Abstract
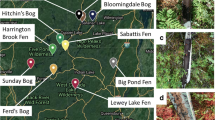
Peatlands store approximately one-half of terrestrial soil carbon and one-tenth of non-glacial freshwater. Some of these important ecosystems are located near heavy metal emitting smelters. To improve the understanding of smelter impacts and potential recovery after initial pollution controls in the 1970s (roughly 50 years of potential recovery), we sampled peatlands along a distance gradient of 134 km from a smelter in Sudbury, Ontario, Canada, an area with over a century of nickel (Ni) and copper (Cu) mining activity. This work is aimed at evaluating potential shifts in bacterial and archaeal community structures in Sphagnum moss and its underlying peat within smelter-impacted poor fens. In peat, total Ni and Cu concentrations were higher (0.062–0.067 and 0.110–0.208 mg/g, respectively) at sites close to the smelter and exponentially dropped with distance from the smelter. This exponential decrease in Ni concentrations was also observed in Sphagnum. 16S rDNA amplicon sequencing showed that peat and Sphagnum moss host distinct microbiomes with peat accommodating a more diverse community structure. The microbiomes of Sphagnum were dominated by Proteobacteria (62.5%), followed by Acidobacteria (11.9%), with no observable trends with distance from the smelter. Dominance of Acidobacteria (32.4%) and Proteobacteria (29.6%) in peat was reported across all sites. No drift in taxonomy was seen across the distance gradient or from the reference sites, suggesting a potential microbiome recovery toward that of the reference peatlands microbiomes after decades of pollution controls. These results advance the understanding of peat and Sphagnum moss microbiomes, as well as depict the sensitivities and the resilience of peatland ecosystems.





Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Data Availability
Data inquiries can be provided by the corresponding author: James Seward.
References
Tarnocai C (2006) The effect of climate change on carbon in Canadian peatlands. Glob Planet Chang 53(4). https://doi.org/10.1016/j.gloplacha.2006.03.012
Clymo RS (1984) The limits of peat bog growth. Philos Trans R Soc Lond Ser B Biol Sci 303(1117)
Erwin KL (2009) Wetlands and global climate change: the role of wetland restoration in a changing world. Wetl Ecol Manag 17(1). https://doi.org/10.1007/s11273-008-9119-1
Gorham E (1991) Northern peatlands: role in the carbon cycle and probable responses to climatic warming. Ecol Appl 1(2). https://doi.org/10.2307/19418110248.2007.01139.x
Waddington JM, Day SM (2007) Methane emissions from a peatland following restoration. Journal of Geophysical Research. Biogeosciences 112(3). https://doi.org/10.1029/2007JG000400
Juottonen H, Hynninen A, Nieminen M, Tuomivirta TT, Tuittila ES, Nousiainen H, Kell DK, Yrjälä K, Tervahauta A, Fritze H (2012) Methane-cycling microbial communities and methane emission in natural and restored peatlands. Appl Environ Microbiol 78(17). https://doi.org/10.1128/AEM.00261-12
Kitson E, Bell NGA (2020) The response of microbial communities to peatland drainage and rewetting. A review. Front Microbiol 11. https://doi.org/10.3389/fmicb.2020.582812
Lin X, Tfaily MM, Green SJ, Steinweg JM, Chanton P, Imvittaya A, Chanton JP, Cooper W, Schadt C, Kostkaa JE (2014) Microbial metabolic potential for carbon degradation and nutrient (nitrogen and phosphorus) acquisition in an ombrotrophic peatland. Appl Environ Microbiol 80(11). https://doi.org/10.1128/AEM.00206-14
Pankratov TA, Ivanova AO, Dedysh SN, Liesack W (2011) Bacterial populations and environmental factors controlling cellulose degradation in an acidic Sphagnum peat. Environ Microbiol 13(7). https://doi.org/10.1111/j.1462-2920.2011.02491.x
Dieleman CM, Branfireun BA, Mclaughlin JW, Lindo Z (2015) Climate change drives a shift in peatland ecosystem plant community: implications for ecosystem function and stability. Glob Chang Biol 21(1). https://doi.org/10.1111/gcb.12643
Yu Z, Beilman DW, Jones MC (2013) Sensitivity of northern peatland carbon dynamics to Holocene climate change. Carbon Cycling in Northern Peatlands. https://doi.org/10.1029/2008GM000822
Luke S, Preston MD, Basiliko N, Watmough SA (2015) Microbial communities, biomass, and carbon mineralization in acidic, nutrient-poor peatlands impacted by metal and acid deposition. Water Air Soil Pollut 226(2). https://doi.org/10.1007/s11270-014-2265-6
Clarke D, Rieley J (2010) Strategy for responsible peatland management. International Peat Society
Gignac LD, Beckett PJ (1986) The effect of smelting operations on peatlands near Sudbury, Ontario, Canada. Can J Bot 64(6). https://doi.org/10.1139/b86-157
Freedman B, Hutchinson TC (1980) Long-term effects of smelter pollution at Sudbury, Ontario, on forest community composition. Can J Bot 58(19). https://doi.org/10.1139/b80-245
Hutchinson TC, Whitby LM (1974) Heavy-metal pollution in the Sudbury mining and smelting region of Canada, I. Soil and vegetation contamination by nickel, copper, and other metals. Environ Conserv 1(2). https://doi.org/10.1017/S0376892900004240
Bhalerao SA, Sharma AS, Poojari AC (2015) Toxicity of nickel in plants. Int J Pure Appl Biosci 3(2):345–355
Flemming CA, Trevors JT (1989) Copper toxicity and chemistry in the environment: a review. Water Air Soil Pollut 44:143–158
Fitzpatrick CR, Mustafa Z, Viliunas J (2019) Soil microbes alter plant fitness under competition and drought. J Evol Biol 32(5). https://doi.org/10.1111/jeb.13426
Gilbert, D., & Mitchell, E. A. D. (2006). Chapter 13 Microbial diversity in Sphagnum peatlands. Developments in Earth Surface Processes, 9(C), 287–318. https://doi.org/10.1016/S0928-2025(06)09013-4
Kotsyurbenko OR, Glagolev MV, Merkel AY, Sabrekov AF, Terentieva IE (2019) Methanogenesis in soils, wetlands, and peat. Biogenesis of Hydrocarbons. https://doi.org/10.1007/978-3-319-78108-2_9
Van Der Heijden MGA, Bardgett RD, Van Straalen NM (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11(3). https://doi.org/10.1111/j.1461-0248.2007.01139.x
Bragina A, Cardinale M, Berg C, Berg G (2013) Vertical transmission explains the specific Burkholderia pattern in Sphagnum mosses at multi-geographic scale. Front Microbiol 4(DEC). https://doi.org/10.3389/fmicb.2013.00394
Bragina A, Oberauner-Wappis L, Zachow C, Halwachs B, Thallinger GG, Müller H, Berg G (2014) The Sphagnum microbiome supports bog ecosystem functioning under extreme conditions. Mol Ecol 23(18). https://doi.org/10.1111/mec.12885
Stough JMA, Kolton M, Kostka JE, Weston DJ, Pelletier DA, Wilhelm SW (2018) Diversity of active viral infections within the Sphagnum microbiome. Appl Environ Microbiol 84(23). https://doi.org/10.1128/AEM.01124-18
Souter L, Watmough SA (2016) The impact of drought and air pollution on metal profiles in peat cores. Sci Total Environ 541:1031–1040
Pucher M, Wünsch U, Weigelhofer G, Murphy K, Hein T, Graeber D (2019) StaRdom: versatile software for analyzing spectroscopic data of dissolved organic matter in R. Water 11(11). https://doi.org/10.3390/w11112366
Massicotte, P. (2019). “eemR: tools for pre-processing emission-excitation-matrix (EEM) fluorescence data.” R package version 1.1 (2019).
RStudio Team (2021) RStudio: integrated development for R. RStudio, Inc., Boston, MA
Bushnell B (2015) BBMap. https://Sourceforge.Net/Projects/Bbmap/AQ
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP (2016) DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 13(7). https://doi.org/10.1038/nmeth.3869
McLaren MR, Callahan BJ (2021) Silva 138.1 prokaryotic SSU taxonomic training data formatted for DADA2, vol 1. Zenodo
McMurdie PJ, Holmes S (2013) Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8(4). https://doi.org/10.1371/journal.pone.0061217
Newman JE et al (2023) The impact of severe pollution from smelter emissions on carbon and metal accumulation in peatlands in Ontario, Canada. Environ Pollut 320:121102
Kamal S, Varma A (2008) Peatland microbiology. Microbiology of Extreme Soils. https://doi.org/10.1007/978-3-540-74231-9_9
Kostka JE, Weston DJ, Glass JB, Lilleskov EA, Shaw AJ, Turetsky MR (2016) The Sphagnum microbiome: new insights from an ancient plant lineage. New Phytol 211(1). https://doi.org/10.1111/nph.13993
Barrett SE, Watmough SA (2015) Factors controlling peat chemistry and vegetation composition in Sudbury peatlands after 30 years of pollution emission reductions. Environ Pollut 206:122–132. https://doi.org/10.1016/J.ENVPOL.2015.06.021
Putkinen A, Larmola T, Tuomivirta T, Siljanen HMP, Bodrossy L, Tuittila ES, Fritze H (2012) Water dispersal of methanotrophic bacteria maintains functional methane oxidation in Sphagnum mosses. Front Microbiol 3(JAN). https://doi.org/10.3389/fmicb.2012.00015
Opelt K, Berg G (2004) Diversity and antagonistic potential of bacteria associated with bryophytes from nutrient-poor habitats of the baltic sea coast. Appl Environ Microbiol 70(11). https://doi.org/10.1128/AEM.70.11.6569-6579.2004
Matthies C, Erhard HP, Drake HL (1997) Effects of pH on the comparative culturability of fungi and bacteria from acidic and less acidic forest soils. J Basic Microbiol 37(5). https://doi.org/10.1002/jobm.3620370506
Rousk J, Bååth E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, Knight R, Fierer N (2010) Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J 4(10). https://doi.org/10.1038/ismej.2010.58
Seward J, Carson MA, Lamit LJ, Basiliko N, Yavitt JB, Lilleskov E, Schadt CW, Smith DS, Mclaughlin J, Mykytczuk N, Willims-Johnson S, Roulet N, Moore T, Harris L, Bräuer S (2020) Peatland microbial community composition is driven by a natural climate gradient. Microb Ecol 80(3). https://doi.org/10.1007/s00248-020-01510-z
Bear SE, Seward JD, Lamit LJ, Basiliko N, Moore T, Lilleskov E, Yavitt JB, Schadt CW, Smith DS, McLaughlin J, Siljanen H, Mykytczuk N, Williams S, Roulet N, Harris L, Carson MA, Watmough S, Bräuer SL (2021) Beyond the usual suspects: methanogenic communities in eastern North American peatlands are also influenced by nickel and copper concentrations. FEMS Microbiol Lett 368(21–24). https://doi.org/10.1093/femsle/fnab151
Kujala K et al (2018) Microbial diversity along a gradient in peatlands treating mining-affected waters. FEMS Microbiol Ecol 94(10):fiy145
Ohno T (2002) Fluorescence inner-filtering correction for determining the humification index of dissolved organic matter. Environ Sci Technol 36(4). https://doi.org/10.1021/es0155276
Garcia JL, Ollivier BE, Whitman WB (2006) The order methanomicrobiales. Prokaryotes 3:208–230
Belova SE, Ravin NV, Pankratov TA, Rakitin AL, Ivanova AA, Beletsky AV, Mardanov AV, Damsté JSS, Dedysh SN (2018) Hydrolytic capabilities as a key to environmental success: chitinolytic and cellulolytic Acidobacteria from acidic sub-arctic soils and boreal peatlands. Front Microbiol 9(NOV). https://doi.org/10.3389/fmicb.2018.02775
Pankratov TA, Serkebaeva YM, Kulichevskaya IS, Liesack W, Dedysh SN (2008) Substrate-induced growth and isolation of Acidobacteria from acidic Sphagnum peat. ISME J 2(5). https://doi.org/10.1038/ismej.2008.7
Urbanová Z, Bárta J (2016) Effects of long-term drainage on microbial community composition vary between peatland types. Soil Biol Biochem 92. https://doi.org/10.1016/j.soilbio.2015.09.017
Ward NL, Challacombe JF, Janssen PH, Henrissat B, Coutinho PM, Wu M, Xie G, Haft DH, Sait M, Badger J, Barabote RD, Bradley B, Brettin TS, Brinkac LM, Bruce D, Creasy T, Daugherty SC, Davidsen TM, DeBoy RT et al (2009) Three genomes from the phylum Acidobacteria provide insight into the lifestyles of these microorganisms in soils. Appl Environ Microbiol 75(7). https://doi.org/10.1128/AEM.02294-08
Garrity GM, Holt JG, Castenholz RW, Pierson BK, Keppen OI, Gorlenko VM (2001) Phylum BVI. Chloroflexi phy. nov. In Bergey’s manual® of systematic bacteriology. https://doi.org/10.1007/978-0-387-21609-6_23
Lee JZ, Burow LC, Woebken D, Craig Everroad R, Kubo MD, Spormann AM, Weber PK, Pett-Ridge J, Bebout BM, Hoehler TM (2014) Fermentation couples Chloroflexi and sulfate-reducing bacteria to Cyanobacteria in hypersaline microbial mats. Front Microbiol 5(FEB). https://doi.org/10.3389/fmicb.2014.00061
St. James AR, Lin J, Richardson RE (2021) Relationship between peat type and microbial ecology in Sphagnum-containing peatlands of the Adirondack Mountains, NY, USA. Microb Ecol 82(2). https://doi.org/10.1007/s00248-020-01651-1
Acknowledgements
We would like to recognize Emily Smenderova, Mikkealla Hurley, Kevin Adkinson, and Edward Kellaway for their help and contributions to this project.
Funding
Funding was provided from the Natural Sciences and Engineering Research Council (Collaborative Research and Development Grant CRDPJ/509182-2017) to Nathan Basiliko, Shaun Watmough, Peter Beckett, Erik Emilson, and others. James Seward is also grateful for international tuition fee waiver support from the Faculty of Graduate Studies at Laurentian University.
Author information
Authors and Affiliations
Contributions
James Seward, Nathan Basiliko, and Peter Beckett contributed to the study conception and design. Material preparation, data collection, and analyses were performed by James Seward, Suzanna Bräuer, Erik Emilson, Shaun Watmough, and Pascale Roy-Léveillée. The first draft of the manuscript was written by James Seward, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
N/A.
Consent to Participate
N/A.
Consent for Publication
N/A.
Conflict of Interest
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Seward, J., Bräuer, S., Beckett, P. et al. Recovery of Smelter-Impacted Peat and Sphagnum Moss: a Microbial Perspective. Microb Ecol 86, 2894–2903 (2023). https://doi.org/10.1007/s00248-023-02289-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-023-02289-5