydroxybutyrate accumulation in cyanobacteria and in purple non-sulfur bacteria. FEMS Microbiol Lett 103(2–4):187–194
Dean AP, Nicholson JM, Sigee DC (2008) Impact of phosphorus quota and growth phase on carbon allocation in Chlamydomonas reinhardtii: an FTIR microspectroscopy study. Eur J Phycol 43(4):345–354
De-Bashan LE, Bashan Y (2004) Recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997–2003). Water Res 38(19):4222–4246
Eaton AD, Clesceri LS, Greenberg AE (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association/American Water Works Association/Water Environment Federation, Washington DC, USA
Fanta SE, Hill WR, Smith TB, Roberts BJ (2010) Applying the light: nutrient hypothesis to stream periphyton. Freshw Biol 55:931–940
Feng P, Deng Z, Fan L, Hu Z (2012) Lipid accumulation and growth characteristics of Chlorella zofingiensis under different nitrate and phosphate concentrations. J Biosci Bioeng 114(4):405–410
Gómez C, Escudero R, Morales MM, Figueroa FL, Fernández-Sevilla JM, Acién FG (2013) Use of secondary-treated wastewater for the production of Muriellopsis sp. Appl Microbiol Biotechnol 97(5):2239–2249
González CV, Cerón Mdel C, Acien FG, Segovia CS, Chisti Y, Fernández JM (2010) Protein measurements of microalgal and cyanobacterial biomass. Bioresour Technol 101:7587–7591
González-Fernández C, Ballesteros M (2012) Linking microalgae and cyanobacteria culture conditions and key-enzymes for carbohydrate accumulation. Biotechnol Adv 30(6):1655–1661
Guieysse B, Plouviez M, Coilhac M, Cazali L (2013) Nitrous oxide (N2O) production in axenic Chlorella vulgaris microalgae cultures: evidence, putative pathways, and potential environmental impacts. Biogeosciences 10(10):6737–6746
Harold FM (1966) Inorganic polyphosphates in biology: structure, metabolism, and function. Bacteriol Rev 30(4):772–794
He PJ, Mao B, Lü F, Shao LM, Lee DJ, Chang JS (2013) The combined effect of bacteria and Chlorella vulgaris on the treatment of municipal wastewaters. Bioresour Technol 146:562–568
Kamiya A, Kowallik W (1987) Photoinhibition of glucose uptake in Chlorella. Plant Cell Physiol 28:611–619
Kamiya A, Saitoh T (2002) Blue-light-control of the uptake of amino acids and of ammonia in Chlorella mutants. Physiol Plant 116:248–254
Kessler E, Oesterheld H (1970) Nitrification and induction of nitrate reductase in nitrogen deficient algae. Nature 228:287–288
Kim KH, Choi IS, Kim HM, Wi SG, Bae HJ (2014) Bioethanol production from the nutrient stress-induced microalga Chlorella vulgaris by enzymatic hydrolysis and immobilized yeast fermentation. Bioresour Technol 153:47–54
Kulaev I, Vagabov V, Kulakovskaya T (1999) New aspects of inorganic polyphosphate metabolism and function. J Biosci Bioeng 88(2):111–129
Li Y, Fei X, Deng X (2012) Novel molecular insights into nitrogen starvation induced triacylglycerols accumulation revealed by differential gene expression analysis in green algae Micractinium pusillum. Biomass Bioenergy 42:199–211
Liu ZY, Wang GC, Zhou BC (2008) Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour Technol 99(11):4717–4722
Markou G, Nerantzis E (2013) Microalgae for high-value compounds and biofuels production: a review with focus on cultivation under stress conditions. Biotechnol Adv 31(8):1532–1542
Mesquita DP, Leal C, Cunha JR, Oehmen A, Amaral AL, Reis MA, Ferreira EC (2013) Prediction of intracellular storage polymers using quantitative image analysis in enhanced biological phosphorus removal systems. Anal Chim Acta 770:36–44
Muñoz R, Guieysse B (2006) Algal–bacterial processes for the treatment of hazardous contaminants: a review. Water Res 40(15):2799–2815
Panda B, Mallick N (2007) Enhanced poly-β-hydroxybutyrate accumulation in a unicellular cyanobacterium, Synechocystis sp. PCC 6803. Lett Appl Microbiol 44(2):194–198
Pérez-García O, de-Bashan LE, Hernández JP, Bashan Y (2010) Efficiency of growth and nutrient uptake from wastewater by heterotrophic, autotrophic, and mixotrophic cultivation of Chlorella vulgaris immobilized with Azospirillum brasilense. J Phycol 46(4):800–812
Pérez-García O, Escalante FME, de-Bashan LE, Bashan Y (2011a) Heterotrophic cultures of microalgae: metabolism and potential products. Water Res 45(1):11–36
Pérez-García RO, Bashan Y, Puente ME (2011b) Organic carbon supplementation of municipal wastewater is essential for heterotrophic growth and ammonium removing by the microalgae Chlorella vulgaris. J Phycol 47(1):190–199
Powell N, Shilton AN, Pratt S, Chisti Y (2008) Factors influencing luxury uptake of phosphorus by microalgae in waste stabilization ponds. Environ Sci Technol 42(16):5958–5962
Powell N, Shilton A, Chisti Y, Pratt S (2009) Towards a luxury uptake process via microalgae—defining the polyphosphate dynamics. Water Res 43(17):4207–4213
Prajapati SK, Kaushik P, Malik A, Vijay VK (2013) Phycoremediation coupled production of algal biomass, harvesting and anaerobic digestion: possibilities and challenges. Biotechnol Adv 31(8):1408–1425
Sharma L, Mallick N (2005) Accumulation of poly-β-hydroxybutyrate in Nostoc muscorum: regulation by pH, light–dark cycles, N and P status and carbon sources. Bioresour Technol 96(11):1304–1310
Sigee DC, Bahrami F, Estrada B, Webster RE, Dean AP (2007) The influence of phosphorus availability on carbon allocation and P quota in Scenedesmus subspicatus: a synchrotron-based FTIR analysis. Phycology 46(5):583–592
Simionato D, Block MA, La Rocca N, Jouhet J, Maréchal E, Finazzi G, Morosinotto T (2013) The response of Nannochloropsis gaditana to nitrogen starvation includes de novo biosynthesis of triacylglycerols, a decrease of chloroplast galactolipids, and reorganization of the photosynthetic apparatus. Eukaryot Cell 12(5):665–676
Sournia A (1978) Phytoplanton Manual. Museum National d’ Historie Naturelle, París. United Nations Educational. Scientific and Cultural Organization (Unesco)
Subashchandrabose SR, Ramakrishnan B, Megharaj M, Venkateswarlu K, Naidu R (2011) Consortia of cyanobacteria/microalgae and bacteria: biotechnological potential. Biotechnol Adv 29(6):896–907
Takagi M, Karseno, Yoshida T (2006) Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J Biosci Bioeng 101(3):223–226
Venkata Mohan S, Devi MP, Subhash GV, Chandra R (2014) Algae oils as fuels. Biofuels from Algae, Elsevier 8:155–187
Zúñiga C, Morales M, Le Borgne S, Revah S (2011) Production of poly-hydroxybutyrate (PHB) by Methylobacterium organophilum isolated from a methanotrophic consortium in a two-phase partition bioreactor. J Hazard Mater 190(1–3):876–882
Acknowledgments
This research was supported by the regional government of Castilla y León and the European Social Fund (Contract N° E-47-2011-0053564 and Project Ref. GR76). The financial support of the Ministry of Economy and Competitiveness and the National Institute for Agricultural Research and Technology and Food is also gratefully acknowledged (Project Ref. RTA2013-00056-C03-02).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(docx 45.1 kb)
Rights and permissions
About this article
Cite this article
Alcántara, C., Fernández, C., García-Encina, P.A. et al. Mixotrophic metabolism of Chlorella sorokiniana and algal-bacterial consortia under extended dark-light periods and nutrient starvation. Appl Microbiol Biotechnol 99, 2393–2404 (2015). https://doi.org/10.1007/s00253-014-6125-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6125-5






 ) species and PHB content (–●–) in C. sorokiniana (a) and algal-bacterial (b) cultures
) species and PHB content (–●–) in C. sorokiniana (a) and algal-bacterial (b) cultures

 ), protein (□), lipid (■), and PHB content (–●–) during the mixotrophic cultivation of C. sorokiniana (a) and the algal-bacterial consortium (b) under nutrient deprivation
), protein (□), lipid (■), and PHB content (–●–) during the mixotrophic cultivation of C. sorokiniana (a) and the algal-bacterial consortium (b) under nutrient deprivation