Abstract
Arbuscular mycorrhizal fungi (AMF) are symbiotic fungi with a broad distribution, and many taxa have physiological and ecological adaptations to specific environments, including semiarid ecosystems. Our aim was to address regional distribution patterns of AMF communities in such semiarid environments based on spore morphological techniques. We assessed AMF spores at the bottom and top of inselbergs distributed throughout the tropical dry forest in the Northeast region of Brazil. Across 10 replicate inselbergs and the surrounding area, spanning a range of altitude between 140 and 2000 m, we scored the AMF soil diversity and properties in 52 plots. We fitted parsimonious ordination analyses and variance partitioning models to determine the environmental factors which explained the variation in AMF community, based on morphological spore analysis. The diversity of AMF was similar at the bottom and top of inselbergs; however, we detected high variation in abundance and richness across sites. We formulated a parsimonious richness model that used physical soil factors as predictors. The AMF community structure could be best explained through the variables coarse and total sand, iron, organic matter, potassium, silt, and sodium which together accounted for 17.8% of total variance. Several AMF species were indicators of either deficiency or high values of specific soil properties. We demonstrated that habitat isolation of the inselbergs compared with surrounding areas did not trigger differences in AMF communities in semiarid regions of Brazil. At the regional scale, soil predictors across sites drove the distribution of symbiotic mycorrhizal fungi.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The tropical dry forest biome extends over more than 40% of the total surface area of the tropics and subtropics [1]. In Brazil, this biome occurs mainly in the Northeast region of the country, where the dominant vegetation is caatinga shrubs, influenced by topographical parameters and soil type [2]. Often, these landscapes are characterized by rocky outcrops known as inselbergs that result in unique physical and biotic conditions. In the inselbergs, limiting resources including moisture and soil nutrients influence the plant communities which form particular vegetation ecotones [3, 4]. These distinct plant communities experience a relatively high degree of isolation and often experience adverse weather conditions. Both these two properties make it likely that the plants occurring at inselbergs associate with distinct communities of symbiotic microbes and depend on them for survival. We thus believe that symbiotic microbes occurring at the top of inselbergs warrant further scientific attention.
Caatinga environments usually suffer the simultaneous stress of low nutrient availability, soil organic matter, and irregular precipitation. As a result, arbuscular mycorrhizal fungi (AMF) are considered an indispensable component of the ecosystem [5]. AMF are formed by a monophyletic group of fungi; these fungi are obligate plant-root mutualists of the vast majority of terrestrial plant species influencing plant community structure [6] and ecosystem processes [7]. The symbiosis is classified as nutritional, where phosphorus is delivered to the plant in return for carbon. However, AMF also convey an entire range of non-nutritional benefits to their plant hosts [8] such as tolerance to water stresses [9] and protection against pathogens [10]. Moreover, arbuscular mycorrhiza influence the cycling rates of C, N and possibly other nutrients (e.g. [11, 12]) and improve soil structure, soil aggregation and water infiltration [13]. In this sense, AMF may permit plants to establish in habitats where they would otherwise be absent [14], influencing plant community dynamics and diversity [6, 15].
In many semiarid ecosystems, researchers have detected exceptionally diverse AMF assemblages in the dual niche occupied by these fungi, plant roots [16, 17] and soil [18]. The AMF community characterization is historically based on measurement of morphological features of spores, although in recent years, this technique has been often replaced with DNA-based methods. Both approaches have limitations, but can provide complementary information for taxonomic and ecological studies. Spore-based approaches yield important information concerning land management on AMF propagules, abundances and richness in the soil [19, 20]. Furthermore, morphological spore identification may allow better differentiation of fungal taxa and can be quite sensitive in terms of detecting changes in AMF community composition and diversity [21].
The factors that influence the structure of AMF communities at both local and regional scales have not yet been studied in sufficient depth. Studying the influence of environmental drivers on AMF community structure at different spatial scales (i.e. local, regional and global) can give us a more robust overview of the ecology of arbuscular mycorrhizal systems [22]. A factor that may influence the distribution of AMF taxa is environmental filtering, including the abiotic factors in a particular location that prevent the establishment or persistence of species. Through studying AMF taxa at higher taxonomic levels, it became apparent that taxa belonging to different AMF families appear to have distinct ecological preferences [23]. As an example, species of the family Glomeraceae appear to thrive in soils with high availability of nutrients, while fungi in the Gigasporaceae prevail in soils with low nutrients [24]. This may reflect physiological differences found across AMF groups and, depending on the relative composition of the AMF community, give rise to systematic differences in the kind of ecosystem services the plant hosts receive from their mycosymbionts across different environments [25].
Studies conducted at a regional scale have found that the structure of AMF communities is influenced by a broad range of factors including the environment, interspecific competition and regional spatial dynamics [24]. The relative influence of the abovementioned factors may change for studies carried out at different spatial scales [22]. When working at larger spatial scales, it is considerably easier to accurately assess the influence of the different factors that influence the distribution of AMF when working in relatively uniform climatic conditions [26]. In semiarid regions, the climatic conditions are relatively static and it appears that the biogeography of microorganisms is determined to a larger extent by environmental factors than by geographic distance and spatial distribution patterns [27]. Our study aims at addressing how drivers of AMF community structure described above might differ across sites in semiarid communities when they are assessed based on spore morphological techniques. Thus, we hypothesize that we will detect more diverse AMF communities at the bottom than at the top of inselbergs (1). We also expected that the harsh environmental conditions on top of the inselbergs would result in plants colonized by distinct assemblages of AMF (2). As we argue earlier, AMF communities are subject to a strong environmental filtering. This is why we thought that, at larger spatial scales (i.e. regional scale), we would find a strong relationship between the community structure of AMF and environmental drivers. We additionally expect AMF occurrence in our system to be less stochastic than in moist or temperate systems [28]. We justify this because plants in the semiarid region experience a wider range of stresses, such as drought and extreme temperatures. We thus hypothesized that we can explain the environmental variability with a variance partitioning approach at a regional scale (3). Finally, we wanted to make our results comparable to older surveys of AMF in the region. For many of these studies, we had to infer environmental parameters. This is why we concluded our study through identifying AMF taxa that could be informative of environmental properties. We hypothesize that we could classify several of our AMF taxa into groups of indicator species (4).
Methods
Study Area
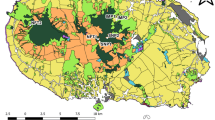
The semiarid region of Brazil is covered by a tropical dry forest biome that comprises part of the Brazilian Northeast and North of the State of Minas Gerais. The plant community is characterized by caatinga vegetation, and climatic conditions are described by low levels of humidity, an irregular and brief rainy season (average 750 mm/year) and long periods of drought [29, 30]. The climate variation explains most of the differences in the composition of the caatinga vegetation between the inselbergs with plants of the families Cyperaceae, Poaceae, Euphorbiaceae, Fabaceae, Orchidaceae, Bromeliaceae and Cactaceae being relatively abundant [31]. The sampling was carried out in 13 areas in the semiarid, where in each area we defined four sampling sites, separated at least 1 km from each other (Fig. 1). In each site, one 100 m2 plot was chosen, from which 10 soil samples were randomly collected to obtain a representative composite sample of the plot. In those sampled areas, 10 were in inselbergs with altitudes ranging from 140 to 2130 m. For each of these inselbergs, one sample was obtained at the top and three at the base. We additionally included three sites that were not centred on an inselberg, aiming to get a more representative picture of the AMF communities’ structure away of inselbergs. For each of these three sites, we implemented a comparable protocol to what we used for the inselbergs, each consisting of four samples at a distance of approximately 1 km from each other. In total, 52 plots were sampled. The sampling was performed between August and September 2014.
Soil Analysis and AMF Identification
Glomerospores were extracted from 50 g of soil by decanting and wet sieving followed by centrifugation in water and in a 50% sucrose solution [32, 33]. The spores were separated under the microscope into morphotypes based on colour and size. The taxonomic identification was based on the morphology of the AMF spores (spores of each species were counted to assess their abundance), consulting publications with descriptions of new species and databases (http://invam.caf.wvu.edu; http://www.zor.zut.edu.pl/Glomeromycota/Taxonomy.html). Soil properties including copper (Cu), aluminium (Al), zinc (Zn), manganese (Mn), phosphorus (P), soil pH, potassium (K), sodium (Na), calcium (Ca), magnesium (Mg), hydrogen (H), sum of bases (S.B), cation exchange capacity (C.E.C), bases saturation (V), carbon (C), aluminium saturation (m), organic matter (OM), sand, clay and silt were measured at the Estação Experimental de Cana-de-Açúcar do Carpina, of the Universidade Federal Rural de Pernambuco, Brazil. Climate data, temperature and precipitation were extracted from the Global Climate Data [34] at a resolution of 30 s.
Data Analysis
The differences in AMF alpha diversity and species abundance (spores number) from the top and bottom of each inselberg were compared through paired t tests of each sample mean values. The sampling intensity was normalized by standardizing the soil sample from which spores were extracted to 50 g. To measure the influence of environmental factors on richness (response variable), a general purpose full model was built. In this full model, we used a blocking factor (a categorical factor with discrete values for each inselberg—i.e. four samples), climatic data and a range of abiotic variables describing soil texture, nutrient availability and altitude. We simplified this model with a forward and backward (bidirectional) AIC-minimizing selection approach that was implemented through the command stepAIC. The null model in our case consisted of a single predictor, the blocking factor (inselbergs).
We first Hellinger transformed [35] the spore community data to correct for double absences and we applied a principal coordinates of neighbour matrices approach as implemented with the command pcnm in the package ‘vegan’ [36] to correct for spatial autocorrelation. Then, we fitted a full model with all the abiotic predictors we measured, which included soil texture, altitude, latitude, longitude and nutrient status of the soil. We then applied a forward and backward (bidirectional) selection approach with a p < 0.05 condition to identify the soil variables that we would further consider in our model. We also tried PERMANOVA as an alternative approach to assess significant effects of latitude and altitude the fungal spore community using the function adonis in the ‘vegan’ package. We use a manual forward-and-backward (bidirectional) selection procedure at p ≤ 0.05.
We finally carried out a variance partitioning of our community matrix according to [37]. We first simplified the RDA model to retain the subset of predictors that influenced significantly the community table. That way, we also addressed issues of collinearity across our predictors. We then used an indicator species analysis to establish whether any particular AMF taxa were characteristic of particular levels of soil properties. For each of the significant soil properties, we identified instances of values lower than the first quantile (i.e. low) and higher than the third quantile (i.e. high). We carried out the indicator species analysis with the command multipatt function in the ‘indicspecies’ package [38]. The analyses were performed with the statistical program R, version 3.2.3 [39]. The packages are available at https://cran.r-project.org.
Results
AMF Diversity
In the tropical dry forest of Brazil, we recorded 82 morphospecies of AMF, belonging to nine families and 18 genera, distributed among the inselbergs tops and surrounding areas (Table 1). Twenty-six morphospecies were shared by the top and bottom of the inselbergs. Eight species were exclusively detected at the tops of the inselbergs, and the most frequent taxa were within Acaulospora, Glomus and Dominikia genera. In the surrounding areas of the outcrops, we found 48 morphospecies that were not found at any inselberg top. Acaulospora and Glomus were the most representative genera in these areas, encompassing 24 and 11 taxa, respectively. The most strongly represented families were Acaulosporaceae containing 24 morphospecies and Glomeraceae with 23 across the sample sites. The most dominant AMF species occurred in more than 40% of the total sites sampled and they belonged to Acaulospora, Ambispora and Glomus genera. The morphospecies most abundant and with frequent occurrence records were Glomus macrocarpum which was found in 95% of the samples, followed by Acaulospora excavata (61%), Glomus brohultii (56%) and Claroideoglomus sp.1 occurred in 52% of the sites. The identified species are classified according to type of spore formation as acaulosporoid, entrophosporoid, gigasporoid, glomoid and scutellosporoid. The most abundant species groups were acaulosporoid, glomoid and scutellosporoid. The abundance and richness of AMF species were similar between the tops and bottoms of inselbergs; however, when the top and surrounding area were considered together, the abundance differed between the 13 regions sampled, as well the richness (p < 0.001), by block effect (Figs. 2 and 3). According to PERMANOVA results, the AMF community composition differed significantly between the latitude (R 2 = 0.06, p < 0.001) and altitudes (R 2 = 0.03, p = 0.03) of sites (Fig. 4). In our optimal richness model, richness was influenced positively by clay content (p = 0.03).
RDA redundancy analysis showing the variation in AMF community composition across latitudes (F = 3.7660, R 2 = 0.06764, P = 0.0003) and an altitude range. (F = 1.8873, R 2 = 0.03390, P = 0.0411). The PERMANOVA was calculated using Bray-Curtis distance and sample relative abundance data. Colours indicate different altitudes. Dispersion ellipses represent one standard deviation of points around group centroids
Predictors of the Structure of AMF Communities and Indicator Species Analysis
Comparison of three different sets of predictors of AMF communities showed that environmental factors were responsible for explaining 9% of the AMF community patterns in the dry tropical forest of Brazil, while the geographical distance and climate factors did not contribute to explaining the AMF community composition (Fig. 5). The effects of individual predictors within each group were first evaluated independently; thereafter, we compared the effects across groups with variation partitioning. The group of predictor variables (soil, geography and climate) shared little contribution and showed that variability in AMF community depended on unique soil effects. Redundancy analysis explained approximately 17.8% of the variation of the data (Fig. 6) with the main portion for axis 1 (3.6%) then to axis 2 (3.3%). The community of arbuscular mycorrhiza fungi was structured according to the soil variables in the axis 1, iron (0.59), base saturation (0.36), organic matter (−0.38) and coarse sand content (−0.53). The variables sodium (− 0.64), potassium (0.19), silt (0.17) and total sand content (−0.35) were strongly correlated with the axis 2. The analysis of indicator species showed that several species were indicators of soil properties. Acaulospora morrowie (p = 0.05), Claroideoglomus etunicatum (p = 0.03) and Gigaspora decipiens (p = 0.03) were indicators of high values of iron in the soil. G. decipiens (p = 0.04) and Glomus microcarpum (p = 0.03) were indicators of high sand content in the soil. G. microcarpum (p = 0.01) was an indicator of low sodium contents, and Acaulospora scrobiculata (p = 0.04) and Acaulospora sp.1 (p = 0.03) indicated the portions of the soil with high sodium content. Acaulospora sp.1 (p = 0.04) and Ambispora appendicula (p = 0.03) were indicators for low carbon content, and Glomus glomerulatum (p = 0.01) and A. appendicula (p = 0.02) indicated low potassium levels. Gigaspora margarita (p = 0.02), Gigaspora sp. (p = 0.02) and Paradentiscutata maritima (p = 0.04) were indicators of low values of silt contents on soil, and Acaulospora morrowiae (p = 0.03) indicated the high amounts of silt.
Discussion
The vast majority of existing studies that have addressed the extent to which AMF communities are influenced by environmental factors are molecular studies [27, 40,41,42,43] and few use spore-based identification, as our study does [20, 44, 45]. This method is a historically predominant approach to characterize species, allowing a better differentiation of fungal taxa and a more sensitive detection of changes in AMF diversity and community composition [21], yielding also important information about soil management [20] but only for the sporulation fraction of the AMF assemblage [46]. Spore identification to a species level is an intricate procedure and, when conducted by non-experts, can result in individual spore morphotypes matching up with more than one AMF species [47]. Simultaneously, though, spore-based community studies can yield superior estimates of relative abundances [21, 48] and can better control for confounding issues arising from variable sampling depth. In the tropical dry forest of Brazil, AMF communities on the top of the inselbergs did not differ in richness from those in the surrounding soil, not corroborating our first hypothesis, and showing no effects of rocky outcrops isolation in AMF community and the lack of historical processes in the distribution of AMF. This latter point implies that community members of AMF communities around the inselbergs could also disperse within the specific rocky habitats and that there was no isolation. Results may also be related to the weak host specificity of AMF, as well with the strong similarities of the vegetation on inselbergs with the surrounding matrix, due to the overall harsh overall conditions in these semiarid environments [49].
We were expecting based on our second hypothesis that we would detect different AMF communities at the top and at the bottom of inselbergs. To assess whether the AMF communities differed, we had to first explore and when necessary correct for other confounding drivers of AMF community structure. Because we experimented over a broad geographical range, we had to consider the fact that AMF communities follow a latitudinal diversity gradient [43]. Although our sampling design here was carried out within the limits of a single country, as expected, we detected evidence in support of such a latitudinal gradient at a regional scale, in the case of the species richness of AMF on the top of the inselbergs. Nevertheless, the low species richness of AMF that we found at low latitudes is incongruent with our expectations from the inferred latitudinal gradient for AMF taxa. The AMF community was sampled from ten different inselbergs with different altitude, which may have influenced the result from the latitudinal gradient. Altitude is a factor that influences the geography and ecology of species [50] because higher-altitude sites experience pronounced environmental differences from lowland sites such as lower temperatures, slowing down decomposition and nutrient cycling [51]. Studies on the dynamics of the AMF community as a function of latitude have rarely taken into consideration the possible confounding influence of altitude. A global approach to species richness of AMF did not yield evidence in support of a latitudinal gradient of diversity [40]. However, it is known that the AMF communities are influenced by latitude [52]. The Tropical Conservatism Hypothesis [53] postulates that the basal fungal groups predominate in low latitudes, and that this pattern is related with phylogenetic patterns, since key morphological or physiological traits determine fungal taxa response to temperature and precipitation [54]. There is evidence that the latitudinal gradient observed for AMF is the outcome of host selectivity by the plant hosts [52]; higher plant richness per unit area at low latitudes means a higher number of hosts, each with unique preferences for AMF associates which in the end result in an apparent latitudinal gradient in AMF richness. The diversity of microorganisms in relation to the latitudinal gradient could also be influenced by frequency of disturbance, higher productivity and environmental heterogeneity [55]. Even after correcting for these factors, we could not find any evidence that AMF communities at the top and bottom of inselbergs differed.
In agreement to our third hypothesis, we detected that environmental conditions exerted a strong influence on the AMF community structure. We used variance partitioning for this purpose, a commonly used tool in microbial ecology which is particularly reliable [56, 57]. In microbial communities, soil properties have been found in multiple studies to have pervasive effects on environmental filtering [26, 58,59,60]. Our results support these previous studies; the soil edaphic factors were responsible for the explained variation of the AMF community within the Brazilian semiarid region. The main soil predictor of AMF richness was clay content. Other evidence also suggests that soil texture is an important predictor of AMF community structure [20, 26, 44, 61], and the low clay content in the soil is a limiting factor for several species of the family Gigasporaceae and Acaulosporaceae [24]. In relatively homogeneous areas, differences in soil texture may override other predictor variables such as weather, host plant, soil management practices and seasonality as structuring of AMF communities [24]. In semiarid ecosystems, clay content is an important factor governing water infiltration [62]. In these systems, mobility of nutrients such as iron and aluminium is relatively low as there is limited diffusion. These soils also are characterized by low cation exchange capacity, clay and organic material content in the topsoil. Evidence accrues to suggest that soil texture has multifaceted effects on many soil properties such as porosity, water holding capacity of the soil, cation exchange capacity [59, 63] and, consequently, the habitat of soil microorganisms. The understanding of how soil properties can influence the diversity of AMF is important to predict the biogeography and functionality of these microorganisms. For instance, the organic matter in the soil is related to the rate of production of external mycelium by the AMF species [63, 64]. Although studies predicting AMF niche spaces are still scarce, our results show that niche processes mediate the distribution of AMF and that more investigation in this regard could elucidate issues related to ecological role in different biomes.
Of particular concern in our area was the condition with regard to Na availability and the way AMF taxa responded to it. The levels of Na in the soil vary geographically. In semiarid regions, crystalline characteristics of the soil induce Na limitation of plant communities [65]. Sodium is considered a functional nutrient of plants, and partially replaces K activity, regulating ion balance of plants and improving water balance via regulation of stomatal conductance with indirect participation on carbon sequestration [66, 67]. The mechanisms that regulate photosynthesis are of the utmost importance to the establishment and survival of plants; thus, the amount of sodium in the soil indirectly affects the communities of AMF via effects on plant growth. Studies have shown that sodium content influences soil fungal communities [59] and mycorrhizal symbionts [68, 69]. In the semiarid region of Brazil, the low precipitation and the high evapotranspiration, linked to deforestation, have led to salinization [70]. Nevertheless, the mechanisms responsible for the changes in the community of microorganisms still remain little understood. Some species of AMF are able to overcome high levels of soil salinity effectively helping the host plant [71]. In conditions of high salinity, species of Glomus assist in plant development by increasing the acquisition of water and nutrients [72, 73].
To address hypothesis four, we carried out an indicator species analysis and found, in agreement with our hypothesis, several indicator species. G. decipiens was found to be a good indicator of high sand content, corroborating prior studies observing this same pattern [24, 74]. Some species of the genus Acaulospora, Gigaspora and Rhizoglomus were indicators of high levels of soil iron. The ability of these different groups of AMF to serve as indicators of different soil properties is reported [20, 75] and could reflect the functional role of these symbionts in the environment. Different strategies of colonization have been reported for members of the groups with acaulosporoid and glomoid spores, which show rapid colonization [76], while more tolerance to environmental disturbance [61, 77] is ascribed to species which produce gigasporoid spores. The occurrence of species of Acaulospora and Glomus in soil with high iron content manifests the benefit that these groups offer for the plants in the initial processes of colonization, while Gigaspora species, due to delayed colonization, tend to be involved in the processes of maintenance of plant species [76]. This shows that taxonomic/morphological parameters are related to the ecological role of species of AMF, reflected in functional terms.
In conclusion, our work addresses the factors that shape AMF communities in semiarid regions, by evaluating the morphological diversity of these symbionts. We found no differences between AMF communities at the top and bottom of the inselbergs; we also found soil characteristics as the main predictor of the distribution of AMF communities in Brazilian tropical dry forest. The pattern of diversity of the AMF along the latitudinal gradient is incongruent with the known diversity patterns in low latitudes, suggesting effects of altitude. At a regional scale, physical soil properties (e.g. clay content) shape AMF community and reflect the functional role of these fungi. There is evidence that different AMF species play different functional roles [76, 78]; thus, studies of the distribution of AMF at the landscape-scale can increase the understanding of biogeography and predict potential ecosystem services provided by the species of the Glomeromycota.
References
Santos R, Barbosa A, Almeida H, et al. (2011) Structure and floristics of a remnant of arboreous caatinga in Juvenília, northern Minas Gerais, Brazil. Cerne 17:247–258
Prado D (2003) As Caatingas da América do Sul. In: Lea I, Tabarelli M, Silva J (eds) Ecol. e Conserv. da Caatingas, 1st ed. Editora Universitária da UFPE, Recife, pp 3–74
Porembski S, Martinelli G, Ohlemuller R, Barthlott W (1998) Diversity and ecology of saxicolous vegetation mats on inselbergs in the Brazilian Atlantic rainforest. Divers Distrib 4:107–119. doi:10.1046/j.1365-2699.1998.00013.x
Pires GG, dos Santos RM, Tristão RA, et al. (2014) Influência de variáveis ambientais na comunidade arbórea de inselbergs. Cerne 20:97–104. doi:10.1590/S0104-77602014000100013
Camargo-Ricalde SL, Esperón-Rodríguez M (2005) Efecto de la heterogeneidad espacial y estacional del suelo sobre la abundancia de esporas de hongos micorrizógenos arbusculares en el valle semiárido de Tehuacán-Cuicatlán, México. Rev Biol Trop 53:339–352. doi:10.4067/S0718-16202007000300006
Klironomos J, Zobel M, Tibbett M, et al. (2011) Forces that structure plant communities: quantifying the importance of the mycorrhizal symbiosis. New Phytol 189:366–370. doi:10.1111/j.1469-8137.2010.03550.x
Rillig MC (2004) Arbuscular mycorrhizae and terrestrial ecosystem processes. Ecol Lett 7:740–754. doi:10.1111/j.1461-0248.2004.00620.x
Newsham KK, Fitter AH, Watkinson AR (1995) Multi-functionality and biodiversity in arbuscular mycorrhizas. Trends Ecol Evol 10:407–411. doi:10.1016/S0169-5347(00)89157-0
Augé RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11:3–42. doi:10.1007/s005720100097
Veresoglou SD, Rillig MC (2012) Suppression of fungal and nematode plant pathogens through arbuscular mycorrhizal fungi. Biol Lett 8:214–217. doi:10.1098/rsbl.2011.0874
Veresoglou SD, Halley JM, Rillig MC (2015) Extinction risk of soil biota. Nat Commun 6:1–10. doi:10.1038/ncomms9862
Averill C, Turner BL, Finzi AC (2014) Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 505:543–545. doi:10.1038/nature12901
Rillig MC, Mummey DL (2006) Mycorrhizas and soil structure. New Phytol 171:41–53. doi:10.1111/j.1469-8137.2006.01750.x
Klironomos JN, McCune J, Hart M, Neville J (2000) The influence of arbuscular mycorrhizae on the relationship between plant diversity and productivity. Ecol Lett 3:137–141. doi:10.1046/j.1461-0248.2000.00131.x
Rosendahl S (2008) Communities, populations and individuals of arbuscular mycorrhizal fungi. New Phytol 178:253–266. doi:10.1111/j.1469-8137.2008.02378.x
Wubet T, Weiß M, Kottke I, et al. (2004) Molecular diversity of arbuscular mycorrhizal fungi in Prunus africana, an endangered medicinal tree species in dry Afromontane forests of Ethiopia. New Phytol 161:517–528. doi:10.1046/j.1469-8137.2003.00924.x
Martínez-García LB, Richardson SJ, Tylianakis JM, et al. (2015) Host identity is a dominant driver of mycorrhizal fungal community composition during ecosystem development. New Phytol 205:1565–1576. doi:10.1111/nph.13226
Xu T, Veresoglou SD, Chen Y, et al. (2016) Plant community, geographic distance and abiotic factors play different roles in predicting AMF biogeography at the regional scale in northern China. Environ Microbiol Rep 8:1048–1057. doi:10.1111/1758-2229.12485
Overby ST, Owen SM, Hart SC, et al. (2015) Soil microbial community resilience with tree thinning in a 40-year-old experimental ponderosa pine forest. Appl Soil Ecol 93:1–10. doi:10.1016/j.apsoil.2015.03.012
Säle V, Aguilera P, Laczko E, et al. (2015) Impact of conservation tillage and organic farming on the diversity of arbuscular mycorrhizal fungi. Soil Biol Biochem 84:38–52. doi:10.1016/j.soilbio.2015.02.005
Wetzel K, Silva G, Matczinski U, et al. (2014) Superior differentiation of arbuscular mycorrhizal fungal communities from till and no-till plots by morphological spore identification when compared to T-RFLP. Soil Biol Biochem 72:88–96. doi:10.1016/j.soilbio.2014.01.033
Vályi K, Mardhiah U, Rillig MC, Hempel S (2016) Community assembly and coexistence in communities of arbuscular mycorrhizal fungi. ISME J 10:2341–2351. doi:10.1038/ismej.2016.46
Veresoglou SD, Caruso T, Rillig MC (2013) Modelling the environmental and soil factors that shape the niches of two common arbuscular mycorrhizal fungal families. Plant Soil 368:507–518. doi:10.1007/s11104-012-1531-x
Lekberg Y, Koide RT, Rohr JR, et al. (2007) Role of niche restrictions and dispersal in the composition of arbuscular mycorrhizal fungal communities. J Ecol 95:95–105. doi:10.1111/j.1365-2745.2006.01193.x
Sikes BA, Powell JR, Rillig MC (2010) Deciphering the relative contributions of multiple functions within plant–microbe symbioses. Ecol Model 91:1591–1597
Jansa J, Erb A, Oberholzer HR, et al. (2014) Soil and geography are more important determinants of indigenous arbuscular mycorrhizal communities than management practices in Swiss agricultural soils. Mol Ecol 23:2118–2135. doi:10.1111/mec.12706
Pasternak Z, Al-Ashhab A, Gatica J, et al. (2013) Spatial and temporal biogeography of soil microbial communities in arid and semiarid regions. PLoS One 8:e69705. doi:10.1371/journal.pone.0069705
Dumbrell AJ, Nelson M, Helgason T, et al. (2010) Idiosyncrasy and overdominance in the structure of natural communities of arbuscular mycorrhizal fungi: is there a role for stochastic processes? J Ecol 98:419–428. doi:10.1111/j.1365-2745.2009.01622.x
Ab’Sáber AN (1999) Dossiê Nordeste seco. Estud Avançados 13:5–59
Velloso AL, Giulietti AM, Oren DC, et al (2002) Ecorregiões—Propostas para o Bioma Caatinga. In: Recife APNE, Nat. Conserv. do Bras. p 80
Gomes P, Alves M (2009) Floristic and vegetational aspects of an inselberg in the semi-arid region of Northeast Brazil Edinburgh. J Bot 66:329. doi:10.1017/S0960428609005241
Gerdemann JW, Nicolson TH (1963) Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans Br Mycol Soc 46:235–244. doi:10.1016/S0007-1536(63)80079-0
Jenkins W (1964) A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Dis Rep 48:692
Hijmans RJ, Cameron SE, Parra JL, et al. (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978. doi:10.1002/joc.1276
Legendre P, Gallagher ED (2001) Ecologically meaningful transformations for ordination of species data. Oecologia 129:271–280. doi:10.1007/s004420100716
Oksanen AJ, Blanchet FG, Friendly M, et al (2016) The Vegan Package. Community Ecol. Packag
Legendre P (2008) Studying beta diversity: ecological variation partitioning by multiple regression and canonical analysis. J Plant Ecol 1:3–8. doi:10.1093/jpe/rtm001
Cáceres M, Jansen F (2015) Package “ indicspecies ”—relationship between species and groups of sites
Core Team R (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna,
Öpik M, Vanatoa A, Vanatoa E, et al. (2010) The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytol 188:223–241. doi:10.1111/j.1469-8137.2010.03334.x
Kivlin SN, Hawkes CV, Treseder KK (2011) Global diversity and distribution of arbuscular mycorrhizal fungi. Soil Biol Biochem 43:2294–2303. doi:10.1016/j.soilbio.2011.07.012
Martínez-García LB, Armas C, Miranda J de D, et al. (2011) Shrubs influence arbuscular mycorrhizal fungi communities in a semi-arid environment. Soil Biol Biochem 43:682–689. doi:10.1016/j.soilbio.2010.12.006
Davison J, Moora M, Opik M, et al. (2015) Global assessment of arbuscular mycorrhizal fungus diversity reveals very low endemism. Science 349:970–973. doi:10.1126/science.aab1161
Pontes J, Oehl F, Marinho F, et al. (2017) Diversity of arbuscular mycorrhizal fungi in Brazil’s Caatinga and experimental agroecosystems. Biotropica, In Press
Chaudhary VB, O’Dell TE, Rillig MC, Johnson NC (2014) Multiscale patterns of arbuscular mycorrhizal fungal abundance and diversity in semiarid shrublands. Fungal Ecol 12:32–43. doi:10.1016/j.funeco.2014.06.003
Öpik M, Davison J (2016) Uniting species- and community-oriented approaches to understand arbuscular mycorrhizal fungal diversity. Fungal Ecol 24:106–113. doi:10.1016/j.funeco.2016.07.005
Taylor A, Walker C, Bending GD (2013) Dimorphic spore production in the genus Acaulospora. Mycoscience 55:1–4. doi:10.1016/j.myc.2013.03.001
Antoninka AJ, Ritchie ME, Johnson NC (2015) The hidden Serengeti—mycorrhizal fungi respond to environmental gradients. Pedobiologia (Jena) 58:165–176. doi:10.1016/j.pedobi.2015.08.001
Porembski S (2007) Tropical inselbergs: habitat types, adaptive strategies and diversity patterns. Rev Bras Bot 30:579–586. doi:10.1590/S0100-84042007000400004
Diaz HF, Grosjean M, Graumlich L (2003) Climate variability and change in high elevation regions: past, present and future. Clim Chang 59:1–4. doi:10.1023/A:1024416227887
Soethe N, Lehmann J, Engels C (2008) Nutrient availability at different altitudes in a tropical montane forest in Ecuador. J Trop Ecol 24:397–406. doi:10.1017/S026646740800504X
Koske RE, Tews LL (1987) Vesicular-arbuscular mycorrhizal fungi of Wisconsin sandy soils. Mycologia 79:901. doi:10.2307/3807694
Wiens JJ, Donoghue MJ (2004) Historical biogeography, ecology and species richness. Trends Ecol Evol 19:639–644. doi:10.1016/j.tree.2004.09.011
Treseder KK, Maltz MR, Hawkins BA, et al. (2014) Evolutionary histories of soil fungi are reflected in their large-scale biogeography. Ecol Lett 17:1086–1093. doi:10.1111/ele.12311
Brown J (1995) Macroecology. The University of Chicago Press, Chicago,
Svenning J-C, Skov F (2005) The relative roles of environment and history as controls of tree species composition and richness in Europe. J Biogeogr 32:1019–1033. doi:10.1111/j.1365-2699.2005.01219.x
Duivenvoorden JF (2002) ECOLOGY: beta diversity in tropical forests. Science 295(80):636–637. doi:10.1126/science.295.5555.636
Lauber CL, Strickland MS, Bradford MA, Fierer N (2008) The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol Biochem 40:2407–2415. doi:10.1016/j.soilbio.2008.05.021
de Gannes V, Eudoxie G, Bekele I, Hickey WJ (2015) Relations of microbiome characteristics to edaphic properties of tropical soils from Trinidad. Front Microbiol 6:1–13. doi:10.3389/fmicb.2015.01045
Thougnon Islas AJ, Hernandez Guijarro K, Eyherabide M, et al. (2016) Can soil properties and agricultural land use affect arbuscular mycorrhizal fungal communities indigenous from the Argentinean Pampas soils? Appl Soil Ecol 101:47–56. doi:10.1016/j.apsoil.2016.01.005
Oehl F, Laczko E, Bogenrieder A, et al. (2010) Soil type and land use intensity determine the composition of arbuscular mycorrhizal fungal communities. Soil Biol Biochem 42:724–738. doi:10.1016/j.soilbio.2010.01.006
Pumpanen J, Ilvesniemi H, Hari P (2003) A process-based model for predicting soil carbon dioxide efflux and concentration. Soil Sci Soc Am J 67:402. doi:10.2136/sssaj2003.4020
Chaudhary VB, Lau MK, Johnson NC (2008) Macroecology of microbes—biogeography of the Glomeromycota. Mycorrhiza. Springer, Berlin, pp. 529–563
Hu Y, Rillig MC, Xiang D, et al. (2013) Changes of AM fungal abundance along environmental gradients in the arid and semi-arid grasslands of northern China. PLoS One 8:e57593. doi:10.1371/journal.pone.0057593
Marschner H (2011) Mineral nutrition of higher plants, third. Academic Press, London,
Subbarao GV, Ito O, Berry WL, Wheeler RM (2003) Sodium—a functional plant nutrient. CRC Crit Rev Plant Sci 22:391–416. doi:10.1080/748638747
Gattward JN, Almeida AAF, Souza JO, et al. (2012) Sodium-potassium synergism in Theobroma cacao: stimulation of photosynthesis, water-use efficiency and mineral nutrition. Physiol Plant 146:350–362. doi:10.1111/j.1399-3054.2012.01621.x
Campanelli A, Ruta C, De MG, Morone-Fortunato I (2013) The role of arbuscular mycorrhizal fungi in alleviating salt stress in Medicago sativa L. var icon. Symbiosis 59:65–76. doi:10.1007/s13199-012-0191-1
Krishnasamy K, Bell R, Ma Q (2014) Wheat responses to sodium vary with potassium use efficiency of cultivars. Front Plant Sci 5:631. doi:10.3389/fpls.2014.00631
Castelletti C, Santos A, Tabarelli M, Silva J (2003) Quanto ainda resta da Caatinga? Uma estimativa preliminar. In: Leal I, Tabarelli M, Silva J (eds) Ecol. e Conserv. da Caatinga, First. Recife, pp 719–734
Caravaca F, Alguacil MM, Vassileva M, et al. (2004) AM fungi inoculation and addition of microbially-treated dry olive cake-enhanced afforestation of a desertified Mediterranean site. Land Degrad Dev 15:153–161. doi:10.1002/ldr.600
Miransari M (2010) Contribution of arbuscular mycorrhizal symbiosis to plant growth under different types of soil stress. Plant Biol 2:563–569. doi:10.1111/j.1438-8677.2009.00308.x
Mardukhi B, Rejali F, Daei G, et al. (2015) Mineral uptake of mycorrhizal wheat ( Triticum aestivum L.) under salinity stress. Commun Soil Sci Plant Anal 46:343–357. doi:10.1080/00103624.2014.981271
Johnson NC, Tilman D, Wedin D (1992) Plant and soil controls on mycorrhizal fungal communities. Ecology 73:2034–2042. doi:10.2307/1941453
de Assis DMA, Oehl F, Gonçalves CM, et al. (2016) Community structure of arbuscular mycorrhizal fungi in fluvial and maritime dunes of Brazilian Northeast. Appl Soil Ecol 108:136–146. doi:10.1016/j.apsoil.2016.07.018
Hart MM, Reader RJ (2002) Does percent root length colonization and soil hyphal length reflect the extent of colonization for all AMF? Mycorrhiza 12:297–301. doi:10.1007/s00572-002-0186-5
Varela-Cervero S, López-García Á, Barea JM, Azcón-Aguilar C (2016) Differences in the composition of arbuscular mycorrhizal fungal communities promoted by different propagule forms from a Mediterranean shrubland. Mycorrhiza 26:489–496. doi:10.1007/s00572-016-0687-2
Klironomos JN, Hart MM (2002) Colonization of roots by arbuscular mycorrhizal fungi using different sources of inoculum. Mycorrhiza 12:181–184. doi:10.1007/s00572-002-0169-6
Acknowledgements
The authors would like to thank Coordenação de aperfeiçoamento de pessoal de nível superior (CAPES), proc.: 1374510, and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), proc.: 206415/2014-1 for financial support of the research and authors’ collaboration.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Sousa, N.M.F., Veresoglou, S.D., Oehl, F. et al. Predictors of Arbuscular Mycorrhizal Fungal Communities in the Brazilian Tropical Dry Forest. Microb Ecol 75, 447–458 (2018). https://doi.org/10.1007/s00248-017-1042-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-017-1042-7