Abstract
The short time-scale dynamics of three families of Bdellovibrio and like organisms (i.e. Bdellovibrionaceae, Peredibacteraceae, and Bacteriovoracaceae) were studied on the surface waters of Lake Geneva in summer. Using mesocosms deployed nearshore in July 2019, we simulated an extreme climatic event (an input of carbon from the watershed in response to runoff from the catchment, light reduction, and mixing in response to stormy conditions) and aimed to study the impact of both abiotic and biotic factors on their dynamics. The three families of Bdellovibrio and like organisms (BALOs) showed different dynamics during the experiment. Peredibacteraceae was the most abundant group, whereas Bacteriovoracaceae was the least abundant. Compared with the other two families, the abundance of Bdellovibrionaceae did not fluctuate, remaining relatively stable over time. Environmental variables only partially explained the dynamics of these families; in particular, temperature, pH, and chloride concentrations were positively correlated with Bacteriovoracaceae, Bdellovibrionaceae, and Peredibacteraceae abundance, respectively. Prokaryote-like particles (PLPs), such as those with high DNA content (HDNA), were strongly and positively correlated with Peredibacteraceae and Bacteriovoracaceae. In contrast, no relationships were found between Bdellovibrionaceae and PLP abundance, nor between the virus-like particles (VLPs) and the different BALOs. Overall, the experiment revealed that predation was stable in the face of the simulated climatic events. In addition, we observed that Peredibacteraceae and Bacteriovoracaceae share common traits, while Bdellovibrionaceae seems to constitute a distinct category.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among microbial predators, Bdellovibrio and like organisms (BALOs) are obligate predatory bacteria with a broad spectrum of prey that are mainly Gram-negative bacteria. BALOs have been reported to act as “population-balancers” [1] and “microbial alpha diversity drivers” [2], highlighting their potential important roles in microbial ecosystems. They are classified into two distinct polyphyletic clades, Oligoflexia and Alphaproteobacteria, and are characterised by two well-described modes of reproduction [3,4,5]. BALOs are found in many habitats such as soil and aquatic environments [5,6,7], and they have recently been discovered and studied in peri-alpine lakes [8,9,10,11]. In general, the water column is not a favourable environment for the multiplication of BALOs, which thrive better in biofilms, sediments, or closed environments such as in aquaculture [12,13,14]. Given their predation obligation and distribution in nature and diversity, BALOs may have a strong impact on the dynamics and structure of bacterial communities. However, studies related to the ecological impact of BALOs on microbial communities are scarce.
BALOs have been seldom studied in freshwater lakes. Recently, these bacteria have been shown to favour eutrophic conditions, both in terms of abundance and diversity, due to a higher number and diversity of potential prey [15]. It is noteworthy that BALOs have also been detected in less nutrient-replete lakes, such as Lake Geneva, Bourget, and Annecy, characterised by meso- to oligotrophic status [8,9,10]. However, in these lakes, BALOs have been studied away from shore, at reference stations of the lakes, on a monthly or seasonal time scale, i.e. at a temporal scale unsuited to studying microbial predator dynamics. In Lake Geneva, probably one of the most studied lakes regarding BALOs, Bdellovibrionaceae seems to be the most diverse family in terms of OTUs, while Peredibacteraceae are the most abundant, as measured by quantitative PCR (qPCR). It was also found that potential prey mainly belonged to the genus Pseudomonas [11, 15] are predated by Bdellovibrionaceae.
Assessing the dynamics of BALOs and the impact of environmental forcing on this functional group can be significantly facilitated by analysing them on short time scales and under relatively controlled experimental conditions. This is what in situ deployed mesocosms can typically provide, as they constitute an ideal experimental tool, intermediate between microcosms commonly used in laboratories and in situ ecological surveys [16,17,18]. In the past, in situ deployed mesocosms have proved to be a relevant type of instrumentation for observing changes in complex planktonic communities while controlling environmental conditions such as brightness, concentrations of nutrients or dissolved gases, water mixing, and predatory effects, among others. [19,20,21,22]. We conducted a mesocosm experiment in Lake Geneva, designated project “MESOLAC,” which aimed at analysing the response of surface planktonic populations to extreme events caused by global climatic change. The latter has already resulted in an increase in the number and strength of the so-called extreme events, including storms, heavy rainfall, floods, or drought [23]. Ecosystems are likely to be massively impacted by such events, and lakes are no exception [24]. For example, Woolway and Merchant [25] reported alterations in the thermal regime of lakes, which could result in planktonic diversity reduction and the inability of lake ecosystems to return to their original state due to the loss of their functional redundancy. Therefore, addressing issues regarding lacustrine functions and their resilience is of major importance to better understand the impact of climate change, including global warming, on their state and evolution in the near future.
The focus of this work was to study (i) the dynamics and diversity of three main BALO families belonging to the Oligoflexia group (e.g. Bdellovibrionaceae, Bacteriovoracaceae and Peredibacteraceae) on a short time scale and (ii) their response to simulated extreme events and abiotic and biotic factors. We hypothesised that the addition of dissolved organic carbon (simulating an input of carbon from the watershed in response to runoff from the catchment), light reduction, and mixing (simulating stormy conditions) would stimulate bacterial growth and thus the predation by bacterial predators such as BALOs. Using flow cytometry, allowing us to discriminate between two groups of bacteria, namely low DNA content [26] or low nucleic acid content [27] (LDNA) and high DNA content [26] or high nucleic acid content [27] (HDNA), we also hypothesised that the supply of nutrients may favour the HDNA, likely to constitute preferential prey for BALOs.
Materials and Methods
Experimental Setup
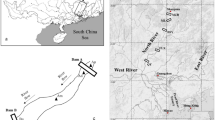
The MESOLAC experiment was conducted on Lake Geneva over 4 weeks in July 2019. Nine experimental mesocosms (Supplementary Fig. 1) were placed approximately 100 m away from the coast and at a depth of 7 m near the Alpine Centre for Research on Lake Ecosystems and Food Webs (CARRTEL, Thonon-les-Bains; 46° 22′ 09.1″ N, 6° 27′ 15.3″ E). An ecological anchoring system, set up by professional scuba divers, including the corresponding author, ensured the fixation of each mesocosm and good preservation of the bottom (i.e. sediments and plants) of the lake. Mesocosms consisted of reinforced polythene bags of 4.5 m height and 1.4 m diameter at their upper level with a finishing conical part towards the bottom (Insinööritoimisto Haikonen Oy, Norra Paipis, Finland). The mesocosms were supported at every metre by a plastic frame and a double system of buoys on top. All bags were filled with water simultaneously on the same day (1 July) within a few hours and were untouched for 3 days to acclimate. The total volume of water in each mesocosm was approximately 4 m3. The experiment started on 4 July, designated T0. The experimental design included three treatments, each replicated thrice. Each bag was covered by filters (Lee filters) applied on the surface of the mesocosm, to protect embedded water from bird droppings and other external elements, so that they were all treated similarly. The control treatment (C) consisted of minimal light reduction (i.e. 5%). Treatment M consisted of a medium stress situation, i.e. 30% light reduction, dissolved organic carbon (i.e. total DOC ~ 2 mg L−1; using autoclaved peat soil extract) at a concentration × 1.5 times that of the control and regular manual mixing for 5 min daily for 2 weeks. Treatment H consisted of a shorter but stronger intensity stress simulation. For 5 days, light was reduced by ~ 85%, DOC concentration was increased fivefold (i.e. total DOC ~ 6 mg L−1), and daily mixing was performed for 15 min. After 5 days, treatment H was exposed to the control conditions. More details can be found in [28].
Sampling Strategy and Analysed Parameters
Water samples were collected at a depth of 2 m in each bag, with a 2–4-day interval from 4 July to 22, July 2019. The sampling dates are 4th, 8th, 11th, 16th, 18th, and 22nd of July and are, respectively, relabelled as 0 day, 4 days, 7 days, 12 days, 14 days, and 18 days. Physicochemical parameters such as pH, dissolved oxygen concentration, turbidity, phosphorus, and nitrogen concentrations were measured following standard protocols [29]. Chlorophyll a concentration was obtained using spectrophotometry after pigment extraction in 90% acetone [28]. Meteorological data were collected daily via the CLIMATIK platform, which is reserved for INRAE research. For DNA analysis, 200–350 mL of water was filtered through 0.2-µm PC filters. All filters were stored at − 20 °C until DNA extraction. Water samples were also taken for delayed flow cytometry analyses and consisted of 2 mL of water fixed with glutaraldehyde (15 min, 0.5%), flash-frozen in liquid nitrogen, and stored at − 80 °C.
DNA Extraction
The 0.2-µm filters were subjected to DNA extraction using a homemade protocol. In a 2-mL Eppendorf tube, 300 µL of TE buffer (TRIS, 1 M [pH 8] and EDTA, 0.5 M [pH 8]) was added to each filter. Next, a lysis step was performed by adding 200 µL of lysis solution (TRIS, 1 M [pH 8]; EDTA, 0.5 M [pH 8], and sucrose: 0.7 M). After a thermic shock at − 80 °C for 15 min and at 55 °C for 2 min, 50 µL of 10% sodium dodecyl sulphate (SDS), and 10 µL of proteinase K (20 mg/mL) were added. The samples were then incubated at 37 °C for 1 h with gentle stirring and placed on a heating block at 55 °C for 20 min. After a quick centrifugation step (13,000 rpm at 4 °C for 3 min), the supernatant was collected. Afterwards, 50 µL of sodium acetate (3 M [pH 5.2]) and 1.5 µL of 25 µg/µL GenEluteTM-LPA (Sigma-Aldrich, St Louis, MO, USA) were added. Subsequently, one volume of isopropanol was added, and the tubes were centrifuged for 10 min at 12,000×g at 4 °C. Following this step, two rounds of ethanol (80%) washing were carried out to clean the DNA pellet. The remaining ethanol was evaporated using a SpeedVac for 20 min. Finally, 30 µL of TE was added, and samples were incubated at 37 °C for 1 h to allow the pellet to gently dissolve into the TE buffer. The DNA concentration was measured using a NanoDrop 1000 spectrophotometer. For DNA concentrations greater than 25 ng µL−1, a dilution was performed. All DNA preparations were stored at − 20 °C until further analysis.
Quantification of BALOs by qPCR
Qiagen’s Rotor-Gene Q machine and QuantiTect SYBR Green PCR kit (Qiagen, Hilden, Germany) were used to amplify the BALOs. Standard curves were prepared using identified clones of Bdellovibrionaceae, Peredibacteraceae, and Bacteriovoracaceae from our previous study [9]. Briefly, plasmids were extracted and purified using a NucleoSpin Plasmid kit (Macherey–Nagel GmbH & Co. KG, Düren, Germany) according to the manufacturer’s instructions. Plasmids were then digested using a BamHI restriction enzyme (Takara Bio Inc., Shiga, Japan) following the manufacturer’s instructions. The digested plasmid concentrations were measured using a Quant-iT PicoGreen ds DNA Reagent kit (Invitrogen, Carlsbad, CA, USA), and fluorescence was read using a Fluoroskan Ascent FL plate reader. The number of copies for each BALO clone was calculated using the following formula:
Serial dilutions were then conducted from 109 to 100 copies. Diluted DNA from 107 to 100 was duplicated and amplified by the BALOs’ qPCR set of primers to construct the standard curve. The list of primers used to amplify the 16S rDNA gene is shown in Supplementary Table 1 [9, 30]. All primer sets for BALO amplification were optimised in our previous study [9]. Two controls were added at each time point. The Bdellovibrionaceae standard curve had an efficiency of 0.92, R2 of 0.996, and the threshold was set to 0.02. For Peredibacteraceae, the efficiency was 0.87, R2 was 0.87, and the threshold was fixed at 0.015. For Bacteriovoracaceae, the efficiency was 0.96, R2 was 0.993, and the threshold was set to 0.02. The qPCR mixture volume was 25 µL and consisted of final concentration: 1 X Master Mix (QuantiTect SYBR Green PCR kit, Qiagen), 0.3 mg mL−1 of BSA, 0.2 µM of forward and reverse primers, and 1 µL of template DNA (25 ng µL−1). The program was as follows: 95 °C for 15 min followed by 40 cycles of 95 °C for 45 s, 60 °C for 45 s, and 72 °C for 45 s, with + 1 °C every 5 s from 60 to 95 °C. For all environmental samples, those that failed to amplify or were outside the standard curve limits were not considered in the analysis. BALO abundances were obtained in copy per reaction and were transformed to copy per millilitre of filtered water using the following formula:
Flow Cytometry Analysis
We used a FACSCalibur flow cytometer (BD BioSciences, Franklin Lakes, NJ, USA) to determine the total prokaryote abundance. After each water sample was thawed at ambient temperature, 2.5 µL was added to 245 µL filtered (< 0.02 µm) TE buffer and 2.5 µL of SYBR Green I (diluted 10,000 times). The sample was then heated for 10 min at 75 °C before the FCM analysis [31]. “Listmode” files were exported and analysed using CYTOWIN [32]. The analysis provided information on prokaryote-like particles (PLPs), which encompassed two subgroups designated “high” (HDNA) and “low” DNA contents (LDNA), indicating possibly active and less productive/active bacterial populations, respectively [27]. Virus-like particles (VLPs) and the virus-to-prokaryote ratio (VPR) were also analysed [33].
Statistics
Statistical analyses were performed using R software version 3.6.3 [34], and figures were produced using the ggplot2 package [35] or ggpubr [36]. One-way ANOVA (p < 0.05) or Kruskal–Wallis test followed by Dunn’s test [37] or Tukey’s HSD post hoc tests were performed to compare the mean values of each treatment throughout the experiment. NMDS and Adonis tests were also performed to test the differences between groups, i.e. replicates, treatments, and dates. Spearman’s rank correlation test was conducted to examine the correlation between variables, and a correlogram was drawn using the corrplot package [38] following the Antoine Soetewey code (https://statsandr.com/blog/correlation-coefficient-and-correlation-test-in-r/.). The correlogram shows the correlation coefficient for all pairs of variables (with more intense colours for more correlations), and correlations not statistically significant are represented by a white box. Moreover, to visualise the variation in microbial community structure and the relationship between environmental variables and sample clusters, we performed a redundancy analysis (RDA). All RDAs performed were significant according to the ANOVA test and chosen according to the detrended correspondence analysis (DCA) [39]. RDA was performed using the Vegan package [40]. Environmental parameters were selected using the envfit function (p < 0.05). Multicollinear variables were removed using variance inflation factors (VIF) [40].
Results
BALOs’ Abundance and Dynamics
qPCR measurements indicated that Peredibacteraceae abundance was the highest, followed by Bdellovibrionaceae and Bacteriovoracaceae. The abundance of Bdellovibrionaceae (Fig. 1A) in the H and C treatments seemed to follow the same decreasing pattern until day 14, with Bdellovibrionaceae being less abundant in treatment H. The abundance of Bdellovibrionaceae slightly increased in treatment M until day 4 before decreasing as the others. Bdellovibrionaceae abundance varied from 4.27 × 102 to 4.30 × 102 copies mL−1 in C, from 6.24 × 101 to 1.91 × 102 copies mL−1 in H, and from 1.68 × 102 to 2.06 × 102 copies mL−1 in M. While statistical analyses revealed that treatments H and M were different (Dunn’s test p value = 0.02), no significant difference was observed between treatments C and H, or between C and M (Fig. 2A). Peredibacteraceae (Fig. 1B) and Bacteriovoracaceae (Fig. 1C) dynamics showed a similar pattern to those of Bdellovibrionaceae, with a decrease in abundance that was more marked in Bacteriovoracaceae, Peredibacteraceae varied from 4.74 × 103 to 2.59 × 102 copies mL−1 in C, from 9.61 × 102 to 8.27 × 101 copies mL−1 in H, and from 2.46 × 103 to 1.88 × 102 copies mL−1 in M. For Bacteriovoracaceae abundance varied from 2.88 × 103 to 5.51 × 100 copies mL−1 in C, from 8.38 × 102 to 5.41 × 100 copies mL−1 in H, and from 2.12 × 103 to 8.39 × 100 copies mL−1 in M. Kruskal–Wallis test showed no significant difference between treatments for both Peredibacteraceae (p value = 0.19) and Bacteriovoracaceae (p value = 0.34). The Adonis test confirmed that the treatments were not responsible for the disparity (p value = 0.196). Moreover, the same test confirmed that the replicate mesocosms did not differ (p value = 0.588). However, differences existed between dates (p value < 0.01), and three different clusters could be defined: days 0–4, 7, and 12–18 (Fig. 3).
Dynamics of BALOs as measured by qPCR using adequate set of primers that amplify the 16S rRNA gene of each family (A–C) and heterotrophic prokaryotes as measured using Flow cytometry (D–F) for the three treatments (Control (C)—green circle, Moderate (M)—red triangle, and High (H)—blue square). PLP prokaryote-like particles; HDNA high DNA content; LDNA low DNA content
Box plot of Bdellovibrionaceae abundances measured by qPCR (A) and prokaryote-like particles (PLP) measured by Flow cytometry (B) for each treatment over time. The solid line in each box plot corresponds to the median value. The letters C, M, and H denote the treatment types, i.e. control, moderate, and high, respectively. The statistical tests used are displayed at the top of the figures. The significance of the tests is represented by stars and the non-significance by the letters “ns”
Non-metric multidimensional scaling (NMDS) illustrating separation of samples based on dates in the ordination space with 95% ellipses (k = 2; stress = 0.00009). 0 day, 4 days, 7 days, 12 days, 14 days, and 18 days correspond, respectively, to 4th, 8th, 11th, 16th, 18th, and 22nd of July. Permutational multivariate analysis of variance (Adonis) showed that the dates are statistically different
Relationships with Abiotic Factors
The dynamics of the main environmental parameters during the experiment are described in Supplementary Text Box 1 and presented in Supplementary Fig. 2. On the one hand, the correlogram (white boxes for non-significant correlation; Fig. 4) shows that Bdellovibrionaceae, unlike the other two BALOs, displayed very few relationships with measured environmental variables. Correlations were observed between pH (ρ = 0.30) and water hardness (ρ = 0.27). On the other hand, Peredibacteraceae and Bacteriovoracaceae were positively and significantly correlated with water hardness, nitrite (NO2−), total nitrogen (Ntot), phosphate (PO43−), total phosphorus (Ptot), chloride (Cl−), temperature, and particulate phosphorus (Ppart), and negatively correlated with ammonium (NH4+), silica (SiO2), and chlorophyll a (Chl a). The RDA, which explained 65% of the variance (Fig. 5A), showed that Bdellovibrionaceae correlated positively with water hardness, pH, and day 18; Peredibacteraceae with nitrite (NO2−) and chloride (Cl−), and Bacteriovoracaceae with temperature and day 0. The correlogram confirmed these observations. Overall, environmental parameters explained, to some extent, the variation in abundance observed for Peredibacteraceae and Bacteriovoracaceae but not for Bdellovibrionaceae.
This correlogram is based on 21 observations that represent environmental variables, PLPs, VLPs, and BALOs. Positive and negative correlations are displayed in blue and red, respectively. The intensity of the colour is proportional to the correlation coefficient. The white boxes indicate that the correlation is not significantly different from 0 at the 5% level tested with a correlation test. Abbreviation are as follows: ammonium (NH4+), chloride (Cl−), chlorophyll a (Chla), nitrate (NO3−), nitrite (NO2−), particulate phosphorus (Ppart), silica (SiO2), sulphate (SO42−), phosphate (PO43−), total carbon (Ctot), total phosphorus (Ptot), total nitrogen (Ntot), temperature (temp), water hardness (Wt hardness), prokaryote-like particles (PLP), high DNA content (HDNA), low DNA content (LDNA), virus-like particles (VLP), and virus-to-prokaryote ratio (VPR)
RDA representation indicating the relationship between environmental variables (arrows) and BALOs (A) or PLP (B) sampled for 6 days. 0 day, 4 days, 7 days, 12 days, 14 days, and 18 days correspond, respectively, to 4th, 8th, 11th, 16th, 18th, and 22nd of July. Treatments are represented with shapes i.e. circle for C, triangle for M, and square for H. The days are highlighted with colours. Abbreviations are as follows: ammonium (NH4+), chloride (Cl−), chlorophyll a (Chla), nitrate (NO3−), nitrite (NO2−), particulate phosphorus (Ppart), silica (SiO2), sulphate (SO42−), phosphate (PO43−), total carbon (Ctot), total phosphorus (Ptot), total nitrogen (Ntot), temperature (temp), water hardness (Wt hardness), prokaryote-like particles (PLP), high DNA content (HDNA), low DNA content (LDNA), virus-like particles (VLP), and virus-to-prokaryote ratio (VPR)
Relationships Between Biotic Variables
The correlogram (Fig. 4) suggested that Bdellovibrionaceae was not significantly correlated with Prokaryote-like particles (PLP), HDNA, or LDNA. Contrastingly, both Peredibacteraceae and Bacteriovoracaceae had a significant positive relationship with PLP and HDNA and a significant negative relationship with LDNA. More specifically, Peredibacteraceae was more correlated with HDNA (ρ = 0.81) than Bacteriovoracaceae (ρ = 0.60), whereas the opposite was true for LDNA (ρ = − 0.51 vs − 0.66). Furthermore, Peredibacteraceae had a superior relationship with PLP (ρ = 0.53) than Bacteriovoracaceae (ρ = 0.30). Considering the evolution of PLP, HDNA, and LDNA during the experiment (Fig. 1D–F), we observed that the trend was generally similar to that observed for BALOs. The curve of the M treatment was slightly higher than that of the C and H treatments. Moreover, except for LDNA, abundance tended to decrease during the experiment—LDNA abundance increased during the experiment until days 13–14. PLPs (Fig. 1D) decreased during the experiment in all mesocosms, from 5.44 × 106 to 4.10 × 106 cells mL−1 for C, from 5.14 × 106 to 2.63 × 106 cells mL−1 for H, and from 5.13 × 106 to 4.26 × 106 cells mL−1 for M. The same pattern was also observed for the HDNAs (Fig. 1E) with a decrease in abundance from 3.80 × 106 to 1.65 × 106 cells mL−1 for C, from 3.67 × 106 to 1.10 × 106 cells mL−1 for H, and from 3.65 × 106 to 1.18 × 106 cells mL−1 for M. The LDNA dynamics were completely different (Fig. 1F) since the proportion of this group increased significantly during the experiment (from 1.63 × 106 to 2.5 × 106 cells mL−1 for C, from 1.47 × 106 to 1.58 × 106 cells mL−1 for H, and from 1.59 × 106 to 2.00 × 106 cells mL−1 for M). It is also noteworthy that the HDNAs were more abundant than the LDNAs at the beginning of the experiment for all treatments, which was reversed at the end. The ANOVA test showed that HDNA and LDNA mean abundances were not significantly different between the treatments (p value = 0.37 and 0.11, respectively). However, treatments impacted PLP, and differences were significant between C and H (p value = 0.01) and between M and H (p value = 0.007). As shown in Figs. 1D and 2B, the H treatment had less PLP than C and M. Moreover, the correlogram in Fig. 4 shows that HDNA and PLP were positively and significantly correlated (ρ = 0.77). In contrast, LDNA was not significantly correlated with PLP and was significantly and negatively correlated with HDNA. PLP, LDNA, and HDNA had 8, 12, and 14 positive or negative significant relationships with environmental variables, respectively. LDNA had mostly negative relationships with environmental parameters, whereas HDNA had mostly positive relations. The RDA (Fig. 5B), which explained 70% of the variance in PLP, HDNA, and LDNA, revealed that PLP correlated positively with water hardness and day, HDNA with nitrite and chloride, and LDNA with chlorophyll a and day 12.
The present study also allowed us to examine VLP dynamics that were found to be opposite to those of BALOs and prokaryotes (Supplementary Fig. 3). VLP abundance increased more in H than in the C and M treatments; however, the ANOVA test revealed that the differences were not significant (p value = 0.5). Moreover, the correlogram (Fig. 4) suggested the absence of a significant correlation between VLP and BALOs. No significant correlations were observed between VLP and PLP, HDNA, or LDNA. When considering the virus-to-prokaryote ratio (VPR), H favoured an increase in VPR at the beginning of the experiment (until day 12; Supplementary Fig. 3B), and statistical analyses indicated a significant difference between H and M, but not between C and H or between C and M. The correlogram (Fig. 4) indicated significant and negative correlations between VPR and Peredibacteraceae (ρ = − 0.49) and Bacteriovoracaceae (ρ = − 0.32), but not with Bdellovibrionaceae. VPR also displayed a strong negative relationship with PLP (ρ = − 0.86) and HDNA (ρ = − 0.67), but not with LDNA.
Discussion
With global climate change, episodic extreme events (such as floods and violent storms) are expected to become more frequent, causing changes in lake subsidies and exposing them to nutrient pulses and transfer imbalances. The effects of allochthonous dissolved organic matter inputs on aquatic organisms, biotic interactions and metabolic processes feedback, and the impact on the littoral versus pelagic communities remain largely underexplored. Our experimental approach made it possible to simulate such a scenario with the main aim of investigating the response of a specific functional group of bacteria.
To study microbial interactions, especially predator–prey relationships, the time scale is important. For BALOs, predation is known to be rapid under controlled and favourable conditions, but the impacts of bacterial predators on bacterial prey dynamics, distribution, and composition under natural conditions have been poorly investigated until now. Using a 2–4-day sampling strategy and an outdoor mesocosm approach, we aimed to assess, for the first time, the dynamics of the main BALO families belonging to Oligoflexia, highlighting their response to abiotic and biotic factors.
After observing the BALO dynamics and performing statistical analyses, it appears that the simulated effects did not have a strong impact on BALOs’ abundance. Said differently, BALOs were not affected by the stresses or modifications caused by extreme events. As predation is an important contributor to community functional dynamics and diversity, observing that the predators’ dynamics is stable in the face of such disturbance is rather reassuring. We are aware, however, that further testing would be useful to confirm such results. Our result may be an extra observation that lake responses to extremes disturbance can be dependent on lake condition antecedents [41,42,43,44,45] and, in some situations, although exposed to severe climatic conditions (e.g. storms), lakes may not be strongly modified because lake condition antecedents shape resistance and resilience of the ecosystem [46].
When the treatment effects are not considered, it is clear that each family of BALOs displayed a very different dynamic, suggesting a variety of interactions and responses to forcing (abiotic and biotic interactions). Many factors and/or processes could be responsible for these differences (e.g. the predation spectrum, prey presence/absence, resistance genes or other attributes, etc.). The Peredibacteraceae family was the most abundant among the studied BALOs. Despite their abundance decreasing slightly during the experiment, this was consistent with our previous studies on BALOs in Lake Geneva [8,9,10,11], suggesting that this group is the dominant one among the targeted BALOs, especially, in lake surface waters. In contrast, Bacteriovoracaceae were less abundant and decreased strongly during the experiment. This is also in accordance with another study of our team [47]. Bdellovibrionaceae abundance remained relatively stable.
Bdellovibrionaceae displayed only relationships with pH and water hardness. It has been established in the literature that some bacterial species are vastly dependent on pH [48, 49]. In laboratory conditions, Bdellovibrio sp. motility has been shown to be inhibited at pH < 5 and pH > 9. The optimum attachment and growth for Bdellovibrio sp. ranged from neutrality to slightly alkaline pH [50,51,52]. In Lake Geneva, pH varied slightly, i.e. between 7.3 and 8.7, corresponding to the optimal range for Bdellovibrio growth. Further laboratory experiments will be welcomed to provide more pieces of information on pH optima for Bdellovibrionaceae and other BALOs of Lake Geneva. Peredibacteraceae had marked relationships with nitrite and chloride ions, what no other studies have shown before. These variables also varied very little during the experiment. For the chloride ion, Roeßler et al. (2003) [53] demonstrated that chloride ions are not necessary for growth in 44 different bacterial strains, except in very salty environments. Naturally, this is not the case for Lake Geneva. The only ions reported to be beneficial to the growth of BALOs are calcium and magnesium ions because they are important for BALO penetration into prey [54]. For nitrite, Peredibacteraceae may be associated with this variable because they could consume nitrite-oxidising bacteria or use nitrite to survive under anoxic conditions. In fact, other BALOs such as Bdellovibrio bacteriovorus UP and Micavibrio spp. are capable of preying on Nitrospira in sludge flocs [55, 56]. Also, Sockett and Lambert (2004) [57] found that within the B. bacteriovorus HD100 genome there are genes that encode for nitrite reductase and nitric oxide reductase. This result suggests that B. bacteriovorus HD100 strain could use nitrite as a terminal electron acceptor instead of oxygen. In fact, Monnappa et al. (2013) [58] confirmed that B. bacteriovorus HD100 predated upon Escherichia coli under anoxic conditions when at least 1 mM of nitrate was available in the culture medium. Looking into the genome of Peredibacteraceae of Lake Geneva could help to understand better such a relationship with nitrogen. For Bacteriovoracaceae temperature seemed to be important, and literature has indicated temperature’s impact on the growth and abundance of BALOs [12, 14, 59]. Bacteriovoracaceae abundance decreased with increasing temperature; however, the temperature difference in the present study was 3 °C, which is small considering that BALOs generally tolerate a wide temperature range [5, 60]. A study by Kandel et al. (2014) [14] showed that a variation of 4 °C in a closed system (aquaculture) does not affect BALO abundance. In general, it takes large temperature fluctuations, as in natural systems, to observe effects on the BALO population.
We know very little about BALOs’ prey preference and interactions with other microorganisms in general. This study did not cover this issue, among the Prokaryote-like particles (PLP), we discriminated bacteria with high (HDNA) vs low (LDNA) DNA or nucleic acid content, some of which might be considered as potential BALO prey. Indeed, BALOs have a prey minimum size [61] and benefit more from prey with high amount of nutrient (more progeny) such as nucleic acids. Hence, we hypothesised that BALOs may preferably prey on HDNA, which would also benefit from the initial load of nutrients in treatments H and M. It is also noteworthy that the obligate oligotrophic LDNA cells could get richer in nucleic acids and shift to HDNA category [62, 63]. After eliminating the treatment effect, the statistical tests’ results did not show significant differences in the abundances of HDNA and LDNA. However, during the experiment, LDNA tended to increase while the reverse trend was observed for HDNA. Clearly, different dynamics occurred on different days as shown in Fig. 3. On the other hand, there was a significant difference in PLP abundances between C and H and M and H. The events that the H mesocosms were exposed to induced a significant decrease in PLPs compared with the M treatment and the control. This result was the opposite of what we expected. Our initial hypothesis was that our experimental conditions, especially the increase in dissolved organic carbon, would enhance bacterial growth and reproduction if temperature and inorganic nutrients were not limiting at that time, and by extension, promote the growth of BALOs and other bacterial predators. In fact, BALOs have been reported to be more abundant and diverse in eutrophic lakes owing to the abundance and diversity of the population of prey bacteria [15]. To explain the overall absence of significant differences between treatments, we first surmised that one cannot rule out the possibility that a weak increase in organic matter input will have little or no effect on prokaryotic communities, as already shown elsewhere [64]. Secondly, in another study with smaller mesocosms than the ones deployed here [65], a difference in microorganisms population could be observed but with DOC concentration considerably higher, i.e. 428.7 mg L−1. Thirdly, it is possible that other elements were limiting for prokaryotic growth [66]. However, it was not the case for inorganic nutrients because ammonium, nitrite, nitrate, nitrogen, and phosphorus concentrations were at sufficient concentrations to sustain microorganism growth [28]. We do not think that autoclaving the peat extract soil did any damage on the nutrient composition as reported by Williams-Linera and Ewel (1984) [67]. However, heterotrophic bacteria might not have efficiently assimilated the organic carbon from the soil peat extract. Indeed, Guillemette et al. (2016) [68] showed that the terrestrial organic carbon which contains more humic substances and polysaccharides is more recalcitrant to bacterial degradation, while on the contrary algal-derived organic carbon is more labile and easier to incorporate. Additionally, Zhou et al. (2020) [69] reported that soil-derived dissolved organic matter has a higher environmental filtering on bacteria where only those who have the adequate arsenal to degrade such compounds can benefit. Finally, we assumed that the frequency of mixing the mesocosms might not have been enough to let microorganisms benefit from DOC, as pointed out by Striebel et al. (2015) [70] who advised to mix three times the mesocosms when using a disc and to preferably instal an automatic system to mix the water more frequently. Regardless of the small decrease in PLPs, their abundance remained relatively high during the experiment. In general, the PLPs and HDNAs decreased slightly over time. Conversely, LDNA slightly increased. This was unexpected as stated above. Regarding the decrease in PLPs and HDNAs, we surmised that the disruption in the number of prokaryotes, and by extension of potential prey, especially the most active one, HDNA, caused a decrease in BALO abundance. BALOs also obviously did not benefit from the increase in LDNAs, which contain less nucleic acid. This was supported by the correlogram results (Fig. 4). Bacteriovoracaceae and Peredibacteraceae showed a strong positive correlation with PLPs, especially HDNAs, and conversely, they both had a negative relationship with LDNAs, which is consistent with our hypothesis that HDNAs could be more preyed upon. The observed decrease in PLPs, particularly HDNAs, could thus have disturbed BALO dynamics in the mesocosms. Bdellovibrionaceae was not significantly correlated with PLP, HDNA, or LDNA. Other factors seemed to explain the dynamics over time. Furthermore, BALOs are known to mainly hunt for Gram-negative bacteria; however, not all Gram-negative bacteria are susceptible to BALO predation. Moreover, BALOs’ species and strains have different prey spectrum and can be either generalist, specialist, or versatilist [71, 72]. Unfortunately, the prey spectrum of BALOs in Lake Geneva is unknown. However, we could suggest that the strategy of each studied BALOs resulted probably in a different outcome with an important decline of Bacteriovoracaceae, a relative stability of Bdellovibrionaceae, and an entre-deux for Peredibacteraceae. In addition to the predation spectrum issue, another explanation can be proposed for the striking absence of BALOs’ concentration increase i.e. decoy microorganisms. It is known that non-prey and decoy cells can impact negatively BALOs’ attachment and growth [73,74,75]. The peat soil extract could have introduced decoy cells that hindered BALOs’ predation, typically heated cells with intact cell that could be infected by BALOs, likely to result in various BALOs’ species growth efficiency [76].
On the other hand, the decrease in HDNA and especially PLP was partly explained by the treatments, but it seemed that other factors were also at play, particularly for the increase in LDNA. The increase in LDNA was strongly and positively correlated with chlorophyll a and negatively correlated with temperature. These two factors seemed to play a role in LDNA dynamics, despite their variations being minimal. Furthermore, LDNA was negatively correlated with HDNA, implying that the dynamics of one probably influenced that of the other. For the decrease of PLP, especially HDNA, the environmental variables of the correlogram and RDA did not appear to show a clear pattern. Both were positively correlated and showed several other correlations with other factors. In contrast, LDNA had more negative relationships with the environmental variables.
Other interactions may explain the dynamics of Bdellovibrionaceae, PLP, and HDNA. Among the other parameters, we were able to study the dynamics of viruses (PLP). According to literature, some bacteriophages are capable of infecting BALOs [77], and in Lake Geneva PLPs and HDNAs [78, 79]. PLP dynamics were observed to increase over time. Statistical tests did not show any treatment effect on PLP abundance. Despite the relative increase in VLPs, there was no correlation with BALOs or PLPs, HDNA, and LDNA. The presence and abundance of VLPs had no visible impact on the prokaryotic community and BALOs. In contrast, the virus-to-prokaryote ratio (VPR), which can be used as a proxy for host–parasite interactions [80], showed the opposite trend. This ratio increased strongly in the H treatment, with statistical analysis showing a significant difference between the H and M treatments. This suggests that phages affected prokaryotic growth and dynamics in the H treatment through cell lysis. In contrast, the M and C treatments did not seem to favour a “boom” of phages.
We also noted a negative correlation between VPRs and Peredibacteraceae and Bacteriovoracaceae but no significant correlation with Bdellovibrionaceae. Finally, we observed that Peredibacteraceae and Bacteriovoracaceae appeared to share common trends, and these two families were positively correlated with each other, and Bdellovibrionaceae had a weaker positive correlation. The current study could not explain Bdellovibrionaceae dynamics.
We are aware that mesocosms can have a significant detrimental effect on planktonic populations but we do not think that Bacteriovoracaceae could be more susceptible to such effect, compared to the other predators. Firstly, we did not measure inhibitors that could have affected Bacteriovoracaceae DNA detection but also all the other BALOs’ DNA. Secondly, from previous studies, we know that Bacteriovoracaceae are less abundant than Peredibacteraceae and Bdellovibrionaceae in the water column [8,9,10]. It is possible that variations are likely to be more important and visible for such low abundances. We can wonder, however, why Bacteriovoracaceae are less numerous than Peredibacteraceae and Bdellovibrionaceae? As pointed out above, Bacteriovoracaceae may prefer other types of habitats such as biofilm, sediment, wastewater treatment plant effluent, etc.… Another nonexclusive possibility is that these bacteria are less competitive than the other two BALOs and/or have a limited prey range. Also, they could be preferably more predated or lysed than other BALOs.
We hypothesise that factors other than those measured here, such as the importance and role of other predators, e.g. heterotrophic nanoflagellates, ciliates, and metazooplankton, could be determinants of the observed effect. These groups could likely exert considerable control over bacteria, explaining the observed dynamics of both PLPs and BALOs. It would be interesting to specifically target BALO prey using an adequate set of qPCR primers instead of looking at the general trend of prokaryotes. However, we only identified one BALO prey species in Lake Geneva, i.e. Pseudomonas spp. [11]. Further efforts to pinpoint prey strain and type are needed to fully understand BALO dynamics in Lake Geneva and peri-alpine lakes.
Conclusions
Bacteria play key roles in ecosystem functioning, as they are involved in nutrient cycling and are prey for a variety of predators, among other functions. Therefore, it is essential to accurately predict their relationships with other species and general patterns governing their adaptation to the environment. Here, we shed some light on a group of bacteria that are still poorly understood in terms of their ecology, particularly, in natural freshwater systems such as lakes. Although BALOs were not significantly affected by our treatment conditions, which were supposed to mirror the advent of extreme events on the surface waters of Lake Geneva, they were shown to be a dynamic community, and that constitutive families of this functional group exhibited different patterns. As the main environmental physicochemical parameters measured in the present study did not seem responsible for the observed dynamics, the importance of biotic interactions is proposed. These interactions were likely predation and parasitism through the action of phages and protozoans, but also possibly the scarcity of specific prey for BALOs. Next steps should include more (biotic) factors, focus on the quantity and quality of prey, and examine different types of predation on both total and specific groups of bacteria.
Data Availability
All data can be made available. It is noteworthy that two data papers have been published on the MESOLAC data (https://doi.org/10.1016/j.dib.2020.106255 and https://doi.org/10.1016/j.dib.2021.107150).
References
Iebba V, Santangelo F, Totino V et al (2013) Higher prevalence and abundance of Bdellovibrio bacteriovorus in the human gut of healthy subjects. PLoS ONE 8:e61608. https://doi.org/10.1371/journal.pone.0061608
Johnke J, Fraune S, Bosch TCG et al (2020) Bdellovibrio and like organisms are predictors of microbiome diversity in distinct host groups. Microb Ecol 79:252–257. https://doi.org/10.1007/s00248-019-01395-7
Hahn MW, Schmidt J, Koll U et al (2017) Silvanigrella aquatica gen. nov., sp. nov., isolated from a freshwater lake, description of Silvanigrellaceae fam. nov. and Silvanigrellales ord. nov., reclassification of the order Bdellovibrionales in the class Oligoflexia, reclassification of the families Bacteriovoracaceae and Halobacteriovoraceae in the new order Bacteriovoracales ord. nov., and reclassification of the family Pseudobacteriovoracaceae in the order Oligoflexales. Int J Syst Evol Microbiol 67:2555–2568. https://doi.org/10.1099/ijsem.0.001965
Koval SF, Williams HN, Colin Stine O (2015) Reclassification of Bacteriovorax marinus as Halobacteriovorax marinus gen. nov., comb. nov. and Bacteriovorax litoralis as Halobacteriovorax litoralis comb. nov.; description of Halobacteriovoraceae fam. nov. in the class Deltaproteobacteria. Int J Syst Evol Microbiol 65:593–597. https://doi.org/10.1099/ijs.0.070201-0
Jurkevitch E, Davidov Y (2006) Phylogenetic diversity and evolution of predatory prokaryotes. In: Jurkevitch E (ed) Predatory prokaryotes. Microbiology monographs. Springer, Berlin, pp 11–56
Williams HN, Li N, Scientists E (2018) Data report: exploring the presence of Bdellovibrio and like organisms in deep-sea sediment by culture-independent and culture-dependent methods. Proc Int Ocean Discov Progr 349:202
Sutton DC, Besant PJ (1994) Ecology and characteristics of Bdellovibrios from three tropical marine habitats. Mar Biol 119:313–320. https://doi.org/10.1007/BF00349571
Paix B, Ezzedine JA, Jacquet S (2019) Diversity, dynamics and distribution of Bdellovibrio and like organisms in peri-alpine lakes. Appl Environ Microbiol 85:e02494-e2518. https://doi.org/10.1128/AEM.02494-18
Ezzedine JA, Chardon C, Jacquet S (2020) New 16S rRNA primers to uncover Bdellovibrio and like organisms diversity and abundance. J Microbiol Methods 175:105996. https://doi.org/10.1016/j.mimet.2020.105996
Ezzedine JA, Jacas L, Desdevises Y, Jacquet S (2020) Bdellovibrio and like organisms in Lake Geneva: an unseen elephant in the room? Front Microbiol 11:1–14. https://doi.org/10.3389/fmicb.2020.00098
Ezzedine J, Pavard G, Gardillon M, Jacquet S (2020) Bdellovibrio sp.: an important bacterial predator in Lake Geneva? J Microbiol Biotechnol 5:1–11. https://doi.org/10.23880/oajmb-16000157
Williams HN, Piñeiro S (2006) Ecology of the predatory Bdellovibrio and like organisms. In: Jurkevitch E (ed) Predatory prokaryotes. Microbiology monographs. Springer, Berlin, pp 214–247
Li H, Chen C, Sun Q et al (2014) Bdellovibrio and like organisms enhanced growth and survival of Penaeus monodon and altered bacterial community structures in its rearing water. Appl Environ Microbiol 80:6346–6354. https://doi.org/10.1128/AEM.01737-14
Kandel PP, Pasternak Z, van Rijn J et al (2014) Abundance, diversity and seasonal dynamics of predatory bacteria in aquaculture zero discharge systems. FEMS Microbiol Ecol 89:149–161. https://doi.org/10.1111/1574-6941.12342
Chauhan A, Fortenberry GZ, Lewis DE, Williams HN (2009) Increased diversity of predacious Bdellovibrio-like organisms (BLOs) as a function of eutrophication in Kumaon Lakes of India. Curr Microbiol 59:1–8. https://doi.org/10.1007/s00284-009-9385-z
Mcallister CD, Parsons TR, Stephens K, Strickland JDH (1961) Measurements of primary production in coastal sea water using a large volume plastic sphere. Limnol Oceanogr 6:237–258
Antia NJ, McAllister CD, Parsons TR et al (1963) Further measurements of primary production using a large-volume plastic sphere. Limnol Oceanogr 8:166–183. https://doi.org/10.4319/lo.1963.8.2.0166
Egge JK, Heimdal BR (1994) Blooms of phytoplankton including Emiliania huxleyi (Haptophyta). Effects of nutrient supply in different N:P ratios. Sarsia 79:333–348. https://doi.org/10.1080/00364827.1994.10413565
Mostajir B, Demers S, De Mora S et al (1999) Experimental test of the effect of ultraviolet-B radiation in a planktonic community. Limnol Oceanogr 44:586–596. https://doi.org/10.4319/lo.1999.44.3.0586
Thingstad TF, Rassoulzadegan F (1999) Conceptual models for the biogeochemical role of the photic zone microbial food web, with particular reference to the Mediterranean Sea. Prog Oceanogr 44:271–286. https://doi.org/10.1016/S0079-6611(99)00029-4
Jacquet S, Prieur L, Avois-Jacquet C et al (2002) Short-timescale variability of picophytoplankton abundance and cellular parameters in surface waters of the Alboran Sea (western Mediterranean). J Plankton Res 24:635–651. https://doi.org/10.1093/plankt/24.7.635
Havskum H, Schlüter L, Scharek R et al (2004) Routine quantification of phytoplankton groups—microscopy or pigment analyses? Mar Ecol Prog Ser 273:31–42. https://doi.org/10.3354/meps273031
Lavell A, Oppenheimer M, Diop C et al (2012) Climate change: new dimensions in disaster risk, exposure, vulnerability, and resilience. In: Managing the risks of extreme events and disasters to advance climate change adaptation. A special report of working groups I and II of the intergovernmental panel on climate change. https://doi.org/10.1017/CBO9781139177245.004
Jenny JP, Anneville O, Arnaud F et al (2020) Scientists’ warning to humanity: rapid degradation of the world’s large lakes. J Great Lakes Res 46:686–702. https://doi.org/10.1016/j.jglr.2020.05.006
Woolway RI, Merchant CJ (2019) Worldwide alteration of lake mixing regimes in response to climate change. Nat Geosci 12:271–276. https://doi.org/10.1038/s41561-019-0322-x
Gasol JM, Li Zweifel U, Peters F et al (1999) Significance of size and nucleic acid content heterogeneity as measured by flow cytometry in natural planktonic bacteria. Appl Environ Microbiol 65:4475–4483. https://doi.org/10.1128/aem.65.10.4475-4483.1999
Lebaron P, Servais P, Agogué H et al (2001) Does the high nucleic acid content of individual bacterial cells allow us to discriminate between active cells and inactive cells in aquatic systems? Appl Environ Microbiol 67:1775–1782. https://doi.org/10.1128/AEM.67.4.1775-1782.2001
Tran-Khac V, Quetin P, Domaizon I et al (2020) In situ pelagic dataset from continuous monitoring: a mesocosm experiment in Lake Geneva (MESOLAC). Data Br 32:106255. https://doi.org/10.1016/j.dib.2020.106255
Rimet F, Anneville O, Barbet D et al (2020) The Observatory on LAkes (OLA) database: sixty years of environmental data accessible to the public. J Limnol 79:164–178. https://doi.org/10.4081/jlimnol.2020.1944
Van Essche M, Sliepen I, Loozen G et al (2009) Development and performance of a quantitative PCR for the enumeration of Bdellovibrionaceae. Environ Microbiol Rep 1:228–233. https://doi.org/10.1111/j.1758-2229.2009.00034.x
Jacquet S, Dorigo U, Personnic S (2013) A few tests prior to flow cytometry and epifluorescence analyses of freshwater bacterio- and virioplankton communities. Flow Cytom 1:1–30
Vaulot D, Courties C, Partensky F (1989) A simple method to preserve oceanic phytoplankton for flow cytometric analyses. Cytometry 10:629–635
Marie D, Brussaard CPD, Thyrhaug R et al (1999) Enumeration of marine viruses in culture and natural samples by flow cytometry. Appl Environ Microbiol 65:45–52. https://doi.org/10.1128/aem.65.1.45-52.1999
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online at https://www.R-project.org/
Wickham H, Chang W, Henry L et al (2018) ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics. Available at https://ggplot2.tidyverse.org/
Kassambara A (2020) ggpubr: “ggplot2” Based Publication Ready Plots. Available at https://github.com/kassambara/ggpubr
Alexis Dinno A (2017) Dunn’s Test of Multiple Comparisons Using Rank Sums. pp 1–7. Available at https://cran.rproject.org/web/packages/dunn.test/dunn.test.pdf
Wei T, Simko V, Levy M et al (2017) Visualization of a correlation matrix. Statistician 56:316–324
Ramette A (2007) Multivariate analyses in microbial ecology. FEMS Microbiol Ecol 62:142–160. https://doi.org/10.1111/j.1574-6941.2007.00375.x
Oksanen J, Blanchet FG, Friendly M et al (2019) Package “vegan” title community ecology package. Community Ecol Packag 2:1–297
Havens KE, Schelske CL (2001) The importance of considering biological processes when setting total maximum daily loads (TMDL) for phosphorus in shallow lakes and reservoirs. Environ Pollut 113:1–9. https://doi.org/10.1016/S0269-7491(00)00235-9
Havens KE, Beaver JR (2011) Composition, size, and biomass of zooplankton in large productive Florida lakes. Hydrobiologia 668:49–60. https://doi.org/10.1007/s10750-010-0386-5
Havens KE, Fulton RS III, Beaver JR et al (2016) Effects of climate variability on cladoceran zooplankton and cyanobacteria in a shallow subtropical lake. J Plankton Res 38:418–430. https://doi.org/10.1093/plankt/fbw009
Perga M-E, Bruel R, Rodriguez L et al (2018) Storm impacts on alpine lakes: antecedent weather conditions matter more than the event intensity. Glob Chang Biol 24:5004–5016. https://doi.org/10.1111/gcb.14384
Stockwell JD, Doubek JP, Adrian R et al (2020) Storm impacts on phytoplankton community dynamics in lakes. Glob Chang Biol 26:2756–2784. https://doi.org/10.1111/gcb.15033
Thayne MW, Kraemer BM, Mesman JP et al (2021) Antecedent lake conditions shape resistance and resilience of a shallow lake ecosystem following extreme wind storms. Limnol Oceanogr. https://doi.org/10.1002/lno.11859
Ezzedine JA, Scheifler, Mathilde Desdevises Y, Jacquet S (2021) Exploring the diversity, abundance, structure of Bdellovibrio and like organisms in four contrasted ecosystems over a year. In Press
Ruber J, Geist J, Hartmann M et al (2018) Spatio-temporal distribution pattern of the picocyanobacterium Synechococcus in lakes of different trophic states: a comparison of flow cytometry and sequencing approaches. Hydrobiologia 811:77–92. https://doi.org/10.1007/s10750-017-3368-z
Stockner JG, Shortreed KS (1991) Autotrophic picoplankton: community composition, abundance and distribution across a gradient of oligotrophic British Columbia and Yukon Territory Lakes. Int Rev Hydrobiol 76:581–601
Dashiff A, Junka RA, Libera M, Kadouri DE (2011) Predation of human pathogens by the predatory bacteria Micavibrio aeruginosavorus and Bdellovibrio bacteriovorus. J Appl Microbiol 110:431–444. https://doi.org/10.1111/j.1365-2672.2010.04900.x
Varon M, Shilo M (1968) Interaction of Bdellovibrio bacteriovorus and Host Bacteria. J Bacteriol 95:744–753
Seidler RJ, Starr MP (1969) Isolation and characterization of host-independent Bdellovibrios. J Bacteriol 100:769–785. https://doi.org/10.1128/jb.100.2.769-785.1969
Roeßler M, Sewald X, Müller V (2003) Chloride dependence of growth in bacteria. FEMS Microbiol Lett 225:161–165. https://doi.org/10.1016/S0378-1097(03)00509-3
Huang JCC, Starr MP (1973) Effects of calcium and magnesium ions and host viability on growth of bdellovibrios. Antonie Van Leeuwenhoek 39:151–167. https://doi.org/10.1007/BF02578850
Feng S, Tan CH, Constancias F et al (2017) Predation by Bdellovibrio bacteriovorus significantly reduces viability and alters the microbial community composition of activated sludge flocs and granules. FEMS Microbiol Ecol 93:1–12. https://doi.org/10.1093/femsec/fix020
Dolinšek J, Lagkouvardos I, Wanek W et al (2013) Interactions of nitrifying bacteria and heterotrophs: identification of a Micavibrio-like putative predator of Nitrospira spp. Appl Environ Microbiol 79:2027–2037. https://doi.org/10.1128/AEM.03408-12
Sockett RE, Lambert C (2004) Bdellovibrio as therapeutic agents: a predatory renaissance? Nat Rev Microbiol 2:669–675. https://doi.org/10.1038/nrmicro959
Monnappa AK, Dwidar M, Mitchell RJ (2013) Application of bacterial predation to mitigate recombinant bacterial populations and their DNA. Soil Biol Biochem 57:427–435. https://doi.org/10.1016/j.soilbio.2012.09.010
Schoeffield AJ, Williams HN, Turng BF, Falkler WA (1996) A comparison of the survival of intraperiplasmic and attack phase bdellovibrios with reduced oxygen. Microb Ecol 32:35–46. https://doi.org/10.1007/BF00170105
Davidov Y, Jurkevitch E (2004) Diversity and evolution of Bdellovibrio-and-like organisms (BALOs), reclassification of Bacteriovorax starrii as Peredibacter starrii gen. nov., comb. nov., and description of the Bacteriovorax-Peredibacter clade as Bacteriovoracaceae fam. nov. Int J Syst Evol Microbiol 54:1439–1452. https://doi.org/10.1099/ijs.0.02978-0
Summers JK, Kreft J-U (2019) Predation strategies of the bacterium Bdellovibrio bacteriovorus result in overexploitation and bottlenecks. bioRxiv 44:1–36. https://doi.org/10.1101/621490
Santos M, Oliveira H, Pereira JL et al (2019) Flow cytometry analysis of low/high DNA content (LNA/HNA) bacteria as bioindicator of water quality evaluation. Ecol Indic 103:774–781. https://doi.org/10.1016/j.ecolind.2019.03.033
Wang Y, Hammes F, Boon N et al (2009) Isolation and characterization of low nucleic acid (LNA)-content bacteria. ISME J 3:889–902. https://doi.org/10.1038/ismej.2009.46
Kankaala P, Peura S, Nykänen H et al (2010) Impacts of added dissolved organic carbon on boreal freshwater pelagic metabolism and food webs in mesocosm experiments. Fundam Appl Limnol 177:161–176. https://doi.org/10.1127/1863-9135/2010/0177-0161
Kim S, Kim JH, Baek SH, Kim J (2020) The influence of dissolved organic carbon on the microbial community associated with Tetraselmis striata for bio-diesel production. Appl Sci 10:3601. https://doi.org/10.3390/app10103601
Jacquet S, Havskum H, Thingstad TF, Vaulot D (2002) Effects of inorganic and organic nutrient addition on a coastal microbial community (Isefjord, Denmark). Mar Ecol Prog Ser 228:3–14. https://doi.org/10.3354/meps228003
Williams-Linera G, Ewel JJ (1984) Effect of autoclave sterilization of a tropical andept on seed germination and seedling growth. Plant Soil 82:263–268. https://doi.org/10.1007/BF02220253
Guillemette F, Leigh Mccallister S, Del Giorgio PA (2016) Selective consumption and metabolic allocation of terrestrial and algal carbon determine allochthony in lake bacteria. ISME J 10:1373–1382. https://doi.org/10.1038/ismej.2015.215
Zhou L, Zhou Y, Tang X et al (2021) Resource aromaticity affects bacterial community successions in response to different sources of dissolved organic matter. Water Res 190:116776. https://doi.org/10.1016/j.watres.2020.116776
Striebel M, Kirchmaier L, Hingsamer P (2015) Different mixing techniques in experimental mesocosms—does mixing affect plankton biomass and community composition? Limnol Oceanogr Methods 11:176–186. https://doi.org/10.4319/lom.2013.11.176.Different
Shemesh Y, Yaacov D, Koval S, Jurkevitch E (2003) Samll eats big: ecology and diversity of Bdellovibrio and like organisms, and their dynamics in predator-prey interactions. Agronomie 23:433–439
Chen H, Athar R, Zheng G, Williams HN (2011) Prey bacteria shape the community structure of their predators. ISME J 5:1314–1322. https://doi.org/10.1038/ismej.2011.4
Wilkinson MHF (2001) Predation in the presence of decoys: an inhibitory factor on pathogen control by bacteriophages or Bdellovibrios in dense and diverse ecosystems. J Theor Biol 208:27–36. https://doi.org/10.1006/jtbi.2000.2197
Shemesh Y, Jurkevitch E (2004) Plastic phenotypic resistance to predation by Bdellovibrio and like organisms in bacterial prey. Environ Microbiol 6:12–18. https://doi.org/10.1046/j.1462-2920.2003.00530.x
Hobley L, King JR, Sockett RE (2006) Bdellovibrio predation in the presence of decoys: three-way bacterial interactions revealed by mathematical and experimental analyses. Appl Environ Microbiol 72:6757–6765. https://doi.org/10.1128/AEM.00844-06
Varon M, Shilo M (1969) Interaction of Bdellovibrio bacteriovorus and Host Bacteria. J Bacteriol 99:136–141
Hashimoto T, Diedrich DL, Conti SF (1970) Isolation of a Bacteriophage for Bdellovibrio bacteriovorus. J Virol 5:97–98
Personnic S, Domaizon I, Sime-Ngando T, Jacquet S (2009) Seasonal variations of microbial abundances and virus- versus flagellate-induced mortality of picoplankton in three peri-alpine lakes. J Plankton Res 31:1161–1177. https://doi.org/10.1093/plankt/fbp057
Jacquet S, Domaizon I, Chardon C, Personnic S (2013) Are small grazers and/or viruses a structuring factor of the free-living bacterial community in Lake Geneva? Adv Microbiol 3:233–248. https://doi.org/10.4236/aim.2013.33035
Parikka KJ, Le Romancer M, Wauters N, Jacquet S (2017) Deciphering the virus-to-prokaryote ratio (VPR): insights into virus–host relationships in a variety of ecosystems. Biol Rev 92:1081–1100. https://doi.org/10.1111/brv.12271
Acknowledgements
The authors would like to thank all those who participated in the MESOLAC project: Laurent Espinat, Clementine Gallot, Pascal Perney, Jean-Christophe Hustache, Philippe Quetin, Viet Tran-Khac, Laura Crepin, and Mathilde Chevallay. We also thank Cécilia Barouillet and Teofana Chonova for their statistical assistance. Finally, professional scuba divers (Jean-Marc Bel, Stéphanie Kocca, Stéphan Jacquet and Eric Mocelin) are gratefully acknowledged for their help in anchoring the eco-friendly (e.g. ecological mooring) mesocosms. We would like to thank Editage (www.editage.com) for English language editing.
Funding
JE (PhD grant) was funded by INRAE and University Savoie-Mont Blanc. AJ (Master grant) was funded by INRAE. The MESOLAC project was funded by INRAE and ANAEE France.
Author information
Authors and Affiliations
Contributions
JE participated to data analysis and writing. AJ helped to analyse the data. SR and ID co-organised the MESOLAC project with SJ. SJ co-organised the MESOLAC project with SR and ID, planned this study, participated to writing, and submitted the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ezzedine, J.A., Janicot, A., Rasconi, S. et al. Short-Term Dynamics of Bdellovibrio and Like Organisms in Lake Geneva in Response to a Simulated Climatic Extreme Event. Microb Ecol 84, 717–729 (2022). https://doi.org/10.1007/s00248-021-01875-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-021-01875-9