Abstract
Background
Young children requiring clinical magnetic resonance imaging (MRI) may be given general anesthesia. General anesthesia has potential side effects, is costly, and introduces logistical challenges. Thus, methods that allow children to undergo awake MRI scans are desirable.
Objectives
To compare the effectiveness of mock scanner training with a child life specialist, play-based training with a child life specialist, and home book and video preparation by parents to allow non-sedated clinical MRI scanning in children aged 3–7 years.
Materials and methods
Children (3–7 years, n=122) undergoing clinical MRI scans at the Alberta Children’s Hospital were invited to participate and randomized to one of three groups: home-based preparation materials, training with a child life specialist (no mock MRI), or training in a mock MRI with a child life specialist. Training occurred a few days prior to their MRI. Self- and parent-reported functioning (PedsQL VAS) were assessed pre/post-training (for the two training groups) and pre/post-MRI. Scan success was determined by a pediatric radiologist.
Results
Overall, 91% (111/122) of children successfully completed an awake MRI. There were no significant differences between the mock scanner (89%, 32/36), child life (88%, 34/39), and at-home (96%, 45/47) groups (P=0.34). Total functioning scores were similar across groups; however, the mock scanner group had significantly lower self-reported fear (F=3.2, P=0.04), parent-reported sadness (F=3.3, P=0.04), and worry (F=3.5, P=0.03) prior to MRI. Children with unsuccessful scans were younger (4.5 vs. 5.7 years, P<0.001).
Conclusions
Most young children can tolerate awake MRI scans and do not need to be routinely anesthetized. All preparation methods tested, including at-home materials, were effective.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The lack of ionizing radiation and the ability to obtain high-resolution images of soft-tissue structures make magnetic resonance imaging (MRI) a favorable choice for pediatric imaging [1]. However, MRI is very sensitive to motion; images can take several minutes to obtain, and an MRI scanner is loud and can be claustrophobic for some [1]. These factors can lead to difficulty (or perceived difficulty) in obtaining useful images in young children [2]. As a result, young children are often given a general anesthetic to undergo clinical MRI [3]. General anesthesia permits MRI images to be obtained without the worry of movement, providing high-quality scans. However, general anesthesia introduces a small risk of side effects [4, 5] and can be a scary procedure itself, and there is ongoing concern about the impact of general anesthesia in early childhood on neurological development [6]. Additionally, MRI scans requiring general anesthesia are more resource-intensive for the healthcare system, requiring much longer appointment times, more staff, and up to a tenfold increase in costs compared to an MRI scan without sedation [7].
Thus, there is a need to develop and evaluate methods to allow young children to undergo MRI scans successfully without sedation. Pediatric neurodevelopmental research using MRI has necessitated the development of methods to help children tolerate awake MRIs [8], including the use of mock MRI scanners, which are similarly sized replicas of MRI scanners without functional magnets, but with the same mirrors, audiovisual set up, and sound effects (Fig. 1). Children can be given a training session in the mock MRI machine, often by a trained research assistant, child life specialist, or play therapist, which allows them to become familiar with the MRI environment and practice lying still while tolerating the MRI sounds [8]. Prior research has reported success frequencies of 88–96% using mock MRI protocols to prepare children for research MRI scans [9,10,11], with the most pronounced benefit in 3–8-year-olds. Two other studies retrospectively reviewed sedation frequencies after instituting a mock MRI training program, finding 16.8% [12] and 34.6% [13] reductions in the need for general anesthesia. One prospective, randomized trial evaluated full instruction (MRI simulator and book) vs partial instruction (book only) immediately prior to an MRI scan and found that general anesthesia was required for significantly fewer children in the full instruction group (27% vs 47%) [14].
Mock MRI scanner used to prepare children for their awake MRI scans. The mock scanner used in this study is an MRI Simulator System (model PST-100355), from Psychology Software Tools, Inc manufactured in 2014. The mock scanner is operated with a small remote to move the bed in and out and uses a personal computer to play movies and simulate MRI sounds using SimFx. MoTrak software was used for head motion tracking
Despite potential benefits, there are barriers to using a mock MRI scanner for some centers, including cost, increased appointments for the patient, physical space for the mock scanner, and lack of appropriately trained staff. Other less resource-intensive methods of preparation for non-sedated MRI scans have also been shown to be successful, including the use of a photobook which reduced the need for sedation by 34% [13], a child-life consultation using low-tech materials prior to the MRI scan which resulted in a 15% reduction in the need for general anesthesia [15], the use of a teddy bear mock MRI machine which reduced anxiety and motion artifact [16], and educational videos, which reduced children’s anxiety (frequency of success not reported) [17, 18]. A recent systematic review and metanalysis that included any type of MRI training program found frequencies of success ranging from 40 to 100% [19].
There is some evidence that preparation methods work, but prior studies have lacked appropriate comparison groups, used very small sample sizes, compared a limited scope of preparation methods, and/or focused only on specific populations. Given the paucity of systematic studies of the efficacy of various preparation techniques for young children and clear motivation to reduce the use of general anesthesia, there is a need to evaluate different preparation methods to provide guidance to pediatric imaging centers and inform research studies. This study aimed to compare the effectiveness of three different preparation methods for non-sedated clinical MRI scanning in children aged 3–7 years: a mock scanner training session with a child life specialist, a play-based training session with a child life specialist, and a home book and video preparation performed by parents. We hypothesized that the mock scanner group would have the highest frequency of success in completing awake MRI scans.
Materials and methods
This study received institutional approval from the University of Calgary’s conjoint health research ethics board. The study design followed a prospective, randomized, open approach with three training arms.
Participants
Children who had been referred for a clinical diagnostic MRI scan were recruited from the Alberta Children’s Hospital Diagnostic Imaging department between March 2016 and September 2019. Inclusion criteria were: age 3–7 years, the child would normally receive general anesthesia for their scan, the child was referred for a low or medium priority clinical MRI scan that involved lying supine head-first in the MRI bore (so children could see the TV), MRI with or without intravenous contrast material, and child and at least one guardian who could speak and understand English. The 3–7 year age range was chosen because it aligned with the age range of highest effects in prior studies [19], overlapped with the age range routinely sedated at the Alberta Children’s Hospital at the time of study design, and excluded children too young to understand the preparation materials. Exclusion criteria were: high priority/urgent scans, orbital scans, previous awake MRI scans, scans requiring breath holds, significant developmental delays such that participants would not be able to understand training information, and scans where general anesthesia was not routinely used due to short duration. Eligible participants were contacted by a child-life specialist, informed of the study, and given the opportunity to consent to participate. We targeted a sample size of 120 participants (40 per group) based on 80% power to detect differences in frequencies of success of approximately 25% at alpha < 0.05.
Of 134 participants originally recruited to the study, 7 withdrew from the study, 2 did not show up for their appointments, and 3 participants had their scans canceled before they occurred as they were no longer required; therefore, 122 participants are included in this analysis.
Randomization and training groups
Enrolled participants were randomized into one of three treatment groups. Participants were randomized using covariate adaptive randomization [20, 21] to balance age and sex among groups. An exception to random allocation was made for a small number of participants who lived more than a 1-h drive from the study site, who were placed in the home training group for practical reasons.
Participants in all groups were provided with home training materials, which included links to online videos about MRI [22], audio files with scanner noises, and a children’s picture book about MRI scans [23], for parents to use at home to prepare their child (At-Home Group). One group also visited the hospital for a training session with a child life specialist prior to the MRI scan, but did not use the mock MRI machine (Child Life Group). The third group received the same at-home training materials and had an appointment with a child-life specialist prior to the MRI scan, which included a session on the mock MRI scanner machine (Mock Scanner Group) (Fig. 1). All training was conducted by one of two trained Child Life specialists (LC, 10 years’ experience; CS, 12 years’ experience) at the Alberta Children’s Hospital. Data regarding the amount of preparation time and resources used were collected at the training session for each participant in the Child Life and Mock Scanner Groups. The child life specialist made a prediction of success at each training session, which was recorded. A child’s self-report of functioning and a parent’s report of the child’s functioning were collected before and after the training session using the Peds QL Present Functioning Visual Analogue Scales (PedsQL VAS). These scales ask participants to rank domains of fear, sadness/upset, anger, worried, tiredness, and pain/discomfort from 1 (very much) to 5 (not at all) and include diagrams of faces to guide participants who cannot read [24].
The mock MRI machine was purchased from Psychology Software Tools, Inc. (Sharpsburg, Pennsylvania) and was manufactured in August 2014 (model # PST-100355). It is operated with a small remote control to move the bed and uses a PC computer to play movies and sounds. Use used SimFX for simulating MRI scanner noises and MoTrak for head motion tracking.
Diagnostic MRI sessions
Participants were booked for one MRI scan without general anesthesia and one with general anesthesia a week later, to ensure no substantial delay in receiving a clinically sufficient scan if the awake scan was unsuccessful. The child and their caregiver met with a member of the study team before and after the non-sedated MRI scan to complete study questionnaires. Prior to undertaking the scan, the MRI technologist made a prediction of scan success, which was recorded. Data collected at the MRI scan appointment included demographic information, scan duration, number of sequences that required repeating, and the child’s self-report and parent report of child functioning before and after the scan using the PedsQL VAS [24]. Scans were performed on either a 1.5 T or 3 T MRI. All participants watched movies or TV shows during their scan. After the completion of the scan, a board-certified pediatric radiologist or neuroradiologist (depending on the area of imaging) reported whether the scan was adequate for the clinical indication or needed to be repeated under general anesthesia; this was used as the final measure of the scan success. If the scan was deemed successful, the general anesthesia appointment was canceled.
Statistical analysis
Collected data was collated and analyzed using SPSS (version 24). Chi-square tests were used to test group differences in categorical data: sex, known developmental disorder, and scan outcome. A one-way ANOVA was used to compare continuous variables across groups: age, scan duration, number of repeated sequences, and child self-report and parent report Peds-QL VAS scores before and after the MRI scans. Data related to training sessions (training session duration, child self-report, and parent report Peds-QL VAS before/after training) was only collected from the child life and mock scanner groups, so a t-test was used to test group differences.
Additional analysis was conducted to compare children with successful vs unsuccessful scans. Chi-square tests were used to compare sex. T-tests were used to compare age, training duration, imaging duration, number of repeated sequences, and functioning scores for parent/child before and after the MRI scan. We also compared success rates across ages and between sexes. For predictions recorded by the child life specialists and MRI technologists, positive and negative predictive values and sensitivity and specificity were calculated, and a Chi-square test was used to determine statistical significance.
Results
Participants
A total of 122 children participated in the study, with 47 participants in the At-Home Group, 39 participants in the child life group, and 36 participants in the mock scanner group (Table 1). Groups did not differ significantly in age or sex. The duration of training was significantly longer for the group which used the mock scanner (61 ± 8.6 min) compared to the child life group (51 ± 9 min; P<0.001). More children with a known developmental disorder (ADHD, speech delay, developmental coordination disorder, sensory processing disorder) were in the At-Home and child life groups (7 in each), compared to zero participants in the mock scanner group. Most scans (n=111; 91%) were of the brain/head and/or spine regions; 11 scans were of other body areas (typically abdomen/pelvis).
Awake scan success
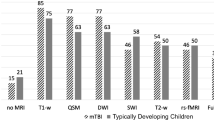
A total of 111 of 122 total participants successfully completed an awake MRI scan (91% success rate). Frequencies of success were 96% (45/47) for the At-Home Group, 88% (34/39) for the child life group, and 89% (32/36) for the mock scanner group; these did not differ significantly by group (Table 2, Fig. 2). The number of repeated sequences did not vary significantly between training groups.
Overall child self-report and parent report PedsQL VAS scores did not differ significantly between the child life and the mock scanner training groups before (P=0.33 and P=0.053, respectively) or after training (P=0.397 and P=0.243). There were small differences in some subscale measures, with the child life training group generally reporting more emotions (lower scores) than the mock scanner group (Supplementary Table 1).
There were no significant differences in overall child-reported functioning or parent-reported functioning between groups before or after the MRI scan (Table 3). On subscores, children in the mock scanner group reported lower fear prior to the MRI scan than the At-Home group (4.59 vs 3.85, P=0.044). Parents in the mock scanner group reported their children feeling significantly less sadness/upset and less worry prior to the MRI scan compared to both the At-Home and child life groups. There were no significant differences for PedsQL VAS scores following the MRI scan (P=0.306 for the child report, P=0.796 for the parent report).
The positive predictive value of predictions by child-life specialists was 95.3% (correctly predicted success in 61 of 64 participants following training, P < 0.01), and by MRI technologists was 94.1% (correctly predicted success in 96 of 102 participants immediately before the MRI, P=0.015). The negative predictive values were lower, at 66.7% for child life (correctly predicted non-success in 6 of 9 participants) and 76.5% for MRI technologist (correctly predicted 13 of 17 participants), respectively.
Children who had an unsuccessful awake MRI were on average 1 year younger than children who had a successful scan (4.51 ± 1.17 vs 5.65 ± 1.14 years, P < 0.001). There were no significant differences in sex, training duration, or the number of repeated sequences (Table 4). There were no significant differences in functioning scores prior to the scan between successful and unsuccessful children (P=0.219 on the child report, P=0.086 on the parent report). Children who were unsuccessful had significantly lower functioning scores on both child and parent report following the MRI scan, compared to children who were successful (P=0.019 on the child report, P < 0.001 on the parent report).
Children aged 3–3.99 years were less successful (69%; 11/16) than the overall group average (91%; 111/122) (P=0.015); there were no significant differences in success for children aged 4 years or older (Fig. 3).
Discussion
Overall, 91% of children aged 3–7 years in our study successfully underwent a clinical MRI scan without general anesthesia following some sort of pre-MRI preparation. The frequency of success was high regardless of the type of training the child underwent before the scan. Performing fewer clinical MRI scans under sedation could lead to substantial cost- and time-saving in pediatric imaging centers and would help children avoid potential negative side effects of general anesthesia.
The frequency of success in our sample was similar to prior studies using mock MRI training for research scans that reported 88–96% success [8,9,10] and higher than prior studies of clinical scans which found only 15–34% of participants avoided sedation after a variety of different preparation methods [12, 13, 15]. The frequency of success may vary with training methods but also likely depends on the hospital environment. All training and scans performed in this study were done in a major children’s hospital with a child-friendly atmosphere (including artwork covering one of the MRI scanners and the ability to watch a video while in the MRI scanner) by trained child life specialists, and all scans were performed by well-trained pediatric MRI technologists who are familiar with attempting non-sedated MRI scans in young children. This may have helped increase the frequency of scan success relative to prior clinical studies and may therefore suggest that specialized training for staff would help reduce sedation and non-dedicated pediatric sites.
An important finding of this study is the lack of significant differences in the frequency of success between training groups (i.e., even most children who prepared exclusively at home were able to tolerate non-sedated MRI scans), which shows that preparation can be done without access to a mock MRI scanner. A prior randomized study found a lower frequency of success in children who prepared immediately before their scan with a photobook compared to children who underwent a mock MRI training session [14]. However, that study defined success and decided whether to use anesthesia based on the children’s behavior prior to the scan, whereas our study allowed all children to attempt a non-sedated scan and evaluated the resulting images to determine success. Success may also be more frequent in our study in part due to the wider range of at-home training materials given to parents (which included a video, children’s book, audio file of MRI noises, suggestions of games to play to practice lying still), as well as the fact that training for our study was done several days prior to the MRI scan potentially allowing more preparation time and familiarization. Providing home training materials is a low-resource intervention that helps address some of the barriers associated with mock MRI training, while still permitting children to have preparation in advance of an unsedated scan.
While all groups had similar frequencies of success, the PedsQL VAS scores suggest that training with a mock MRI scanner reduces a child’s worries and fears about the MRI scan. Children in the mock MRI group reported less fear than the At-Home group, and parents of children in the mock MRI group reported less upset/worry in their children compared to both the child life and At-Home groups. This suggests that the mock MRI scanner may reduce children’s anxiety prior to non-sedated MRI scans.
Child-life specialists and MR technologists were able to predict with great accuracy which children would be successful or not successful at their non-sedated MRI scan (> 66% specificity, > 95% sensitivity). This information could potentially be used after a pre-scan training session with a child-life specialist to help inform the decision of whether to attempt an awake MRI scan or to use general anesthesia. We observed a non-significant trend towards lower parent-reported function scores pre-scan in the group of children that were unsuccessful. Together with prior work [11], this may suggest that parents are good predictors of their child’s comfort and ability to tolerate an awake MRI scan. Future studies could consider evaluating the parental predictive ability.
Children who successfully completed an awake MRI scan were on average 1 year older than those who were unsuccessful. This is similar to other research suggesting that image quality during MRI scans generally increases with age [25,26,27,28]. Nonetheless, the frequency of success was still quite high (> 65%) for the 3-year-olds and did not improve substantially after 4 years. It would be useful to further study MRI training methods in the 3-year-old age group to see how training could be optimized for this age specifically. Some prior studies have shown higher motion and/or decreased scan quality in boys compared to girls [25, 28], but we did not see significant sex differences in this study. Prior studies have also noted associations between scan success and behavioral measures [11], which was not evaluated here, but suggests that a range of factors may help predict scan outcomes.
There are significant potential advantages to the child and family, as well as the healthcare system, when children can tolerate unsedated MRI scan. For the child and family, appointment times would be shorter, and the potential risks associated with general anesthesia would be avoided [4,5,6]. For the healthcare system, awake scans are substantially less expensive [7] and have shorter appointment times, reducing the demand on staff and resources. Particularly in healthcare settings where access to MRI scans is limited, these preparation methods may convey substantial advantages.
Study limitations
Children with significant developmental disabilities were not included in this study, so future studies of the efficacy of training in that population would be beneficial. Some children and parents specifically mentioned fear of the needles required for IV contrast material during the MRI scan. Many children report needle phobia [29], which may have influenced success with awake scanning and/or functioning scores in this study. However, the administration of general anesthesia typically also requires a needle poke, so would not avoid the needle anxiety. This should be considered in future studies and may be useful to incorporate in training methods as well. Data were not collected for time spent training at home; future studies attempting to better quantify this could make clearer recommendations about how to optimize at-home training. It is possible that there was a selection bias where families who thought their children could not complete an awake MRI scan were less likely to consent to participate. Furthermore, the high frequency of success among groups may have limited our abilities to detect group differences. We powered our study to detect differences of ~ 25%, as seen in prior studies. Future studies with larger groups may help further refine preparation methods to train children more efficiently.
Conclusion
Overall, 91% of children aged 3–7 years were able to undergo non-sedated MRI scans after preparation, with no significant differences in scan success based on the type of training they received. This suggests that preparation materials need not be resource intensive and can be administered by either child life specialists or by the child’s parent/caregiver at home. The use of a mock MRI scanner in training may reduce pre-scan anxiety. Institutions that conduct pediatric imaging should consider re-evaluating their policies and procedures for the routine use of sedation in young children requiring MRI scans and may want to adopt training protocols that permit children to attempt a non-sedated scan.
Data Availability
Data is available from the corresponding author upon reasonable request.
References
Marshall SP, Smith MS, Weinberger E (1995) Perceived anxiety of pediatric patients to magnetic resonance. Clin Pediatr (Phila) 34:59–60
Tyc V, Fairclough D, Fletcher B et al (1995) Children’s distress during magnetic resonance imaging procedures. J Child Heal Care 21:5–19
Schulte-Uentrop L, Goepfert M (2010) Anaesthesia or sedation for MRi in children. Curr Opin Anaesthesiol 23:513–517
Slovis T (2011) Sediation and anesthesia issues in pediatric imaging. Pediatr Radiol 41:514
Sanborn P, Michna E, Zurakowski D et al (2005) Adverse cardiovascular and respiratory events during sedation of pediatric patients for imaging examinations. Radiology 237:288–294
Rappaport BA, Suresh S, Hertz S et al (2015) Anesthetic neurotoxicity - clinical implications of animal models. N Engl J Med 372:796–797
Vanderby S, Babyn P, Carter M et al (2010) Effect of anesthesia and sedation on pediatric MR imaging patient flow. Radiology 256:229–237
Hallowell LM, Stewart SE, de Amorim e Silva CT, Ditchfield MR (2008) Reviewing the process of preparing children for MRI. Pediatr Radiol 38:271–279
Barnea-Goraly N, Weinzimer SA, Ruedy KJ et al (2015) High success rates of sedation-free brain MRI scanning in young children using simple subject preparation protocols with and without a commerical mock scanner - the Diabetes Research in Children Network (DirecNet) experience. Pediatr Radiol 44:181–186
de Bie H, Boersma M, Wattjes M et al (2010) Preparing children with a mock scanner training protocol results in high quality structural and functional MRI scans. Eur J Pediatr 169:1079–1085
Thieba C, Frayne A, Walton M et al (2018) Factors associated with successful MRI scanning in unsedated young children. Front Pediatr 6:146
Carter AJ, Greer M-LC, Gray SE, Ware RS (2010) Mock MRI: reducing the need for anaesthesia in children. Pediatr Radiol 40:1368–1374
Khan JJ, Donelly LF, Koch BL, Curtwright LA (2007) A program to decrease the need for pediatric sedation for CT and MRI. Appl Radiol 36:30–33
Rothman S, Gonen A, Vodonos A et al (2016) Does preparation of children before MRI reduce the need for anesthesia? Prospective randomized control trial. Pediatr Radiol 46:1599–1605
Durand D, Young M, Nagy P et al (2015) Mandatory Child Life consultation and its impact on pediatric MRI workflow in an academic medical center. J Am Coll Radiol 12:594–598
Morel B, Andersson F, Samalbide M et al (2020) Impact on child and parent anxiety level of a teddy bear-scale mock magnetic resonance scanner. Pediatr Radiol 50:116–120
McGlashan H, Dineen R, Szeszak S et al (2018) Evaluation of an internet-based animated prepratory video for children undergoing non-sedated MRI. Br J Radiol 91:20170719
Szeszak S, Man R, Love A et al (2016) Animated educational video to prepare children for MRi without sedation: evaluation of the appeal and value. Pediatr Radiol 46:1744–1750
Suzuki A, Yamaguchi R, Kim L et al (2023) Effectiveness of mock scanners and preparation programs for successful magnetic resonance imaging: a systematic review and meta-analysis. Pediatr Radiol 53:142–158
Frane J (1998) A method of biased coin randomization, its implementation, and its validation. Drug Inf J 32:423–432
Kang M, Ragan B, Park J (2008) Issues in outcomes research: an overview of randomization techniques for clinical trials. J Athl Train 43:215–221
Developmental Neuroimaging Lab (2022) Resources. https://www.developmentalneuroimaginglab.ca/participate/resources/. Accessed Dec 2022
Frayne A (2015) Pluto and the MRI rocket ship adventure. Lulu Press, Inc
Sherman SA, Eisen S, Burwinkle TM, Varni JM (2006) The PedsQL present functioning visual analogue scales: preliminary reliability and validity. Health Qual Life Outcomes 4:75
Roalf D, Quarmley M, Elliot M et al (2016) The impact of quality assurance assessment on diffusion tensor imaging outcomes in a large-scale population-based cohort. Neuroimage 125:903–919
Jaimes C, Robson C, Machado-Rivas F et al (2021) Success of nonsedated neuroradiologic MRI in children 1–7 years old. Am J Roentgenol 216:1370–1377
Rajagopal A, Byars A, Schapiro M et al (2014) Success rates for functional MR imaging in children. Am J Neuroradiol 35:2319–2325
Ware A, Shukla A, Guo S et al (2022) Participant factors that contribute to magnetic resonance imaging motion artifacts in children with mild traumatic brain injury or orthopedic injury. Brain Imaging Behav 16:991–1002
Taddio A, Ipp M, Thivakaran S et al (2012) Survey of the prevalence of immunization non-compliance due to needle fears in children and adults. Vaccine 30:4807–4812
Acknowledgements
This project was funded by the Alberta Children’s Hospital Foundation. Sarah Fletcher received a summer studentship from the O’Brien Health Science Summer Student Award fund as part of her work on this project; CL receives salary support from the Canada Research Chair program. We would like to thank the participants and their families for engaging in our study, as well as the numerous MRI technicians who supported and assisted with our study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Research Ethics Board at the University of Calgary.
Consent to participate
Written informed consent was obtained from the legal guardians of all participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fletcher, S., Lardner, D., Bagshawe, M. et al. Effectiveness of training before unsedated MRI scans in young children: a randomized control trial. Pediatr Radiol 53, 1476–1484 (2023). https://doi.org/10.1007/s00247-023-05647-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-023-05647-0