Abstract
Soil microbes may greatly affect plant growth. While plants are commonly associated with diverse communities of soil microbes, complementary roles of different microbial communities that may stimulate synergistic effects on plant growth are not adequately tested. Also, such synergistic effects may vary with environmental conditions such as soil nutrient and water availability. We conducted a greenhouse experiment with a widespread clonal plant Solidago canadensis. The experiment was a factorial design with four levels of soil microbial inoculation (fresh soil inocula from grasslands in northern and southern China that were expected to differ in soil microbial composition, a mixture of the two fresh soil inocula, and a sterilized mixed inoculum control), two levels of nutrient availability (low vs. high), and two levels of water supply (low vs. high, i.e., 1376 vs. 352 mm per year). Irrespective of water supply and nutrient availability, total, aboveground, and belowground mass of S. canadensis were generally higher when the plant grew in soil inoculated with a mixture of soil microbes from the south and north of China (in the mixed inoculum treatment) than when it grew in soil inoculated with soil microbes from only the north or the south or the sterilized control. Such effects of soil microbes on total and aboveground mass were stronger under high than under low nutrient availability and also under high than under low water supply. Our results suggest that interactions of different soil microbial communities can result in a synergistic effect on plant growth and such a synergistic effect depends on environmental conditions. The findings shed light on the importance of plant–microbe interactions during the spreading of some plant species in face of increased atmospheric nutrient deposition coupled with altered rainfall pattern due to global change.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Interactions between plants and soil microbes can have profound impacts on the structure and productivity of plant communities [1,2,3,4,5]. Such biotic interactions also play key roles in global carbon and nutrient cycling [6,7,8,9] and are important for predicting species distribution under ongoing global change [10,11,12,13]. Plants are commonly associated with diverse communities of soil microbes [14,15,16]. Thus, to better understand plant–soil interactions and their impacts, interactions of different soil microbial communities should be considered.
Soil microbial communities vary greatly with climate (e.g., rainfall), soil physico-chemical properties, and vegetation [17]. It is well known that soil microbial communities from different sources (e.g., climatic regions) may have different impacts on plant growth [13]. Furthermore, different soil microbial communities may interact to collectively determine plant growth [18, 19]. If the main functions of different soil microbial communities are complementary, then their collective effect could be synergistic, i.e., greater than the effect of any of the soil microbial communities. For instance, rhizobia and arbuscular mycorrhizal fungi (AMF) showed a synergistic interactive effect on the growth of broad bean Vicia faba [50]. By contrast, if different soil microbial communities compete fiercely for resources such as carbohydrates from plants, then an antagonistic effect may be shown [20, 21]. So far, however, complementary roles of different microbial communities that may stimulate synergistic effects on plant growth are not adequately tested (but see Hestrin et al. [26]).
The synergistic effect of different soil microbial communities may vary with soil water conditions. It is well known that soil microbial communities are sensitive to drought [22, 23] and that soil water conditions can greatly influence the effect of soil microbial communities on plant growth [24, 25]. For example, the mutually beneficial interactions between plant and soil microbial communities under moist conditions could be changed into detrimental interactions under drought [24, 26,27,28]. Given that different soil microbial communities show different dependencies on soil water conditions [25], changing soil water conditions may alter the synergistic effect of different soil microbial communities on plant growth. While many studies have investigated the effects of soil water conditions on plant–microbe interactions [3, 4, 23, 29, 30], to the best of our knowledge, very few studies have tested how soil water conditions affect the synergistic effect of soil microbial communities of different origins on plant growth.
Soil nutrient availability may also affect the synergistic effect of different soil microbial communities on plant growth. Soil nutrient availability can affect the structure and activity of soil microbial communities [31, 32] and influence their impacts on plant growth [33, 34]. As different soil microbial communities may respond differently to soil nutrient availability [35, 36], changing soil nutrient availability may alter the synergistic effect of different soil microbial communities on plant growth. For instance, Mack and Rudgers [20] found that high nutrient availability significantly increased the abundance of endophytic fungi and decreased AMF colonization, resulting in a final increase in plant biomass by ~ 400%. High nutrient availability could also stimulate soil biota interactions that promoted plant growth as the interactions induced a shift in plant–soil feedback from negative to positive [37, 38]. While many studies have tested the effects of soil nutrient availability on plant–microbe interactions [3,4,5, 29, 30], to the best of our knowledge, few have tested how soil nutrient availability affects the synergistic effect of soil microbial communities of different origins on plant growth.
We grew a widespread clonal plant Solidago canadensis in background soil with fresh soil inoculum collected from two different regions (north and south) of China, a mixture of the two types of fresh soil inocula and sterilized soil inoculum (control) under low versus high nutrient availability and under high versus low water supply. Specifically, we addressed (1) whether there is a synergistic interactive effect of the two soil microbial communities (from the two regions) on the growth of S. canadensis, (2) whether such a synergistic effect varies with soil nutrient availability, and (3) whether such a synergistic effect varies with soil water conditions.
Methods
Study Plant
Solidago canadensis L. (Asteraceae), a native of North America, is one of the most destructive invasive weeds in many countries, including China [39,40,41]. In China, it was introduced first to Shanghai as an ornamental plant in 1935 and has since spread into the wild [42]. This species can reproduce both sexually by producing large quantities of viable seeds and asexually by clonal growth from its rhizomes [43]. The seeds are tiny and thus easily dispersed by wind. Plants of S. canadensis can grow over 1.5 m tall. Due to its tall stature and extensive clonal growth, the species has a strong competitive ability and can displace native plant species, especially in habitats disturbed by human activities [44]. It grows mainly along roads, as well as in meadows, pastures, abandoned farmlands, ditches, upland forests, and savannas [45].
Seed Collection and Propagation
In September 2018, we collected seeds of S. canadensis from Taizhou (28° 38′ N, 121° 21′ E) in Zhejiang Province, China, and stored them at 4 °C. On May 25, 2019, the seeds were sterilized with 5% sodium hypochlorite and then sown in a sterilized commercial potting substrate in a tray in a greenhouse of Taizhou University. We covered the seed tray with a plastic lid to increase the humidity to encourage fast germination. We added tap water to the substrate every day to keep it moist. The seedlings were about 10 cm tall with 5–6 leaves before the start of the experiment.
Experimental Design
The experiment was a factorial design with four levels of microbial inoculation, two levels of nutrient availability (low vs. high), and two levels of water supply (low vs. high). The four levels of microbial inoculation were (1) fresh soil (pH = 7.6 ± 0.08, EC = 265 ± 0.33 μS cm−1, clay = 26.4 ± 0.40%, silt = 5.8 ± 0.15%, and sand = 67.8 ± 1.18%; n = 3) obtained from a grassland in Taizhou, Zhejiang Province, in southern China (hereafter referred to as “south inoculum”); (2) fresh soil (pH = 7.2 ± 0.07, EC = 244.3 ± 0.66 μS cm−1, clay = 20.4 ± 0.64%, silt = 9.6 ± 0.23%, sand = 70 ± 0.35%; n = 3) obtained from the Inner Mongolia grassland in northern China (hereafter referred to as “north inoculum”); (3) a mixture of south and north inocula (hereafter referred to as “mixed inoculum”); and (4) a steam-sterilized mixed inoculum soil (hereafter referred to as “control”). In the treatment with high nutrient availability, we added 7.2 g L−1 slow-release fertilizer (14 N: 14 P: 14 K; Osmocote 301, Scotts, USA) to the soil, and in the treatment with low nutrient availability, we added 2.5 g L−1 [20]. In the treatment with high water supply, we added 600 mL water per week to a pot with the target plant, equivalent to the annual precipitation regime of 1376 mm in Zhejiang province. In the treatment with low water supply, we supplied 150 mL water per week to a pot, equivalent to the annual precipitation regime of 351 mm in the Inner Mongolia (https://www.currentresults.com/Weather/China/average-yearly-precipitation.php) [5]. Each treatment had ten replicates.
On 22 July 2019, one seedling of S. canadensis was planted in the middle of a plastic pot (16 cm in diameter × 13.5 cm in height) filled with 2.4 L of a mixture (1:1, v:v) of washed river sand and soil collected from a grassland nearby Taizhou City in Zhejiang province, China. The sand–soil mixture (hereafter referred to as “background soil”) was steam-sterilized at 121 °C for 4 h and then thoroughly mixed with 7.5 g L−1 and 2.5 g L−1 of the slow-release fertilizer to mimic the high and low nutrient soils, respectively.
Fresh soils used as the south and north inocula were collected from the 0–15 cm soil layer from six different points spaced 60 m apart in Taizhou and Inner Mongolia, respectively. In sampling points in Taizhou (28° 38′ N, 121° 21′ E), the dominant vegetations were Phalaris arundinacae, Erigeron canadensis, Solidago canadensis, Bidens pilosa, and Erigeron annuus; in the sampling points in Inner Mongolia (40° 50’ N and 111° 43′ E), the vegetation was dominated by Leymus chinensis, Stipa krylovii, Agropyron cristatum, and Artemisia frigid. The inoculum soils were sampled from these locations so that we could obtain soil microbial communities that differ in species composition as these locations are known to vary with climatic and environmental conditions, and thus, the soil microbes collected were suitable for the aim of the study. The soil collected from each site was pulverized and homogenized to improve the uniformity of soil microbial distribution and stored at 5 °C until use. We homogenized the north and south inocula at the ratio of 1:1 (v:v) to create the mixed inoculum. The inocula were added at 10% (v/v) to the background soil after filling the pots to about two-thirds, and then the pots were capped with another small layer of sterilized background soil [46, 47]. In the control, the sterilized inoculum was added to the sterilized background soil and the pots were capped with a small layer of the sterilized background soil in the same way. This approach of mixing and arranging the substrates enabled us to reduce the effects of the potential differences in soil properties [48].
The pots were arranged randomly on a table in the greenhouse of Taizhou University. The experiment was conducted between July 22 and October 21 2019. During the experiment, we randomly re-arranged the pots every 3 weeks to avoid the potential effects of micro-environmental heterogeneity. The average temperature in the greenhouse was 26.1 °C, and the humidity was maintained at 84.7%. No plant died during the experiment. At the end of the experiment, about 40% of the plants had started flowering and 86.8% had produced offspring ramets.
Measurement and Harvesting
Prior to harvest, we measured the height of mother ramet and recorded number of leaves of the mother ramet and number of ramets in each pot. We harvested the aboveground and the belowground parts (i.e., roots and rhizomes) of each plant separately and then dried them at 70 °C for 72 h before weighing.
Statistical Analysis
We used three-way ANOVAs to examine the effects of soil microbes (control, north inoculum, south inoculum, and mixed inoculum), nutrient levels (low vs. high), and water supply (high vs. low) on biomass (total mass, aboveground mass, belowground mass, and mass per ramet), ramet number, and height and leaf number of the mother ramet of S. canadensis. Before analysis, all data were transformed to square root to improve normality and homogeneity of variance. The data were analyzed, and figures created in R v.3.5.1 [49] through an RStudio platform (v.1.1.442) [50].
Results
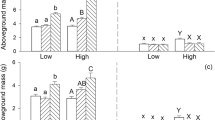
Irrespective of water supply and nutrient availability, total, aboveground, and belowground mass of Solidago canadensis were generally higher in the mixed inoculum treatment than in the control, the north, and the south inoculum treatments (Table 1; Fig. 1). However, such effects on total and aboveground mass were stronger under high and under low water supply and under high than under low nutrient availability (effects of soil microbes × water supply: both P < 0.001; effects of soil microbes × nutrient level: P = 0.006 or 0.001; Table 1; Fig. 1a, b). Such an effect on belowground biomass was also stronger under high than under low water supply (effect of soil microbes × water supply: P = 0.013; Table 1; Fig. 1c) but did not differ between the two nutrient treatments (effect of soil microbes × nutrient level: P = 0.445; Table 1).
Ramet number of S. canadensis was the highest in the mixed inoculum treatment, smallest in the north and the south inoculum treatments, and intermediate in the control (Table 1; Fig. 2a). Mass per ramet and height of the mother ramet of S. canadensis were greater in the south and mixed inoculum treatments than in the control and the north inoculum treatments, and such an effect was stronger under high than under low water supply (effect of soil microbes × water supply: P = 0.021 and 0.030, respectively; Table 1; Fig. 2b, c). Leaf number of the mother ramet of S. canadensis was greater in the south and mixed inoculum treatments than in the control and the north inoculum treatment (Table 1; Fig. 2d). Mass per ramet and height and leaf number of the mother ramet of S. canadensis under high nutrient availability and high water supply tended to be greater in the south inoculum treatment than in the mixed inoculum treatment (Fig. 2b–d).
Discussion
Interactions between different soil microbes may generate a synergistic effect on plant growth [18, 26, 51], and such an effect may vary with abiotic environmental conditions. While there is a large body of evidence that the effect of soil microbes on plant growth can vary with environmental conditions and that different soil microbial communities can have different effects on plant growth [19, 52, 53], there is little evidence that the synergistic effect of different soil microbial communities on plant growth depends on abiotic environmental conditions. Our study provides evidence that there were synergistic interactive effects of soil microbial communities from two different sources on biomass production of Solidago canadensis, and such synergistic effects varied with both soil water supply and soil nutrient availability.
Synergistic Effects of Soil Microbial Communities of Different Sources
We found that averaged across the water and nutrient treatments, inoculation with soil microbes from the north generally reduced the growth of S. canadensis compared to the sterilized control, suggesting that soil microbes from the north were harmful to S. canadensis. While, compared to the control, both inoculation with soil microbes from the south and inoculation with a mixture of soil microbes from the north and the south generally increased biomass production of S. canadensis, the positive effect was much stronger for the latter than for the former. As a result, S. canadensis produced greater biomass when inoculated with a mixture of the soil microbes from the north and the south than when inoculated with soil microbes from either the north or the south. These results suggest that soil microbial communities from the north and the south could interact to produce a synergistic effect on biomass production of S. canadensis.
A synergistic effect of different soil microbial communities on plant growth has also been reported before [18, 54]. For instance, Hestrin et al. [26] found a synergistic positive effect between mycorrhizal fungi and free-living soil microbial communities on the growth of the grass Brachypodium distachyon. Likewise, a recent study showed a synergistic positive effect between rhizobium and AMF on the growth of maize (Zea mays) [55]. However, Bauer et al. [47] found additive effects instead of their expected synergistic effects of the interaction between rhizobia and AMF on the growth of Panicum virgatum when it was grown in monoculture. They, however, attributed their findings to the inability of these microbial functional groups to form a synergy due to the short-term nature of their study period. Similarly, additive effects have also been reported on the interaction between two functionally dissimilar soil organisms (i.e., AMF and root herbivores) on the total productivity of selected grassland species [56]. In that study, Ladygina et al. [55] observed that the positive effects of AMF on the growth of the species were ruled out by the biomass reduction effects that resulted from the activities of wireworms and nematodes (root herbivores).
In the present study, under the conditions of high nutrient availability and high water supply where S. canadensis commonly grows and invades, we found a positive biomass response of S. canadensis to soil microbes from the south, but a negative biomass response to soil microbes from the north (Fig. 1a, b). We suggest that these disparities in growth responses to the different soil types may be due to differences in co-evolutionary histories between plant species and soil microbes [57, 58] and in climatic conditions [59]. In this study, the soil inocula from the south were collected in the invaded area of S. canadensis. Thus, it is not surprising that we found a positive growth response to soil microbes from the south as it is frequently reported that in the invaded range alien invasive species commonly have positive feedback from soil microbes [60,61,62]. By contrast, S. canadensis has not invaded in the Inner Mongolia grasslands where the soil inocula from the north were collected [42]. Due to a lack of co-evolutionary histories and the differences in climatic conditions, especially precipitation, it is reasonable that soil microbes from the north had a harmful effect on the growth of S. canadensis, as also found in previous studies [63,64,65].
Previous studies have indicated that soil microbes from different functional groups or sources can differ greatly in terms of functional characteristics and roles [18, 19]. Based on the finding that S. canadensis showed different growth responses to soil microbes from the north and the south, we hypothesized that soil microbes from these two sources may play different functional roles and some of the functions are also complementary, as reported in a previous study [66, 67]. Due to such complementarity, these soil microbes of different sources could interact to provide more limiting resources such as water and nutrients, which promote the growth of S. canadensis. Consequently, we observed a stronger, positive response of biomass production of S. canadensis to the mixture of soil microbes than to soil microbes from a single source (from the north or the south), demonstrating a synergetic effect on plant growth. The positive feedback from soil microbes in the south where S. canadensis is currently widespread and the synergistic effect (i.e., enhanced positive effect) may highly increase the adaptation and competitive dominance of this invasive plant species in the invaded range [65]. Moreover, the synergistic effects of different microbial communities on the growth performance of S. canadensis could be one of the mechanisms underlying the range expansion for this invasive species in China [41] and possible also for other invasive species [68, 69].
We did not detect a synergistic effect of soil microbes on the size of the S. canadensis ramets as none of the three size measures (mass per ramet, plant height, and leaf number) was larger when S. canadensis grew in soil inoculated with a mixture of soil microbes than when it grew in soil inoculated with soil microbes from the south (Fig. 2b–d). By contrast, under high nutrient availability and high water supply, ramet size of S. canadensis tended to be larger in soil inoculated with microbes from the south than in soil inoculated with a mixture of microbes from the north and the south (Fig. 2b–d), although the number of ramets was smaller (Fig. 2a). This result suggests that the synergistic effect on biomass production of S. canadensis was due to its effect on ramet production, but not its effect on individual ramet size. Similarly, synergistic effects between AMF and rhizobia on biomass of grassland species (e.g., Festuca ovina, Trifolium arvense, and Plantago lanceolata) were also ascribed to the occurrence of the synergistic effects on reproductive output [52].
Environment Dependence of Synergistic Effects
We found that the extent of the synergistic effect of soil microbes from the north and the south on biomass production of S. canadensis varied with environmental conditions. Specifically, the synergistic effect was stronger under high than under low nutrient availability and under high than under low water supply. Given that interactions between soil microbes can result in a synergistic effect on plant growth, if high nutrient and/or high water availability can strengthen such interactions compared to low nutrient or water availability, then a stronger a synergistic effect may occur.
In the current study, we observed a synergic effect of different soil microbes on biomass of S. canadensis. Furthermore, we found a significant positive effect of soil microbes at both high and low nutrient availabilities only when there was a mixture of soil microbes from the north and the south, but the effect of soil microbes from the south and from the north depended on soil water and nutrient availability. Similarly, many studies have shown that soil microbes differ in their responses to soil nutrient availability and soil water availability, which ultimately influences their functional performance and activities [22, 70, 71]. For instance, soil microbial symbionts exchange nutrients for carbon with their host plants; however, in a situation where available nutrients required by soil microbes to offset the carbon cost are insufficient, the interaction often induces inhibitory effects instead of synergistic effects [26, 72]. Moreover, nutrient stress could stimulate microbial communities to compete for the limited available nutrients [73]. As high nutrient and water availability could increase complementary roles of different soil microbes, they increased the synergistic effects of different soil microbes.
In the soil inoculated with soil microbes from the north, a negative response to microbial interaction gradually reduced with increasing water and nutrient availability. Also, in the soil inoculated with soil microbes from the south, a negative growth response at low nutrient availability and under high water supply disappeared under high water supply and nutrient availability. However, soil microbes from the north and south generated a strong positive effect when the two soils were co-inoculated under high water supply and high nutrient availability. A support to our finding is the recent work in which the addition of high nutrients decreases the negative feedback of 24 European grassland species [34]. Also, a low feedback response of Leontodon hispidus to the interaction of AMF at low nutrients gradually increased with the addition of high nutrients [37]. Nutrient availability ultimately shifts feedback from negative to positive due to the overall increase in plant fitness and resistance to pathogenic diseases [63]. Therefore, synergistic effects of microbial interaction under high nutrients and water availability may generally promote the fitness of plants and stimulate strong resistance to pathogenic stress.
Conclusions
Our results suggest that the interaction of soil microbial communities of different sources could have a synergistic effect on plant growth. Our results also provide evidence that water supply and nutrient availability may have a potential impact on interactions between different microbial communities so that they can alter their synergistic effects on plant growth. In this study, we did not quantify the composition and functions of the soil microbial communities in the two soil sources. Thus, we do not know the potential differences between these two soil communities and cannot explore the mechanisms underlying these synergistic effects. Further studies could test whether synergistic effects of different soil microbial communities were due to functional complementary of different soil microbial communities or other mechanisms.
References
Bever JD, Mangan SA, Alexander HM (2015) Maintenance of plant species diversity by pathogens. Annu Rev Ecol Evol Syst 46:305–325
Fry EL, Johnson GN, Hall AL, Pritchard WJ, Bullock JM, Bardgett RD (2018) Drought neutralises plant-soil feedback of two mesic grassland forbs. Oecologia 186:1113–1125
Kaisermann A, de Vries FT, Griffiths RI, Bardgett RD (2017) Legacy effects of drought on plant-soil feedbacks and plant-plant interactions. New Phytol 215:1413–1424
Kannenberg SA, Phillips RP (2017) Soil microbial communities buffer physiological responses to drought stress in three hardwood species. Oecologia 183:631–641
Xi NX, Chu CJ, Bloor JMG (2018) Plant drought resistance is mediated by soil microbial community structure and soil-plant feedbacks in a savanna tree species. Environ Exp Bot 155:695–701
Adair EC, Parton WJ, Del Grosso SJ, Silver WL, Harmon ME, Hall SA, Burke IC, Hart SC (2008) Simple three-pool model accurately describes patterns of long-term litter decomposition in diverse climates. Glob Chang Biol 14:2636–2660
Delgado-Baquerizo M, Reich PB, Trivedi C, Eldridge DJ, Abades S, Alfaro FD, Bastida F, Berhe AA, Cutler NA, Gallardo A, García-Velázquez L, Hart SC, Hayes PE, He J-Z, Hseu Z-Y, Hu H-W, Kirchmair M, Neuhauser S, Pérez CA, Reed SC, Santos F, Sullivan BW, Trivedi P, Wang J-T, Weber-Grullon L, Williams MA, Singh BK (2020) Multiple elements of soil biodiversity drive ecosystem functions across biomes. Nature Ecology & Evolution 4:210–220
Soong JL, Fuchslueger L, Marañon-Jimenez S, Torn MS, Janssens IA, Penuelas J, Richter A (2020) Microbial carbon limitation: the need for integrating microorganisms into our understanding of ecosystem carbon cycling. Glob Chang Biol 00:1–9
Shi M, Fisher JB, Brzostek ER, Phillips RP (2016) Carbon cost of plant nitrogen acquisition: global carbon cycle impact from an improved plant nitrogen cycle in the Community Land Model. Glob Chang Biol 22:1299–1314
van der Putten WH, Macel M, Visser ME (2010) Predicting species distribution and abundance responses to climate change: why it is essential to include biotic interactions across trophic levels. Philosophical Transaction of The Royal Society B 365:2025–2034
Engelkes T, Morriën E, Verhoeven KJF, Bezemer TM, Biere A, Harvey JA, McIntyre LM, Tamis WLM, van der Putten WH (2008) Successful range-expanding plants experience less above-ground and below-ground enemy impact. Nature 456:946–948
Manrubia M, van der Putten WH, Weser C, Veen C (2020) Rhizosphere and litter feedbacks to range-expanding plant species and related natives. J Ecol 108:353–365
Ramirez KS, Snoek LB, Koorem K, Geisen S, Bloem LJ, ten Hooven F, Kostenko O, Krigas N, Manrubia M, Caković D, van Raaij D, Tsiafouli MA, Vreš B, Čelik T, Weser C, Wilschut RA, van der Putten WH (2019) Range-expansion effects on the belowground plant microbiome. Nature Ecology & Evolution 3:604–611
Herman DJ, Firestone MK, Nuccio E, Hodge A (2012) Interactions between an arbuscular mycorrhizal fungus and a soil microbial community mediating litter decomposition. FEMS Microbiol Ecol 80:236–247
Barnard RL, Osborne CA, Firestone MK (2015) Changing precipitation pattern alters soil microbial community response to wet-up under a Mediterranean-type climate. The ISME journal 9:946–957
Fierer N (2017) Embracing the unknown: disentangling the complexities of the soil microbiome. Nat Rev Microbiol 15:579–590
Lehmann A, Zheng W, Rillig MC (2017) Soil biota contributions to soil aggregation. Nature Ecology & Evolution 1:1828–1835
Porter SS, Bantay R, Friel CA, Garoutte A, Gdanetz K, Ibarreta K, Moore BM, Shetty P, Siler E, Friesen ML (2020) Beneficial microbes ameliorate abiotic and biotic sources of stress on plants. Funct Ecol 00:1–12
Larimer AL, Bever JD, Clay K (2010) The interactive effects of plant microbial symbionts: a review and meta-analysis. Symbiosis 51:139–148
Mack K, Rudgers J (2008) Balancing multiple mutualists: asymmetric interactions among plants, arbuscular mycorrhizal fungi, and fungal endophytes. Oikos 117:310–320
Stanton Maureen L (2003) Interacting guilds: moving beyond the pairwise perspective on mutualisms. Am Nat 162:S10–S23
Schimel JP (2018) Life in dry soils: effects of drought on soil microbial communities and processes. Annu Rev Ecol Evol Syst 49:409–432
Manzoni S, Schimel JP, Porporato A (2012) Responses of soil microbial communities to water stress: results from a meta-analysis. Ecology 93:930–938
Rousk J, Smith A, Jones D (2013) Investigating the longterm legacy of drought and warming on the soil microbial community across five European shrubland ecosystems. Glob Chang Biol 19:3872–3884
Huang G, Li Y, Su YG (2015) Divergent responses of soil microbial communities to water and nitrogen addition in a temperate desert. Geoderma 251-252:55–64
Hestrin R, Hammer EC, Mueller CW, Lehmann J (2019) Synergies between mycorrhizal fungi and soil microbial communities increase plant nitrogen acquisition. Communications Biology 2:233
Desbrosses Guilhem J, Stougaard J (2011) Root nodulation: a paradigm for how plant-microbe symbiosis influences host developmental pathways. Cell Host Microbe 10:348–358
Taylor BN, Menge DNL (2018) Light regulates tropical symbiotic nitrogen fixation more strongly than soil nitrogen. Nature Plants 4:655–661
Marasco R, Rolli E, Ettoumi B, Vigani G, Mapelli F, Borin S, Abou-Hadid AF, El-Behairy UA, Sorlini C, Cherif A, Zocchi G, Daffonchio D (2012) A drought resistance-promoting microbiome is selected by root system under desert farming. PLoS One 7:e48479
Ochoa-Hueso R, Collins SL, Delgado-Baquerizo M, Hamonts K, Pockman WT, Sinsabaugh RL, Smith MD, Knapp AK, Power SA (2018) Drought consistently alters the composition of soil fungal and bacterial communities in grasslands from two continents. Glob Chang Biol 24:2818–2827
Yan G, Xing Y, Xu L, Wang J, Dong X, Shan W, Guo L, Wang Q (2017) Effects of different nitrogen additions on soil microbial communities in different seasons in a boreal forest. Ecosphere 8:e01879
Treseder KK (2008) Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol Lett 11:1111–1120
Harrison KA, Bardgett RD (2010) Influence of plant species and soil conditions on plant-soil feedback in mixed grassland communities. J Ecol 98:384–395
Petermann J, Fergus A, Turnbull L, Schmid B (2008) Janzen-Connell effects are widespread and strong enough to maintain diversity in grasslands. Ecology 89:2399–2406
Johnson NC, Rowland DL, Corkidi L, Egerton-Warburton LM, Allen EB (2003) Nitrogen enrichment alters mycorrhizal allocation at five mesic to semiarid grasslands. Ecology 84:1895–1908
Treseder KK (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol 164:347–355
in't Zandt D, van den Brink A, de Kroon H, Visser EJW (2019) Plant-soil feedback is shut down when nutrients come to town. Plant Soil 439: 541–551
van der Putten WH, Bardgett RD, Bever JD, Bezemer TM, Casper BB, Fukami T, Kardol P, Klironomos JN, Kulmatiski A, Schweitzer JA, Suding KN, Van de Voorde TFJ, Wardle DA (2013) Plant-soil feedbacks: the past, the present and future challenges. J Ecol 101:265–276
Abhilasha D, Quintana N, Vivanco J, Joshi J (2008) Do allelopathic compounds in invasive Solidago canadensis s.l. restrain the native European flora? J Ecol 96:993–1001
Zhang CB, Wang J, Qian BY, Li WH (2009) Effects of the invader Solidago canadensis on soil properties. Appl Soil Ecol 43:163–169
Li J, Du L, Guan W, Yu F-H, van Kleunen M (2016) Latitudinal and longitudinal clines of phenotypic plasticity in the invasive herb Solidago canadensis in China. Oecologia 182:755–764
Li B, Hsu PS, Chen JK (2001) Perspectives on general trends of plant invasions with special reference to alien weed flora of Shanghai. Chinese Biodiversity Science 9:446–457
Adomako MO, Ning L, Tang M, Du D-L, van Kleunen M, Yu F-H (2019) Diversity- and density-mediated allelopathic effects of resident plant communities on invasion by an exotic plant. Plant Soil 440:581–592
Chen T, Liu WL, Zhang CB, Wang J (2012) Effects of Solidago canadensis invasion on dynamics of native plant communities and their mechanisms. Chinese Journal of Plant Ecology 36:253–261
Xu Z, Peng H, Feng Z, Abdulsalih N (2014) Predicting current and future invasion of Solidago canadensis: a study from China. Pol J Ecol 62:263–271
Whitaker BK, Bauer JT, Bever JD, Clay K (2017) Negative plant-phyllosphere feedbacks in native Asteraceae hosts—a novel extension of the plant-soil feedback framework. Ecol Lett 20:1064–1073
Bauer J, Kleczewski N, Bever J, Clay KRH (2012) Nitrogen-fixing bacteria, arbuscular mycorrhizal fungi, and the productivity and structure of prairie grassland communities. Oecologia 170:1089–1098
Troelstra SR, Wagenaar R, Smant W, Peters BAM (2001) Interpretation of bioassays in the study of interactions between soil organisms and plants: involvement of nutrient factors. New Phytol 150:697–706
Core Team R (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
RStudio Team (2015) Integrated development environment for R. RStudio, Inc., Boston, MA
Jia Y, Gray VM, Straker CJ (2004) The influence of Rhizobium and arbuscular mycorrhizal fungi on nitrogen and phosphorus accumulation by Vicia faba. Ann Bot 94:251–258
van der Heijden MGA, Sd B, Luckerhoff L, van Logtestijn RSP, Schlaeppi K (2016) A widespread plant-fungal-bacterial symbiosis promotes plant biodiversity, plant nutrition and seedling recruitment. The ISME Journal 10:389–399
Lekberg Y, Bever JD, Bunn RA, Callaway RM, Hart MM, Kivlin SN, Klironomos J, Larkin BG, Maron JL, Reinhart KO, Remke M, van der Putten WH (2018) Relative importance of competition and plant–soil feedback, their synergy, context dependency and implications for coexistence. Ecol Lett 21:1268–1281
Rasmussen PU, Bennett AE, Tack AJM (2020) The impact of elevated temperature and drought on the ecology and evolution of plant-soil microbe interactions. J Ecol 108:337–352
Moreira H, Pereira SIA, Vega A, Castro PML, Marques APGC (2020) Synergistic effects of arbuscular mycorrhizal fungi and plant growth-promoting bacteria benefit maize growth under increasing soil salinity. J Environ Manag 257:109982
Ladygina N, Henry F, Kant MR, Koller R, Reidinger S, Rodriguez A, Saj S, Sonnemann I, Witt C, Wurst S (2010) Additive and interactive effects of functionally dissimilar soil organisms on a grassland plant community. Soil Biol Biochem 42:2266–2275
Callaway RM, Rout ME (2010) Soil biota and plant invasions: biogeographical effects on plant–microbe interactions. In: Richardson, DM (ed.) Fifty years of invasion ecology, pp. 131-142
Richardson DM, Allsopp N, D ‘Antonio CM, Milton SJ, M. R (2000) Plant invasions—the role of mutualisms. Biol Rev Camb Philos Soc 75: 65–93
Wei S, Dai Y, Liu B, Zhu A, Duan Q, Wu L, Zhang Y, Ji D, Ye A, Yuan H, Zhang Q, Chen D, Chen M, Chu J, Dou Y, Guo J, Li H, Li J, Liang L, Liang X, Liu H, Liu S, Miao C, Zhang Y (2013) A China data set of soil properties for land surface modeling. Journal of Advances in Modeling Earth Systems 5:212–224
Callaway RM, Bedmar EJ, Reinhart KO, Silvan CG, Klironomos J (2011) Effects of soil biota from different ranges on Robinia invasion: acquiring mutualists and escaping pathogens. Ecology 92:1027–1035
Klironomos JN (2002) Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 417:67–70
Bever JD (2002) Negative feedback within a mutualism: host specific growth of mycorrhizal fungi reduces plant benefit. Proceedings of the Royal Society B Biological Sciences 269:2595–2601
Ehrenfeld JG, Ravit B, Elgersma K (2005) Feedback in the plant-soil system. Annu Rev Environ Resour 30:75–115
van der Putten WH, Van Dijk C, Peters BAM (1993) Plant-specific soil-borne diseases contribute to succession in foredune vegetation. Nature 362:53–56
Rúa MA, Antoninka A, Antunes PM, Chaudhary VB, Gehring C, Lamit LJ, Piculell BJ, Bever JD, Zabinski C, Meadow JF, Lajeunesse MJ, Milligan BG, Karst J, Hoeksema JD (2016) Home-field advantage? Evidence of local adaptation among plants, soil, and arbuscular mycorrhizal fungi through meta-analysis. BMC Evol Biol 16:122
Ma L, Huang W, Guo C, Wang R, Xiao C (2012) Soil microbial properties and plant growth responses to carbon and water addition in a temperate steppe: the importance of nutrient availability. PLoS One 7:e35165
Hoeksema JD, Chaudhary VB, Gehring CA, Johnson NC, Karst J, Koide RT, Pringle A, Zabinski C, Bever JD, Moore JC, Wilson GWT, Klironomos JN, Umbanhowar J (2010) A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol Lett 13:394–407
Lu X, He M, Ding J, Siemann E (2018) Latitudinal variation in soil biota: testing the biotic interaction hypothesis with an invasive plant and a native congener. The ISME Journal 12:2811–2822
Reinhart KO, Callaway RM (2006) Soil biota and invasive plants. New Phytol 170:445–457
Herman RP, Provencio KR, Torrez RJ, Seager GM (1993) Effect of water and nitrogen additions on free-living nitrogen fixer populations in desert grass root zones. Appl Environ Microbiol 59:3021–3026
Schimel JP, Balser T, Wallenstein M (2007) Microbial stress-response physiology and its implications for ecosystem function. Ecology 88:1386–1394
Johnson NC (2010) Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol 185:631–647
Ho J, Chambers LG (2019) Altered soil microbial community composition and function in two shrub-encroached marshes with different physicochemical gradients. Soil Biol Biochem 130:122–131
Acknowledgments
We thank Dr. Ayub M.O. Odour (Technical University of Kenya, Kenya) and two anonymous reviewers for their comments on an early version of the manuscript. We also thank Miss Angella Nabasirye (Taizhou University) for her assistance with the experiment.
Funding
This study was supported by the NSFC (31761123001, 31870610) and the Ten Thousand Talent Program of Zhejiang Province (2018R52016) and the Joint Fund of Zhejiang Provincial Natural Science Foundation (LTZ20C030001).
Author information
Authors and Affiliations
Contributions
Michael Opoku Adomako (MOA) conceived the idea for the experiment; MOA performed the experiment and collected the data; MOA, Fei-Hai Yu (FHY), and Wei Xue (WX) performed data analyses; MOA drafted the manuscript with input from FHY, Min Tang (MT) and WX; and FHY contributed substantially towards the revision of the manuscript with input from Dao-Lin Du.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Adomako, M.O., Xue, W., Tang, M. et al. Synergistic Effects of Soil Microbes on Solidago canadensis Depend on Water and Nutrient Availability. Microb Ecol 80, 837–845 (2020). https://doi.org/10.1007/s00248-020-01537-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-020-01537-2