Abstract
Viruses are the most abundant components of microbial food webs and play important ecological and biogeochemical roles in aquatic ecosystems. Virioplankton is regulated by several environmental factors, such as salinity, turbidity, and humic substances. However, most of the studies aimed to investigate virioplankton regulation were conducted in temperate systems combining a limited range of environmental variables. In this study, virus abundance and production were determined and their relation to bacterial and limnological variables was assessed in 20 neighboring shallow tropical coastal lagoons that present wide environmental gradients of turbidity (2.32–571 NTU), water color (1.82–92.49 m−1), dissolved organic carbon (0.71–16.7 mM), salinity (0.13–332.1‰), and chlorophyll-a (0.28 to 134.5 μg L−1). Virus abundance varied from 0.37 × 108 to 117 × 108 virus-like-particle (VLP) mL−1, with the highest values observed in highly salty aquatic systems. Salinity and heterotrophic bacterial abundance were the main variables positively driving viral abundances in these lagoons. We suggest that, with increased salinity, there is a decrease in the protozoan control on bacterial populations and lower bacterial diversity (higher encounter rates with virus specific hosts), both factors positively affecting virus abundance. Virus production varied from 0.68 × 107 to 56.5 × 107 VLP mL−1 h−1 and was regulated by bacterial production and total phosphorus, but it was not directly affected by salinity. The uncoupling between virus abundance and virus production supports that the hypothesis that the lack of grazing pressure on viral and bacterial populations is an important mechanism causing virus abundance to escalate with increasing salt concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Viruses have been widely recognized as the most abundant components of the biosphere [1]. Virioplankton reach very high abundances in surface waters of all sorts of aquatic ecosystems but have been found more abundant in inland and coastal waters than oceans [2]. They play important roles in aquatic ecosystems regulating microbial community composition [3] and altering ecosystem functioning and biogeochemical cycles by killing phytoplankton and bacterioplankton [4].
Viruses abundance can be directly affected by environmental factors. High-molecular-weight dissolved organic matter, such as humic substances, has been demonstrated to decrease the abundance of viruses by either binding or destroying virus particles [5]. Organic matter can also decrease virus infectivity and replication rates in freshwater ecosystems [5]. Unsurprisingly, humic lakes have lower virus abundance [6]. High temperatures [7] and exposition to UV radiation [8] have also been suggested to negatively affect virus distribution in aquatic systems.
Most viruses are bacteriophages and their abundance is strongly correlated with bacterial abundance in aquatic systems [9], although the ratio of this relationship is widely variable across ecosystems [2, 10]. Thus, environmental factors that significantly impact bacterial productivity and density also have a strong and indirect effect on virus production and distribution [9]. Salinity has been described as an important indirect virus regulator as high salt concentrations drastically decrease both the prokaryotic community diversity and the eukaryotic grazing pressure on bacteria [11], which increase bacterial abundance and, in turn, virus infection [12]. Viruses are also affected by the trophic status of the ecosystem; there is evidence that eutrophic systems have higher virus abundance than oligotrophic ones as a response to higher bacterial and phytoplankton abundance [13]. Therefore, virus abundance correlates with chlorophyll-a in several aquatic environments such as lakes, estuaries, and marine systems [13,14,15] and high turbidity can negatively affect virus abundance by reducing the phytoplankton abundance [16].
In spite of these investigations about virus responses to different environmental factors in inland aquatic systems, none of them have been performed in aquatic systems covering large environmental gradients. Moreover, most studies were conducted in temperate zones. This bias may underestimate the importance of indirect effects on viruses mediated by bacteria because tropical systems are warmer and sunnier and present higher bacterial metabolic rates [17]. Different patterns of virioplankton abundance and activity might comparatively emerge from these systems.
We investigate virus abundance and production and their major bacterial and environmental drivers in tropical coastal lagoons. Virus-mediated mortality and the amount of carbon released from bacteria lyses by the viruses were also estimated in some of these systems. The tropical coastal lagoons sampled here present broad ranges of trophic state (from oligotrophic to hypereutrophic conditions), salinity (from freshwater to hypersaline systems), turbidity (from clear to highly turbid waters), water color, and dissolved organic carbon concentration (from clear water to extremely humic systems). Besides combining such widely ranging environmental characteristics, these coastal lagoons are relatively close to each other and are subject to similar broad-scale regional factors such as climate and proximity to the sea [18]. In summary, we found that bacterial abundance and salinity are the main drivers of viral abundance among the studied lagoons. However, virus and bacterial production were not directly affected by salinity, bringing about an apparent paradox that is explored throughout this study.
Methods
Study Site
The study took place in 20 tropical coastal lagoons situated in the state of Rio de Janeiro, southeastern Brazil (Fig. 1). Most of them (16 out of 20 lagoons) are located in the Restinga de Jurubatiba National Park (between 22–22° 30′ S and 41° 15′–42° W), which is characterized by patches of shrubs and small trees in sandy dunes. Inter-dune areas are dominated by both seasonally flooded forests and perennial or temporary coastal shallow lagoons. The studied lagoons are located within a maximum distance of 73 km from each other, present nearly the same distance to the sea, and are subject to similar climate factors [18]. Nevertheless, they present a wide range of limnological features such as dissolved organic carbon, chlorophyll-a and nutrient concentrations, salinity, and water color [18]. Water and nutrient inputs to these lagoons are mainly from the water table, the ocean, and small streams; however, the relative importance of these sources greatly depends on each lagoon genesis, position in relation to the ocean, and the watershed and may also vary over a seasonal scale [18]. Some lagoons (lagoons 5, 6, 9, 11, 12, and 19 in Fig. 1) are dystrophic systems with extremely high concentrations of dissolved organic carbon derived from the humic-rich water table [19]. Lagoons 1 and 2 are also humic ecosystems but receive nutrient inputs from both diffusive (agriculture activity) and punctual (sewage runoff from a nearby city) sources [19]. Lagoon 4 is fully inserted in an urban area and receives high nutrient loads from direct sewage discharges. Lagoons 3 and 10 are brackish lagoons [18], while lagoons 16, 17, 20, 13, and 14 are hypersaline (from 43 up to 332‰) because of their reduced water volume in response to the persistent drought periods lately observed in the studied region (Fig. 2). The regional weather is warm, with an average air temperature of 22.6 °C. The historical annual precipitation in the region is 1038 ± 336 mm (Instituto Brasileiro de Meteorologia, INMET). The precipitation accumulated during the year (2014) prior to our sampling was notably low (total of 676 mm, Fig. 2). Additional details about the local climate as well as the limnological and morphological traits of these lagoons are found in Caliman et al. (2010).
Map of the study area with the geographical location of all coastal lagoons (black dots) sampled in this study. Each lagoon is numbered as follows: 1 Iriry, 2 Salgada, 3 Itapebussus, 4 Imboassica, 5 Cabiunas, 6 Comprida, 7 Carcará, 8 Carapebuzinho, 9 Carapebus, 10 Encantada, 11 Paulista, 12 Amarra Boi, 13 Garças, 14 Garcinha, 15 Atoleiro, 16 Visgueiro, 17 Catingosa, 18 Pretinha, 19 Preta, 20 Ubatuba. The black star stands for the city of Macaé, the largest urban area of the northern coast of Rio de Janeiro state, Brazil
Historical average of the monthly-accumulated precipitation (bars) and the monthly-accumulated precipitation of the year (2014) previous to our sampling in January 2015 (dotted line). The precipitation accumulated during this year was 676 mm. Dataset provided by the Instituto Brasileiro de Meteorologia, INMET
Sampling
Sub-surface water samplings and in situ measurements were carried out in a near-shore sampling station of each lagoon in January 2015. Water temperature and dissolved oxygen were in situ evaluated using specific probes (YSI-30 and YSI-61, respectively). Two water samples (1 mL each) for density evaluation of virus, bacteria, and phototrophic bacteria were pre-filtered through a 50-μm membrane mesh to remove large particles. These samples were fixed for 15–30 min at 4 °C into 2-mL cryotubes with paraformaldehyde (1%) and glutaraldehyde (0.05%), or glutaraldehyde (0.5%), or paraformaldehyde (1%), respectively. The fixed samples were then flash frozen in liquid N2 in the field and then stored at −80 °C in the laboratory. Water samples were also collected in polyethylene flasks previously washed with HCl 10% and rinsed with distilled water. These samples were taken to the laboratory within 2 h after sampling for limnological and bacterial production measurements as well as for the time-course experiment set up to determine virus parameters.
Flow Cytometric Measurements: Virus and Bacteria Enumeration
Flow cytometry was used to evaluate virus, bacteria, and phototrophic bacteria numbers [20, 21]. These enumerations were performed separately using a FACSCalibur (BD) Flow Cytometer with standard laser and optics as follows: air-cooled argon ion laser emitting at 488 nm (power at 20 mW), fixed laser alignment, and fixed optical components. A 70-μm nozzle aspirated the sample, and sterile Mill-Q water (18,2 mΩ) was used as sheath fluid. We analyzed the fluorescence emitted by the samples using the forward scatter and the side scatter at 90°. Fluorescence signals were collected by three different photomultipliers: FL1 (530 ± 30 nm), FL2 (585 ± 42 nm), and FL3 (670 LP). Counts were made in triplicate at flow rate of ca. 40 μL min−1, and data were acquired in logarithmic mode. Fluorescent latex beads (Fluoresbrite YG carboxylate 1.0 μm, Polysciences) were added at a known abundance to each sample for calibration of side scatter and green fluorescence signals and as internal standards for cytometric counts and measures.
Fixed viral and bacterial (heterotrophic + phototrophic bacteria) samples were promptly stained with SYBR Green I (Invitrogen) at final concentrations of 0.5 × 10−4 of the commercial stock solution and then mixed in a vortex apparatus for 15 s. Viral samples were then incubated in the dark at 80 °C for 10 min and at room temperature for additional 5 min [21]. All samples were highly concentrated and, hence, they were diluted (Table S1) in sterile TE buffer, pH 8 (10 mM Tris-hydroxymethyl-aminomethane, Roche Diagnostics; 1 mM ethylenediaminetetraacetic acid, Sigma-Aldrich), to reach an ideal working event rate (200–800 events s−1) that avoids electronic coincidence in counts [21].
Viruses were detected, identified, and counted by their signature in a plot of side scatter (X-axis) versus green fluorescence (FL1, Y-axis, green fluorescence from SYBR Green I related to nucleic acid content and virus size) [21]. Bacterial samples were kept for 15 min in the dark until cytometer readings were taken. Total bacterial abundance was determined by their position in a plot of side scatter (X-axis and indicative of cellular size) versus green fluorescence (FL1, Y-axis, related to nucleic acid content) and red fluorescence (FL3) in order to detect different groups of heterotrophic bacterioplankton [20]. Phototrophic bacteria groups were detected by their specific autofluorescence properties and signature in a plot of orange fluorescence (FL2) versus red fluorescence (FL3) [22]. Heterotrophic bacterial abundance (HBA) was determined from difference between total bacterial abundance and phototrophic bacterial abundance. All data were obtained and analyzed by Cell Quest software (BD) using a Macintosh computer.
Bacterial Production
Bacterial production (BP) was measured by using the 3H-leucine incorporation method and trichloroacetic acid (TCA) protein extraction [23], as modified by Miranda et al. [24]. An intracellular isotopic dilution factor of 2 was used in calculations [25]. An aliquot of 1.2 mL of the water sample was incubated in microcentrifuge tubes (25 ± 1 °C) in the dark for 45 min with 20 nM of 3H-leucine (specific activity 58.8 Ci mmol−1). The concentration of 20 nM of 3H-leucine was previously established from saturation curves of bacterial leucine uptake in some of the coastal lagoons studied here (unpublished data). Negative controls were set up by adding 90 μL of TCA to kill microbes prior to starting the incubations. At the end of the incubation, 90 μL of TCA was added to each tube in order to stop 3H-leucine incorporation by bacteria. Bacterial protein was extracted by washing with 5% TCA and 80% ethanol. After protein extraction, liquid scintillation cocktail (EcoLite(+)™) was added to each sample, which was radioassayed by liquid scintillation counting (Beckman LS 6500) after 2 days in the dark to reduce the chemiluminescence. The obtained values in disintegrations per minute were converted to bacterial biomass using a ratio incorporation of 0.86, according to Wetzel and Likens [26].
Time-Course Experiment
Virus production (VP), virus decay (VD), virus turnover (VT), burst size (Bs), and virus-mediated mortality (VMM) were determined for 16 lagoons through the dilution technique in a laboratory time-course experiment [27], as adapted by Hewson et al. [50]. This technique has been widely used and considered the simplest method, while still providing consistent inter-comparison data [28]. This method decreases the virus-bacteria meeting rate, thereby preventing new viral infection, by reducing the amount of viruses in the sample while keeping the original bacterial concentration [27]. First, we obtained virus-free water for every lagoon by filtering water samples (previously filtered through a 50-μm membrane mesh) through a 0.02-μm filter backed by a 2.7-μm filter (25 mm; GF/D Whatman). Second, 300 mL of the unfiltered water samples was vacuum filtered (<250 mmHg) through 0.2-μm pore size polycarbonate filters (47 mm diameter, Millipore). During this filtration procedure, the sample was constantly mixed up by pumping water in and out from a sterilized Pasteur plastic pipette in order to avoid bacteria attachment to the filter. Also during the filtration procedure, 270 mL of the previously obtained virus-free water was gradually added to maintain the initial volume (300 mL) and result in a 1:9 virus dilution. The final 300 mL of diafiltered sample was separated into triplicates of 100-mL polycarbonate flasks and was kept in a BOD chamber (25 ± 1 °C) for a period of 10 h. The polycarbonate flasks used for the incubation were previously autoclaved, washed with HCl 10%, and rinsed with Milli-Q water. Virus-bacterium density sub-samples (1 mL) were collected from each polycarbonate flasks every 2 h and 30 min and then fixed and stored for flow cytometric measurements as described in the section above. Three bacterial production sub-samples (1.2 mL) were also performed after the first 2 h and 30 min of the incubations, when a rapid increase in the virus abundance (VA) is usually observed [29].
VP rates were determined from the mean slope of linear regressions of VA against time for each incubation replicate. VD was obtained from the first-order regression when virus started decreasing just after the main VA increase. VT—the fraction of the viral population that is replaced every hour—was calculated by dividing VP rates by initial abundances of viruses in each replicate. Bs is the amount of virus-like-particles (VLPs) released from a cell lysis event (VLPs lyse−1) and was calculated by dividing the first VA increase by the first HBA decrease of the incubations. The VMM of bacterial cells (cells lysed mL−1 h−1) was calculated by dividing VP values by the Bs (VMM = VP / Bs) [29]. VMM was further divided by the new bacterial cell production rates (cells mL−1 h−1) calculated from 3H-leucine incorporation, to give the virus-induced bacterial mortality (VIBM), expressed as a percentage of bacterial production (VIBM (%) = VMM / NCP × 100) [29]. Finally, estimates of the amount of organic carbon (ng C mL−1 h−1) released by viral lysis of infected bacterial cells were calculated by multiplying VMM by the mean carbon content per cell of 20 fg C cell−1, which is a conversion factor widely used in the literature [30].
Limnological Variables
Water pH was measured using a pH meter (Analion PM 608), turbidity with a turbidimeter (Hanna HI 98703), and salinity with the chlorinity method [31]. Water samples for dissolved organic carbon and dissolved nitrogen evaluation were filtered through 0.7-μm glass fiber filters (47 mm; GF/F Whatman) and the filters were kept frozen in the dark (up to 1 week) for chlorophyll-a concentration analysis. Chlorophyll-a was extracted from these filters with 90% ethanol and absorbance was measured by spectrophotometry at 665 nm [32]. Dissolved organic carbon and dissolved nitrogen concentrations were determined using a Pt-catalyzed high-temperature combustion method with a Total Organic Carbon Analyzer (TOC-5000 with a TNM-L unit; Shimadzu Scientific Instruments). Unfiltered water samples were autoclaved and total phosphorus was spectrophotometrically determined through formation of phosphorus molybdate [33]. Water color was estimated by the absorbance at 440 nm and expressed as the absorption coefficient calculated according to Hu et al. [34]. All spectrophotometric analyses were performed in a 1-cm quartz cuvette (distilled water as a blank) using a Beckman® DU520 spectrophotometer.
Data Analysis
We used linear models to evaluate the main and interactive effects of abiotic (e.g., salinity, temperature, nutrient concentration) and biotic (e.g., chlorophyll-a concentration, HBA, BP) factors on VA and VP using the package nlme [35] in the R Statistical Software (version 3.2.2, <www.r-project.org>). Other viral parameters calculated in this study (e.g., VMM, Bs) were not statistically tested due to the small number of results (see “Results” section). We log-transformed VA and VP to meet normality and homoscedasticity assumptions. We built models containing all variables and used an averaging procedure to identify the best-fitting model (functions aictab and evidence in the Aiccmodavg package; [36]). This model selection procedure retains the best-predicting model by balancing the number of variables and the explanatory power added to the model [37]. Best-predicting models were those that had the smallest AICc scores [37]. We also conducted Pearson’s correlations to test relationship and identify multicollinearity among log-transformed (except pH) environmental variables. For this test, an alpha of 0.05 indicated statistical significance.
Results
VA varied by three orders of magnitude from 0.37 × 108 to 117 × 108 VLP mL−1. The lowest VA values were found in humic lagoons such as lagoons 5, 6, 7, and 16 (Table 1). Eutrophic and hypereutrophic lagoons (lagoons 8 and 4, respectively) were about threefold more abundant in virus than the humic lagoons (Table 1). The highest VAs were recorded in the hypersaline systems (lagoons 13, 14, 16, 17, 20), where VA reached a remarkable maximum of 1.17 · 1010 virus mL−1. HBA varied from 0.14 × 107 to 509 × 107 cells−1 mL−1 and the virus-to-bacteria ratio (VBR) ranged from 2.3 to 144 (Table 1). VP varied by two orders of magnitude, between 0.68 × 107 and 56.5 × 107 VLP mL−1 h−1, whereas BP ranged from 0.26 to 20.87 μM C h−1 (Table 1). VD varied between 0.46 and 39.6 × 107 VLP mL−1 h−1, whereas VT ranged from 0.15 to 2.1 h−1 (Table 1). From a total of 16 lagoons, 10 did not present a bacterial decay alongside the first virus increase recorded in the time-course incubations and, therefore, Bs, VMM, and VIBM were only calculated for six of the lagoons (Table 2). Bs varied from 3.7 to 861 VLP lyse−1, VMM ranged from 2.27 × 106 to 37.6 × 106 cells lysed mL−1 h−1, and VIBM varied from 16 to 1681% (Table 2). We estimated that viruses were responsible for releasing from 45.4 to 751.2 μg C L−1 h−1 of carbon through bacterial cells lyse in surface waters of the tropical coastal lagoons (Table 2).
The environmental conditions in each lagoon during viral and bacterial samplings are shown in Table 3. Chlorophyll-a concentration ranged from 0.28 to 134.5 μg L−1. Carbon concentration varied from 0.71 to 16.7 mM, while nitrogen and phosphorus concentrations varied between 40.3 and 8730.4 μM and from 0.77 to 156 μM, respectively. Water color was recorded between 1.82 and 92.49 m−1. Turbidity was recorded between 2.32 and 571 NTU and pH ranged from 3.22 to 7.59. Temperature was between 23 and 35.2 °C and salinity ranged from 0.13 to 332.1‰. Oxygen saturation in the water column varied from 1.2 and 181.6%.
We observed strong correlation among physical-chemical variables in these lagoons (Table S2). Carbon concentration was positively correlated to phosphorus (r = 0.72) and nitrogen (r = 0.91) concentrations, as well as nitrogen and phosphorus concentrations were positively correlated (r = 0.81). Carbon concentration was also positively correlated to water color (r = 0.63), indicating the strong influence of humic-rich water table to these lagoons. Both carbon and nitrogen concentrations were negatively correlated to oxygen saturation (r = −0.60 and r = −0.56, respectively). All nutrient concentrations were positively correlated to turbidity (r = 0.71, for nitrogen; r = 0.58, for phosphorus; r = 0.70, for carbon) and salinity (r = 0.66, for nitrogen; r = 0.48, for phosphorus; r = 0.50, for carbon).
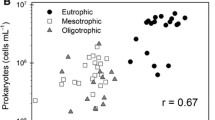
HBA, salinity, and nitrogen concentration were shown to be together the most important explanatory variables for VA (Table 4 and Fig. 3). HBA, salinity, and their interaction constituted the most parsimonious candidate model for positively predicting the VA in these coastal lagoons (Table 4). Adding the interaction of nitrogen concentration and HBA slightly improved the prediction of VA but also increased complexity of the model (Table 4). BP and phosphorus concentration were selected as the best explanatory variables for VP; these variables comprised the only significant models that explained VP (Table 4 and Fig. 4). Adding either single or interactive variables to the model only enhanced complexity but did not significantly improve prediction of VP (Table 4).
Linear regression between virus abundance and heterotrophic bacterial abundance within salinity gradient. The size of the circles represents square-rooted salinity values. The shadow area around the regression line represents 95% confidence interval for the linear regression. The relationship is significant (p < 0.05)
Discussion
Our main aim in this study was investigating which bacterial and environmental factors were most important to drive virus abundance and production in tropical coastal lagoons. Among many environmental factors, HBA and salinity have been clearly shown to be the main drivers of VA in the studied coastal lagoons as evidenced by our best-predicting models (Table 4). VA and HBA were strongly related (Fig. 3); both sharply increased with salinity and reached their highest numbers in the hypersaline systems (Table 3 and Fig. 3), which is in agreement with several previous studies [11, 38,39,40]. Conversely, VP and BP did not increase within a salinity gradient, revealing an apparent paradox of higher abundance despite unaffected production in increasingly salty systems. Nutrient concentration was demonstrated to be also an important factor explaining virus-bacterium interactions in these systems. Other usually important environmental virus regulators in aquatic ecosystems, such as water temperature and concentrations of humic substances, carbon, and chlorophyll-a, were irrelevant for determining virus parameters in our study.
On the one hand, the VA in most of the lagoons was within the previously reported ranges of values for temperate inland waters with similar environmental features [5, 6, 11, 41]. On the other hand, we recorded higher VA than those found in other tropical freshwater ecosystems [16, 42,43,44]. Hence, the general hypothesis that virus would be less abundant in tropical than temperate systems due to higher UV radiation [16] is not supported by our data. We suggest VA would be determined by a combination of several internal environmental factors, without regard to latitude, that affect virus’ hosts. The extremely high VA found in all hypersaline systems is in line with previous studies that recorded VA of up to 109 viruses mL−1 in hypersaline lakes [11, 38, 39], solar salterns [12], and crystallizer ponds [40]. However, we here recorded a remarkable maximum VA of 1.17 1010 virus mL−1 in the hypersaline lagoon 17 (Table 1), which is, to the best of our knowledge, the highest virioplankton abundance ever documented for aquatic systems.
The increasing VA, as a response to higher HBA, within a salinity gradient can be explained by two related factors. First, salinity constrains microzooplankton (i.e., nanoflagellates) presence [12, 45], releasing bacterial populations from predation pressure [46], which should increase HBA. Second, this pattern might be observed due to lower inter-specific competition as a result of a decreasing diversity [11] since a few bacterial groups are capable of surviving in such harshly osmotic conditions [47]. Some studies have shown that the prokaryotic community composition drastically shifts from freshwater to hypersaline conditions [48]. In fact, the bacterial community composition of the lagoons studied here has already been shown to greatly change as salinity increases [49]. The main change is observed with salt concentrations above 150‰, when the prokaryotic assemblage starts being dominated by Archaea instead of the typically dominant Eubacteria [11]. Finally, the higher increase of VA in comparison to HBA in hypersaline systems was probably related to a higher resistance of halophages to environmental virucidal agents [11] and, to a lesser extent, due to a lack of virivorous nanoflagellates or ciliates [12].
This is the first study to measure VP in tropical coastal lagoons. VP was found 1–2 orders of magnitude higher than recorded in sub-tropical [50] and tropical estuaries [51] and coral reefs [52] as well in temperate lakes [53], estuaries [54], and marine systems [27, 28, 55, 56]. VP was mainly explained by BP and phosphorus concentration (Table 4, Fig. 4). Several studies have demonstrated that higher VP is indeed associated to increasing BP rates [53, 55, 57], which is reasonable since the viral development depends on the metabolic state of their hosts [58]. Eutrophic systems usually present higher VP rates than oligotrophic ones [50, 53] because nutrient amendments enhance VP rates [57], which is endorsed by our observations (Table 1). However, the relationship between VP and phosphorus concentration is not mediated by changes in the bacterial metabolism (Fig. 4 and Table 4), which is supported by the fact that phosphorus concentration was not correlated to BP (Table S2). This result contradicts previous findings that bacteria mediate the phosphorus effect on virus production [55] and suggests that P-limitation is potentially an important direct regulator of virus activity. Phosphorus has indeed been described as a direct limiting nutrient for virus proliferation since this element is a key component of DNA [59]. Moreover, the VP coupling with phosphorus concentration could be linked to phytoplankton not bacterial populations as a recent experimental study found higher VP from isolated picoautotrophs (Micromonas pusilla) under P-enriched conditions [60].
The lower BP found in highly salty, yet also nutrient-rich environments (Table 3), is in agreement with previous studies [12, 48]. This pattern is observed because prokaryotes need to protect themselves from the osmotic stress by using several energy-costing strategies [61]. For instance, halobacterial groups present several mechanisms to tolerate the high environmental salinities, such as increasing cytoplasmic salt concentration with or accumulating glycine betaine and other compatible solutes in order to avoid water loss through osmotic instability of their cytoplasm [62]. Such expensive mechanisms may negatively impact the bacterial physiological state and growth rate [11] and, in turn, affect the VP rates since smaller and stressed bacteria produce fewer viruses [63, 64]. Salinity may also constrain VP by slowing down or hampering the virus adsorption processes to their host cells [65]. These results point that VA and HBA increase within a salinity gradient regardless of VP and BP, suggesting that the lack of top-down control on bacterial and viral populations is indeed an important mechanism behind the high abundances in the saltiest ecosystems.
In general, calculated Bs values were mostly recorded to be similar to the range of values previously found in inland waters worldwide [7, 53, 63, 66]. Lagoon 2 was the only coastal lagoon with an extremely high average Bs value (Table 2) and greatly differed from any other system without apparent reason. High Bs values are usually supported by larger cell biovolumes [63] and growth rates [64]. Therefore, Bs was expected to increase with the trophic state of the lagoons, since bacterial cell size and growth rate are commonly larger in eutrophic than in oligotrophic systems [53]. Nevertheless, no consistent pattern regarding lagoon trophic status seems to emerge about the Bs variability among the systems where Bs could be calculated (Table 2). The lack of effect of environmental variables on Bs has already been observed in another study carried out in temperate lakes [6].
There was also no apparent environmental variable driving VIBM (%) values (Table 2). VIBM was recorded to be above 100% of bacterial production in three out of six lagoons where VIBM could be calculated (Table 2). This result is very likely to be overestimated and TEM analysis would be needed to further investigate it. Lagoon 14 presented a VIBM of 16%, which is nearly the same percentage observed in many temperate systems [7, 53, 66, 67], but it is still at least twofold higher than recorded in a tropical inland waters [44, 51]. Lagoons 2 and 8 presented VIBM of >50% (Table 2). This is even higher than observed for tropical systems, but it is realistic if compared to a study conducted in an eutrophic temperate lake [68]. It is worth noticing that because BP is higher in tropical systems [17], VIBM may be of relatively greater importance for the carbon cycling in these ecosystems.
The amount of dissolved organic carbon estimated to be released from bacterial cells lysed by virus (Table 2) represents about 35 to 550% of the bacterial secondary production in the surface waters of these systems. These estimates need to be interpreted with caution since average bacterial carbon content may vary under different environmental conditions [69]. For instance, our calculations could be underestimated because bacteria living in highly productive coastal systems tend to have higher carbon content per cell (30 fg C cell−1) [69]. Here we used a more conservative conversion factor (20 fg C cell−1), and we found high percentages of bacterial production being controlled by virus activity. We thus suggest that viruses have a great impact on the bacterial community carbon cycling in the tropical coastal lagoons. This viral loop would decrease the amount of carbon that reaches higher trophic levels and increases the pool of dissolved carbon in the water column of these lagoons.
The fact that HBA decrease was not recorded in the time-course incubations of 10 out of 16 lagoons indicates that lysogeny is probably prevalent over lytic infection in these microbial abundant systems. This observation is in line with the recently proposed Piggyback-the-Winner model that predicts that lysogeny becomes more frequent with high microbial abundances (>106 microbes mL−1) and growth rates [2]. We also recorded VA increase without HBA decrease in some of the time-course incubations (Table 1), which suggests that VP could have occurred without bacterial lysis. This phenomenon of continuous virus release without cell lysis is known as chronic infection [70], and it has been considered a potential mechanism of virus replication in harsh environments [11]. We could not find an association of these virus life strategies with any specific environmental condition, which is still a research challenge in the field of virus ecology [71]. Further investigations combining TEM, metagenomics, and VP techniques would lead to better insights into the virus life strategies in these systems.
Conclusion
This study provides novel data on virioplankton abundances and production from a large number of tropical coastal lagoons that present a wide range of co-occurring environmental conditions. We found very high virus abundance and production, indicating that virioplankton performs a substantial role in the biogeochemical cycles of tropical coastal lagoons. We conclude that salinity is the strongest environmental factor to positively impact virus abundance by affecting the abundance of bacterial populations. Virus production was mainly controlled by bacterial production and total phosphorus concentration. The uncoupling between virus abundance and production would only be possible because of a decreasing microzooplankton density and, consequently, lower grazing on bacterial and virus populations, in extremely salty ecosystems.
It is worth noticing that wetlands are currently undergoing salinization worldwide because of anthropogenic impacts such as wastewater effluent disposal, irrigation, applications of de-icing salts, and climate change-driven processes such as sea-level rise and shifts in precipitation patterns [72]. Our findings hint that coastal wetlands subjected to these changes could present a future with increasing virus abundances despite unaltered virus production. It is key for future research to experimentally investigate the interactive effects of salinization and eutrophication on the virus-bacterial dynamics in aquatic ecosystems.
References
Cobián Güemes AG, Youle M, Cantú VA, et al. (2016) Viruses as winners in the game of life Annu Rev Virol 3:197–214. doi:10.1146/annurev-virology-100114-054952
Knowles B, Silveira CB, Bailey BA, et al. (2016) Lytic to temperate switching of viral communities Nature 531:466–470. doi:10.1038/nature17193
Pradeep Ram AS, Chaibi-Slouma S, Keshri J, et al. (2016) Functional responses of bacterioplankton diversity and metabolism to experimental bottom-up and top-down forcings Microb Ecol 72:347–358. doi:10.1007/s00248-016-0782-0
Weitz JS, Stock CA, Wilhelm SW, et al. (2015) A multitrophic model to quantify the effects of marine viruses on microbial food webs and ecosystem processes ISME J 9:1352–1364. doi:10.1038/ismej.2014.220
Anesio AM, Hollas C, Granéli W, Laybourn-Parry J (2004) Influence of humic substances on bacterial and viral dynamics in freshwaters Appl. Environ Microbiol 70:4848–4854. doi:10.1128/AEM.70.8.4848-4854.2004
Pradeep Ram AS, Rasconi S, Jobard M, et al. (2011) High lytic infection rates but low abundances of prokaryote viruses in a Humic Lake (Vassivière, Massif Central, France) Appl Environ Microbiol 77:5610–5618. doi:10.1128/AEM.01370-10
Mathias CB, Kirschner AKT, Velimirov B (1995) Seasonal variations of virus abundance and viral control of the bacterial production in a backwater system of the Danube River Appl Environ Microbiol 61:3734–3740
Wilhelm S, Jeffrey W, Dean A, et al. (2003) UV radiation induced DNA damage in marine viruses along a latitudinal gradient in the southeastern Pacific Ocean Aquat Microb Ecol 31:1–8. doi:10.3354/ame031001
Wommack KE, Colwell RR (2000) Virioplankton: viruses in aquatic ecosystems Microbiol Mol Biol Rev 64:69–114. doi:10.1128/MMBR.64.1.69-114.2000
Wigington CH, Sonderegger D, Brussaard CPD, et al. (2016) Re-examination of the relationship between marine virus and microbial cell abundances Nat Microbiol 1:15024. doi:10.1038/nmicrobiol.2015.24
Bettarel Y, Bouvier T, Bouvier C, et al. (2011) Ecological traits of planktonic viruses and prokaryotes along a full-salinity gradient FEMS Microbiol Ecol 76:360–372. doi:10.1111/j.1574-6941.2011.01054.x
Pedrós-Alió C, Calderón-Paz JI, MacLean MH, et al. (2000) The microbial food web along salinity gradients FEMS Microbiol Ecol 32:143–155. doi:10.1016/S0168-6496(00)00025-8
Hanson AM, Berges JA, Young EB (2017) Virus morphological diversity and relationship to bacteria and chlorophyll across a freshwater trophic gradient in the Lake Michigan watershed Hydrobiologia. doi:10.1007/s10750-016-3084-0
Cabral AS, Lessa MM, Junger PC, et al. (2017) Virioplankton dynamics are related to eutrophication levels in a tropical urbanized bay PLoS One 12:e0174653. doi:10.1371/journal.pone.0174653
Wang C, Wang Y, Paterson JS, et al. (2016) Macroscale distribution of virioplankton and heterotrophic bacteria in the Bohai Sea FEMS Microbiol Ecol 92:1–10. doi:10.1093/femsec/fiw017
Barros N, Farjalla VF, Soares MC, et al. (2010) Virus-bacterium coupling driven by both turbidity and hydrodynamics in an Amazonian floodplain lake Appl Environ Microbiol 76:7194–7201. doi:10.1128/AEM.01161-10
Amado AM, Meirelles-Pereira F, Vidal LO, et al. (2013) Tropical freshwater ecosystems have lower bacterial growth efficiency than temperate ones Front. Microbiol. 4:1–8. doi:10.3389/fmicb.2013.00167
Caliman A, Carneiro LS, Santangelo JM, et al. (2010) Temporal coherence among tropical coastal lagoons: a search for patterns and mechanisms Brazilian J Biol 70:803–814. doi:10.1590/S1519-69842010000400011
Suhett AL, Amado AM, Meirelles-Pereira F, et al. (2013) Origin, concentration, availability and fate of dissolved organic carbon in coastal lagoons of the Rio de Janeiro State Acta Limnol Bras 25:326–340. doi:10.1590/S2179-975X2013000300011
Gasol JM, del Giorgio PA (2000) Using flow cytometry for counting natural planktonic bacteria and understanding the structure of planktonic bacterial communities Sci Mar 64:197–224. doi:10.3989/scimar.2000.64n2197
Brussaard CPD (2004) Optimization of procedures for counting viruses by flow cytometry Appl Environ Microbiol 70:1506–1513. doi:10.1128/AEM.70.3.1506-1513.2004
Marie D, Vaulot D, Partensky F (1996) Application of the novel nucleic acid dyes YOYO-1, YO-PRO-1, and PicoGreen for flow cytometric analysis of marine prokaryotes Appl Environ Microbiol 62:1649–1655
Kirchman DL (1993) Leucine incorporation as a measure of biomass production by heterotrophic bacteria. In: Kemp PF, Sherr BF, Sherr EB, Cole JJ (eds.) Handbook of methods in aquatic microbial ecology. Lewis Pub., Boca Raton, pp 509–512
Miranda MR, Guimarães JRD, Coelho-Souza AS (2007) [3H]Leucine incorporation method as a tool to measure secondary production by periphytic bacteria associated to the roots of floating aquatic macrophyte J Microbiol Methods 71:23–31. doi:10.1016/j.mimet.2007.06.020
Simon M, Azam F (1989) Protein content and protein synthesis rates of planktonic marine bacteria Mar Ecol Prog Ser 51:201–213. doi:10.3354/meps051201
Wetzel RG, Likens GE (2000) Limnological analyses, 3rd edn. Springer, New York. doi:10.1007/978-1-4757-3250-4
Wilhelm SW, Brigden SM, Suttle CA (2002) A dilution technique for the direct measurement of viral production: a comparison in stratified and tidally mixed coastal waters Microb Ecol 43:168–173. doi:10.1007/s00248-001-1021-9
Karuza A, Del Negro P, Crevatin E, Fonda Umani S (2010) Viral production in the Gulf of Trieste (Northern Adriatic Sea): preliminary results using different methodological approaches J Exp Mar Bio Ecol 383:96–104. doi:10.1016/j.jembe.2009.12.003
Bellas CM, Anesio AM, Telling J, et al. (2013) Viral impacts on bacterial communities in Arctic cryoconite Environ Res Lett 8:45021. doi:10.1088/1748-9326/8/4/045021
Lee S, Fuhrman JA (1987) Relationships between biovolume and biomass of naturally derived marine bacterioplankton Appl Environ Microbiol 53:1298–1303. doi:10.1016/0198-0254(87)96080-8
Grasshoff K, Kremling K, Erhardt M (1999) Methods of seawater analysis, 3rd edn. Wiley-VCH Verlag, Weinheim
Nusch EA, Palme G (1975) Biologische methoden für die praxis der gewässeruntersuchung. GWF–Wasser/Abwasser
Golterman HL, Clymo RS, Ohnstad MAM (1978) Methods for physical and chemical analysis of fresh waters. Blackwell, Oxford
Hu CM, Muller-Karger FE, Zepp RG (2002) Absorbance, absorption coefficient, and apparent quantum yield: a comment on common ambiguity in the use of these optical concepts Limnol Oceanogr 47:1261–1267. doi:10.4319/lo.2002.47.4.1261
Pinheiro J, Douglas B, DebRoy S, et al. (2015) _nlme: linear and nonlinear mixed effects models_ R Packag version 3:1–122
Mazerolle MJ (2015) AICcmodavg: model selection and multimodel inference based on (Q)AIC(c)
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach Ecol Model. doi:10.1016/j.ecolmodel.2003.11.004
Bettarel Y, Desnues A, Rochelle-Newall E (2010) Lytic failure in cross-inoculation assays between phages and prokaryotes from three aquatic sites of contrasting salinity FEMS Microbiol Lett 311:113–118. doi:10.1111/j.1574-6968.2010.02074.x
Oren A, Bratbak G, Heldal M (1997) Occurrence of virus-like particles in the Dead Sea Extremophiles 1:143–149. doi:10.1007/s007920050027
Guixa-Boixareu N, Calderón-Paz JI, Heldal M, et al. (1996) Viral lysis and bacterivory as prokaryotic loss factors along a salinity gradient Aquat Microb Ecol 11:215–227. doi:10.3354/ame011215
Vrede K, Stensdotter U, Lindström ES (2003) Viral and bacterioplankton dynamics in two lakes with different humic contents Microb Ecol 46:406–415. doi:10.1007/s00248-003-2009-4
Almeida RM, Roland F, Cardoso SJ, et al. (2015) Viruses and bacteria in floodplain lakes along a major Amazon tributary respond to distance to the Amazon River Front Microbiol. doi:10.3389/fmicb.2015.00158
Peduzzi P, Schiemer F (2004) Bacteria and viruses in the water column of tropical freshwater reservoirs Environ Microbiol 6:707–715. doi:10.1111/j.1462-2920.2004.00602.x
Bettarel Y, Bouvy M, Dumont C, Sime-Ngando T (2006) Virus-bacterium interactions in water and sediment of West African inland aquatic systems Appl Environ Microbiol 72:5274–5282. doi:10.1128/AEM.00863-06
Elloumi J, Carrias JF, Ayadi H, et al. (2009) Communities structure of the planktonic halophiles in the solar saltern of Sfax, Tunisia Estuar Coast Shelf Sci 81:19–26. doi:10.1016/j.ecss.2008.09.019
Sherr EB, Sherr BF (2002) Significance of predation by protists in aquatic microbial food webs Antonie Van Leeuwenhoek 81:293–308. doi:10.1023/A:1020591307260
Sime-Ngando T, Lucas S, Robin A, et al. (2011) Diversity of virus-host systems in hypersaline Lake Retba, Senegal Environ Microbiol 13:1956–1972. doi:10.1111/j.1462-2920.2010.02323.x
Bouvier TC, del Giorgio PA (2002) Compositional changes in free-living bacterial communities along a salinity gradient in two temperate estuaries Limnol Oceanogr 47:453–470. doi:10.4319/lo.2002.47.2.0453
Laque T, Farjalla VF, Rosado AS, Esteves FA (2010) Spatiotemporal variation of bacterial community composition and possible controlling factors in tropical shallow lagoons Microb Ecol 59:819–829. doi:10.1007/s00248-010-9642-5
Hewson I, O’Neil JM, Fuhrman JA, Dennison WC (2001) Virus-like particle distribution and abundance in sediments and overlying waters along eutrophication gradients in two subtropical estuaries Limnol Oceanogr 46:1734–1746. doi:10.4319/lo.2001.46.7.1734
Cissoko M, Desnues A, Bouvy M, et al. (2008) Effects of freshwater and seawater mixing on virio- and bacterioplankton in a tropical estuary Freshw Biol 53:1154–1162. doi:10.1111/j.1365-2427.2007.01930.x
Payet JP, McMinds R, Burkepile DE, Vega Thurber RL (2014) Unprecedented evidence for high viral abundance and lytic activity in coral reef waters of the South Pacific Ocean Front Microbiol 5:1–16. doi:10.3389/fmicb.2014.00493
Bettarel Y, Sime-Ngando T, Amblard C, Dolan J (2004) Viral activity in two contrasting lake ecosystems Appl Environ Microbiol 70:2941–2951. doi:10.1128/AEM.70.5.2941-2951.2004
Winget DM, Helton RR, Williamson KE, et al. (2011) Repeating patterns of virioplankton production within an estuarine ecosystem Proc Natl Acad Sci USA 108:11506–11511. doi:10.1073/pnas.1101907108
Bongiorni L, Magagnini M, Armeni M, et al. (2005) Viral production, decay rates, and life strategies along a trophic gradient in the North Adriatic Sea Appl Environ Microbiol. doi:10.1128/AEM.71.11.6644
Poorvin L, Rinta-Kanto JM, Hutchins DA, Wilhelm SW (2004) Viral release of iron and its bioavailability to marine plankton Limnol Oceanogr 49(5):1734–1741. doi:10.4319/lo.2004.49.5.1734
Weinbauer MG, Christaki U, Nedoma J, Simek K (2003) Comparing the effects of resource enrichment and grazing on viral production in a meso-eutrophic reservoir Aquat Microb Ecol 31:137–144
Corinaldesi C, Crevatin E, Del Negro P, et al. (2003) Large-scale spatial distribution of virioplankton in the Adriatic Sea: testing the trophic state control hypothesis Appl Environ Microbiol 69:2664–2673. doi:10.1128/AEM.69.5.2664-2673.2003
Tuomi P, Fagerbakke KM, Bratbak G, Heldal M (1995) Nutritional enrichment of a microbial community: the effects on activity, elemental composition, community structure and virus production FEMS Microbiol Ecol 16:123–134. doi:10.1016/0168-6496(94)00076-9
Maat DS, van Bleijswijk JDL, Witte HJ, Brussaard CPD (2016) Virus production in phosphorus-limited Micromonas pusilla stimulated by a supply of naturally low concentrations of different phosphorus sources, far into the lytic cycle FEMS Microbiol Ecol 92:1–34. doi:10.1093/femsec/fiw136
Oren A (2011) Thermodynamic limits to microbial life at high salt concentrations Environ Microbiol 13:1908–1923. doi:10.1111/j.1462-2920.2010.02365.x
Sleator RD, Hill C (2002) Bacterial osmoadaptation: the role of osmolytes in bacterial stress and virulence FEMS Microbiol Rev 26:49–71. doi:10.1016/S0168-6445(01)00071-7
Weinbauer MG, Höfle MG (1998) Size-specific mortality of lake bacterioplankton by natural virus communities Aquat Microb Ecol 15:103–113. doi:10.3354/ame015103
Middelboe M (2000) Bacterial growth rate and marine virus–host dynamics Microb Ecol 40:114–124. doi:10.1007/s002480000050
Kukkaro P, Bamford DH (2009) Virus-host interactions in environments with a wide range of ionic strengths Environ Microbiol Rep 1:71–77. doi:10.1111/j.1758-2229.2008.00007.x
Hennes KP, Simon M (1995) Significance of bacteriophage for controlling bacterioplankton in a mesotrophic lake Appl Environ Microbiol 61:333–340
Simek K, Pernthaler J, Weinbauer MG, et al. (2001) Changes in bacterial community composition and dynamics and viral mortality rates associated with enhanced flagellate grazing in a mesoeutrophic reservoir Appl Environ Microbiol 67:2723–2733
Fischer UR, Velimirov B (2002) High control of bacterial production by viruses in a eutrophic oxbow lake Aquat Microb Ecol 27:1–12. doi:10.3354/ame027001
Fukuda R, Ogawa H, Nagata T (1998) Direct determination of carbon and nitrogen contents of natural bacterial assemblages in marine environments direct determination of carbon and nitrogen contents of natural bacterial assemblages in marine environments Appl Environ Microbiol 64:3352–3358
Porter K, Russ BE, Dyall-Smith ML (2007) Virus-host interactions in salt lakes Curr Opin Microbiol 10:418–424. doi:10.1016/j.mib.2007.05.017
Knowles B, Bailey B, Boling L, et al. (2017) Variability and host density independence in inductions-based estimates of environmental lysogeny Nat Microbiol 2:17064. doi:10.1038/nmicrobiol.2017.64
Herbert ER, Boon P, Burgin AJ, et al. (2015) A global perspective on wetland salinization: ecological consequences of a growing threat to freshwater wetlands Ecosphere 6:art206. doi:10.1890/ES14-00534.1
Acknowledgments
PCJ is grateful to Brazilian Council for Research, Development and Innovation (CNPq) for a Master scholarship. VFF is grateful to CNPq for research funds and a productivity grant (Project 306448/2011-4). Authors are thankful to Alice Campos and Thiago Benevides for laboratory and fieldwork assistance, Jean Remy Guimarães for assistance in bacterial production analyses, and Fernanda Ferreira and Anderson dos Santos Junior for support in flow cytometry analyses. The authors also thank Andrew MacDonald for language review and Hugo Sarmento for his critical reading of an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
ESM 1
(DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Junger, P.C., Amado, A.M., Paranhos, R. et al. Salinity Drives the Virioplankton Abundance but Not Production in Tropical Coastal Lagoons. Microb Ecol 75, 52–63 (2018). https://doi.org/10.1007/s00248-017-1038-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-017-1038-3