Abstract
Background
Focal nodular hyperplasia (FNH) in children is a rare but benign tumour, which must be differentiated from malignant entities to avoid unnecessary treatment, leading to potential morbidity.
Objectives
To provide data on imaging findings of these lesions with a suggested algorithm for diagnosis, sampling and follow-up.
Materials and methods
This retrospective review evaluated imaging of all patients diagnosed with FNH in two tertiary referral centres in Europe between 1975 and 2018.
Results
One hundred and four patients with 137 tumours were reviewed. The mean age at presentation was 8.2 years. The median tumour size was 5 cm (range: 0.3–29 cm). Multiple lesions were seen in 16.3% of patients. The male-to-female ratio was 1:2.
Conclusion
FNH with typical features on imaging can be safely followed up once the diagnosis has been established. The use of contrast-enhanced ultrasound and magnetic resonance imaging allows accurate characterisation in most cases. Histological sampling is only advised when there is diagnostic doubt. Atypical arterial enhancement of FNH should prompt the search for a congenital portosystemic shunt.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Focal nodular hyperplasia (FNH) is a rare hepatic tumour with a reported prevalence of 0.4–2% in the paediatric population [1, 2]. Although the exact underlying pathogenesis has not been fully elucidated, nodules of hyperplastic hepatocytes are thought to form in response to an imbalance in loco-regional arterial and portal blood flow [3]. Whether this imbalance is congenital or acquired is debated, as is the event that causes the initial microvascular insult [4]. This has been hypothesised to be from a number of potential causes, including vasculitis, thrombosis, portosystemic shunts, chemotherapy and radiotherapy [3, 5,6,7,8,9].
In children, as in adults, FNH discovery is often incidental during imaging examinations for other reasons, although a proportion of patients present with abdominal pain, a palpable abdominal mass or deranged liver function tests [10]. However, in contrast to adults, where they are almost universally considered “do not touch” lesions [11], FNH in children continue to be a source of anxiety for clinicians, patients and their parents, owing to the fact that historically it has been reported that two-thirds of liver tumours in children are malignant in origin [12]. This anxiety is compounded by the fact that FNH in children can often be atypical in radiologic appearance, larger on initial presentation, increase in size and occur in patients who are followed up long term for previous malignancy [8, 13].
Reassuringly, the malignant transformation of FNH has never been documented. Recurrence, intratumoral haemorrhage and rupture are recognised complications, but are rare and have not been reported in the paediatric population [14,15,16,17]. Therefore, establishing the diagnosis is paramount in allaying fears and modifying management.
However, even if a confident diagnosis of FNH can be made on imaging or histologically, there is little high-quality evidence to guide clinicians about definitive management, as robust randomised-controlled trials are difficult given the rarity of this tumour in children [18]. Surgical resection technically remains the gold standard treatment and manner in which to obtain a pathological specimen, but given the morbidity and mortality risk associated with such procedures, coupled with the fact that FNH is a benign indolent lesion, the risk-benefit of this approach has been called into question recently [13, 19, 20].
Today’s imagers have a large array of diagnostic modalities from which to choose, including ultrasound (US), magnetic resonance imaging (MRI) and computed tomography (CT). These can be further augmented with various intravenous contrast agents to give vital supplementary information. The role of contrast-enhanced studies is pivotal in the diagnosis of focal liver lesions, and typical tumour and scar enhancement patterns are well established in contrast-enhanced CT and contrast-enhanced MRI [2, 21,22,23]. Typical enhancement patterns are well-described in contrast-enhanced US [24, 25], but this is still not a routine examination at nonspecialist (and even many specialist) centres.
Five typical features of FNH have been described [26]: similar attenuation/echogenicity/signal to background liver; homogeneity; strong arterial phase enhancement with no washout, a central scar and the absence of a capsule. These findings alone are nonspecific, but if all are present provides up to 98% specificity [14]. Where these are not demonstrated, tissue biopsy (percutaneous or surgical) may be required.
Management of these lesions should be dealt with initially in specialist tertiary hepatobiliary centres, although most of these tumours may be referred in from smaller local centres. Once a diagnosis has been established, patients may be discharged to their local centre for monitoring, so the need for general radiologists and sonographers to recognise these lesions and patterns of evolution is important.
In this paper, we present the imaging findings of FNH lesions in paediatric patients from two European tertiary hepatobiliary referral centres, combining data spanning almost 50 years and based on our findings we suggest an approach to imaging these children. This paper will focus more heavily on radiologic aspects of FNH, but the interested reader can find the clinical and management aspects in a subcohort of these patients already published in the literature [27].
Materials and methods
A retrospective analysis was undertaken of the imaging in all patients < 18 years old referred to our centres’ paediatric radiology departments from 1975 to 2018 with a liver tumour suspicious for FNH. These were comprised of local and nationally and internationally referred patients on a prospectively held database and from searches on the local radiology information systems and Picture Archive and Communication Systems (PACS). T.A., a radiologist with 5 years’ experience, reviewed imaging from their own institute. G.C., a radiologist with 5 years’ experience, reviewed all imaging from both institutes. S.F.-A., a radiologist with 20 years’ experience, and H.W., a radiologist with 19 years’ experience, provided a final consensus opinion, where required, for their respective institutes.
Formal ethics committee approval was waived for this study in both centres, which was considered by the institutional review board to represent evaluation of a routine clinical service. Inclusion criteria were final histological diagnosis of FNH made by an expert pathologist in paediatric liver disease, or where histology was not available/required due to typical imaging features and no clinical or biological red flags, radiologic diagnostic features of FNH confirmed by a radiologist with expertise in paediatric liver disease.
All available cross-sectional modalities were included: MRI, CT and US with or without contrast enhancement. The type of contrast material used was dependent on the licensed use in the respective countries at the time of the image acquisition.
Contrast-enhanced US performed in our institutions used sulfur heaxafluoride suspension in one or more doses up to a maximum of 2.4 ml (SonoVue; Bracco UK Ltd., High Wycombe, UK). Contrast-enhanced MRI was performed in our institutions using various extracellular contrast agents. When hepatobiliary-specific agents were used, this was routinely gadoxetic acid (Primovist; Bayer Plc, Reading, UK) at 0.1ml/kg. Contrast-enhanced CT was performed with various water-soluble intravenous iodinated contrast agents.
Patients with follow-up studies using the same modality had only their baseline studies analysed. Where multiple modalities were used in baseline diagnosis, all modalities were analysed.
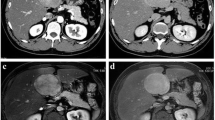
Tumour characteristics recorded included factors such as maximum diameter, number, border definition and presence/absence of a scar. Modality-specific features were recorded for each tumour, respectively: US – echogenicity and enhancement pattern when contrast-enhanced US was performed; CT – density of tumour and scar, and enhancement pattern of tumour/scar; MRI – intensity (T1-W/T2-W) of tumour/scar, diffusion-weighted imaging characteristics, enhancement pattern of tumour/scar and type of contrast used. Tumours were assessed for typical enhancement pattern, which on cross-sectional imaging was defined as arterial phase hyperenhancement above that of the background liver, enhancement equal or just greater than the background liver on portal venous phase, with no washout on delayed imaging (Fig. 1). In addition, retention of hepatobiliary specific contrast agents on delayed-phase MR imaging was documented where used (Fig. 2). On contrast-enhanced US, this was defined as seeing a central arterial feeding vessel, spoke-wheel pattern of enhancement followed by homogeneous centrifugal in-filling with no washout (Fig. 3).
Multiphase axial magnetic resonance imaging using gadoterate meglumine (Dotarem; Guerbet LLC, Villepinte, France) shows typical enhancement of a focal nodule hyperplasia lesion in a 13-year-old girl presenting with nonspecific abdominal pain. a Pre-contrast T1-W axial image shows a large isointense lesion within the right lobe of liver, demonstrating a hypointense central scar (arrow). b Intense enhancement of the tumour is seen in the arterial phase. The scar remains hypointense. c In the portal venous phase, the lesion becomes isointense to the background liver. d In the delayed phase, there is no lesion washout and the scar is hyperintense
Multiphase axial magnetic resonance imaging using gadoxetic acid (Primovist; Bayer Plc, Reading, UK) shows typical enhancement of a focal nodular hyperplasia lesion in a 6-year-old girl presenting with abdominal pain and constipation. a Pre-contrast T1-W axial image shows a large lobulated isointense lesion within the left lobe of liver, demonstrating a hypointense central scar (arrow). b Intense enhancement of the tumour is seen in the arterial phase. The scar remains hypointense. c In the portal venous phase, the lesion is slightly hyperintense to the background liver. d In the hepatobiliary phase, there is retention of Primovist in the tumour and normal liver, indicating a hepatocellular tumour. The scar remains hypointense
Select images from contrast-enhanced ultrasound in transverse section show typical enhancement of an incidental 3-cm focal nodule hyperplasia in a 5-year-old boy. a Radial arterial hyperenhancement with spoke-wheel appearance seen at 8 s. b Rapid in-filling in the arterial phase at 10 s. c and d The lesion becomes isoechoic to background liver (20–28 s) with no washout seen later in the study
Categorical variables are reported as absolute and relative frequencies (%). Continuous variables are reported as mean (95% confidence intervals [CI]) or median (range). Where data were unavailable from the retrospective data collection measure, these points were omitted in the final data analysis.
Results
Overview
One hundred and four patients (median age: 8 years, range: 0.5–15 years) with imaging were included for analysis, yielding 137 tumours. Thirty-five patients were male (33.7%) with a male-to-female ratio of 1:2 (Table 1). The mean tumour diameter was 5.6 cm (95% CI 4.9 to 6.3) and the median diameter was 5.0 cm (range: 0.3 to 29.0 cm). Lesions were solitary in 87 patients (83.7%) and 17 patients (16.3%) had multiple lesions accounting for 47/137 lesions (34.3%). Patients with multiple lesions had a median of 2 tumours (range: 2–6 tumours).
Thirty-three patients (31.7%) had significant comorbidities: congenital portosystemic shunt = 13, portal vein cavernoma = 4, previous malignancy treated with chemotherapy = 6, sickle cell disease = 4. Other isolated comorbidities were biliary atresia, mesenchymal hamartoma, type 1 diabetes, Hashimoto thyroiditis, polycystic ovary disease, complex cardiac malformations, von Willebrand disease, membranoproliferative glomerulonephritis, oesophageal atresia and gallstone pancreatitis. One hundred and eighty-three examinations were performed and analysed: 89 US, 43 CT and 51 MRI. Twenty-one patients (20.2%) underwent US only, 6 patients (5.8%) underwent CT only and 7 patients (6.7%) underwent MR only. A combination of US and CT was used in 26 patients (25.0%), US and MR in 33 patients (31.7%) and MR and CT in 2 patients (1.9%). Nine patients (8.7%) underwent US, CT and MR.
Ultrasound
Eighty-nine patients (85.6%) had US and of these 10/89 (11.2%) received intravenous contrast material (microbubbles). In total, 106/137 total lesions (77.4%) were observed by US. The US imaging findings are summarised in Table 2.
Of 16 lesions with both US (all non-contrast enhanced) and CT examinations available, the visible scar on US was also visible on CT in 6. CT depicted the central scar in the remaining 10 lesions, which were not appreciated on US.
Of 29 lesions with both US (5/29 [17.2%] received US contrast) and MRI, an US visible scar was also seen on MR in 4. Only one lesion showed a scar on contrast-enhanced US, the remainder were seen on B-mode US. A scar was seen on MR in 25 lesions on pre-contrast T1 or T2, which were not visible on US.
Magnetic resonance imaging
Fifty-one patients (49.0%) underwent MR, providing imaging data on 78/137 of the lesions (56.9%) in the data set. Seventy-two of these lesions (92.3%) had pre- and post-contrast imaging, of which 24/72 (33.3%) received hepatobiliary-specific contrast agent. MR imaging features are summarised in Table 3.
Of 11 lesions with both MR and CT, the scar was seen on both modalities in 5/11 (45.5%). A scar was seen on CT but not MR in 1/11 lesions (9.1%) and a visible scar was seen on MR but not on CT in 2/11 lesions (18.2%).
Contrast agents
Of the 45 patients (with 72 lesions) who received intravenous contrast, 14 patients (31.1%) with 25 lesions received Primovist. The remainder (31 patients with 47 tumours) received varying extracellular contrast agents.
With Primovist, atypical tumour enhancement was seen in 2/25 lesions (8%); both in the same patient who was found to have a congenital portosystemic shunt. With extracellular contrast agents, atypical tumour enhancement was seen in 16/47 lesions (34%) in 7 patients. The reason for atypical enhancement in one of these studies (1 lesion) was due to a poor-quality study, the remaining 6 studies (15 lesions) demonstrated poor arterial enhancement of the tumour.
Computed tomography
Forty-three patients (41.3%) underwent CT providing information on 50/137 lesions (36.5%). Forty-seven of these lesions (94.0%) had post-contrast imaging available. CT imaging findings are summarised in Table 4. No patients had dedicated delayed-phase imaging. Typical lesion enhancement was seen in 32/46 lesions (69.6%). In all lesions with atypical enhancement, this was due to poor arterial enhancement.
Discussion
Although paediatric focal liver lesions are rare, the relatively high concern for excluding malignant hepatic masses in this age group makes accurate and timely diagnosis important for prognosis, management and allaying of clinician and patient/family anxiety. FNH has been reported to have typical imaging appearances, which if all are present in a normal liver are highly specific [14, 26]. However, when these features are absent, or appearances are atypical, further investigation is required. Further complicating the diagnosis is the propensity of these lesions to grow (sometimes rapidly) over time whether due to their natural course, via hormonal stimulation or just in proportion to the growing liver. In the face of a rapidly enlarging tumour, routine follow-up is required to reassure, but also to exclude impending compression of important vascular and parenchymal structures within the liver and surrounding organs.
Demographic data
The data show that the clear female predominance in adults is not as large in children (1:2 in children in our study compared to 1:8 − 1:12 in adults) [10]. Typically, the main malignant differential for FNH is fibrolamellar hepatocellular carcinoma (HCC), which shows a slight male preponderance in children [28]. Therefore, it is important to bear in mind that being female is a much weaker predictor of FNH in children than in adults, although both these pathologies remain rare in this population overall.
Our series confirms FNH are often solitary, but can be multiple in 16% of patients, and have a mean diameter of 5.6 cm at diagnosis but can grow to extremely large sizes (up to 29 cm in our cohort). Large lesions are also seen in similar frequency in adults [29]. However, in children, the tumour:liver or tumour:abdomen ratios are much larger, leading to concerns of vascular, digestive and solid organ compromise. Paediatric FNH are almost always well-defined, homogeneous lesions that are iso-/slightly hyperechoic to the background liver on US, iso/hyperintense on T2-W MR and iso/hypodense on pre-contrast CT.
On post-contrast MR and CT, there is homogeneous intense arterial enhancement with lesions becoming iso/slightly hyperintense/dense in the portal venous phase compared to background liver. The features of contrast-enhanced US enhancement are discussed below. No washout is observed. When these typical features are all present, diagnosis is almost certain, but diagnostic difficulty arises when the above features are not all present. In our series, typical FNH appearances were only seen in 35% of cases. This means that in the remaining 65%, there is diagnostic uncertainty between FNH and other entities, requiring further imaging tests or histological sampling.
Presence of a central scar
The most common absent feature was the central scar. A scar is only appreciable in approximately 10% of lesions on US, therefore this is not a reliable sign. However, approximately 50% of lesions on CT or MR demonstrated a scar. Absence of a scar should be considered atypical, especially in larger lesions with our series demonstrating a scar in 90.5% of tumours on CT and 77.8% on MR in lesions > 3 cm.
Scar characteristics are important when trying to differentiate between FNH and fibrolamellar HCC and FNH classically demonstrates a high T2 signal scar, whereas fibrolamellar HCC will demonstrate a low T2 signal stellate scar due to its fibrotic nature [30, 31]. All FNH in this cohort demonstrated T2 hyperintensity of the scar when present.
Delayed-phase enhancement of the central scar on CT and MR is typical in FNH and less common in fibrolamellar HCC when using extracellular contrast agents. However, some reports of delayed scar enhancement in fibrolamellar HCC exist [30]. A pitfall to avoid with Primovist is that the central stellate scar does not enhance due to the absence of functioning hepatocytes expressing the OATP8 receptor. Therefore, non-enhancement in this setting is not indicative of fibrolamellar HCC. Finally, in practice these two entities can often be distinguished by ancillary factors such as local lymphadenopathy, satellite nodules and constitutional symptoms in fibrolamellar HCC (the latter can sometimes be seen in very large FNH).
Enhancement pattern
Contrast-enhanced US appears to be reliable in demonstrating typical FNH enhancement pattern (9/10 patients in this series), whereas MR and CT show this in just over two-thirds of patients [22, 24, 25]. In lesions where this was not the case, the most common reason was poor arterial enhancement.
FNH tend to have a large arterial feeding vessel from the main hepatic artery. Poor arterial enhancement in a hepatic lesion can be due to a number of causes, such as non-arterialised lesion (i.e. metastasis), compromised blood supply (i.e. twisted pedunculated lesion) or decreased relative enhancement compared to the background liver. This last point is important as in patients with a congenital portosystemic shunt, the reduced portal flow in the liver leads to increased arterialisation of the background parenchyma. This, in turn, makes it difficult to differentiate an arterialised focal lesion within a background liver that itself is also arterialised. Hence, if poor arterial enhancement of a tumour is described, a concerted effort to find and exclude a congenital portosystemic shunt is strongly recommended.
Contrast-enhanced US benefits from an extremely high temporal resolution in the arterial phase compared to cross-sectional imaging, which can be advantageous compared to CT and MR. This allows exquisite detail of the classical central spoke-wheel enhancement pattern, representing the central arterial feeder radiating out through the septa to perfuse the lesion, which rapidly fills centrifugally. This is then followed by sustained enhancement in the portal venous phase with the lesion becoming isoechoic to background liver, demonstrating no washout. This arterial filling pattern is highly specific for FNH and the lack of washout is very reassuring. Larger lesions can have multiple feeding vessels and may lack the typical enhancement pattern described above. It should be noted that US microbubble contrast media is being used “off-label” in our institutes and others. Because of this, there are few large studies to establish the sensitivity and specificity of contrast-enhanced US for characterising FNH. However, large adult studies and smaller paediatric series are encouraging [32, 33].
MR has dose-saving advantages compared to CT, but has longer examination times, possibly requiring sedation or general anaesthetic and considerations about gadolinium deposition. However, to diagnose FNH, its use is extremely helpful. MR can provide more detailed tumour and scar characterisation, demonstrate other smaller lesions, as well as allow repeated delayed-phase imaging (for quality or reassurance) in a single examination. One disadvantage is its lower sensitivity in depicting tumoral calcification, which is associated with fibrolamellar HCC.
The utility of hepatobiliary specific contrast agents is a further advantage of MR. Hepatobiliary-specific contrast agent (e.g., Primovist) imaging is useful in determining the presence of hepatocytes (expressing the OATP8 receptor) in lesions and is used to distinguish with accuracy between FNH and adenoma [34, 35]. Although there is some overlap, generally, hepatocellular adenomas (and other non-hepatocyte-containing lesions) will not retain hepatobiliary-specific contrast agents to the same degree as FNH. If contrast retention is seen in the hepatobiliary phase, then this is a reassuring indicator of the lesion being FNH.
In our daily practice, we acquire delayed-phase imaging at 5, 10 and 20 min when using Primovist.
It is important to remember that not all hepatobiliary-specific contrast agents are licenced in every country. Indeed, in this study, all Primovist studies were carried out in one institution where it is used “off-label” but in line with local guidance. In the other, only Multihance (gadobenate dimeglumine Bracco Diagnostics Inc., Monroe Township, NJ) is available, which is not always conducive to paediatric imaging for several logistical reasons. The main issue is that the optimum time for delayed-phase acquisition with this contrast medium is 90–120 min following administration. For patients under general anaesthetic, this is impractical and for non-anaesthetised patients, it may be less efficient to have patients returning for MRI later in the day, especially in the paediatric patient population. Therefore, extracellular contrast agents are used as routine. This may lead to higher biopsy rates, especially in equivocal cases.
When there is a reasonable suspicion of FNH on US, contrast-enhanced US (if solitary) or MRI with hepatobiliary-specific contrast agents can be obtained immediately. If there is doubt, or other differentials including malignancy are present, then MRI with extracellular contrast agents should be the next test. The role for CT is ever diminishing and is limited to patients who cannot tolerate MR imaging or have specific need for vascular/surgical planning. The routine use of CT in our series has gradually decreased over the study period, and the most recent cases represent incidental findings on CT scans performed for other indications.
Lesions that are either nonspecific or have concerning features such as being ill-defined, invasive and heterogeneous and contain fat, haemorrhage or calcification are not typical for FNH and should be further imaged/biopsied. Furthermore, lesions that show the typical features described above but in the presence of a raised alpha-fetoprotein (AFP), deranged liver function tests, lymphadenopathy, contrast washout or satellite lesions should also be further investigated to exclude fibrolamellar HCC.
Lesion growth
In adults, FNH are stable long term and will usually decrease in size later in life (i.e. after menopause) [36], although some appear to grow under the hormonal influence of pregnancy [37]. In a subgroup of 50 patients of whom we published surgical outcomes [27], we documented that approximately 35% of patients had a 50% increase in the diameter of their lesion over a median follow-up of 4.7 years. This increased to 67.5% for 20% increase in diameter. These patients did not show any increased risk of symptoms or complications and therefore we do not recommend extra surveillance if clinical and biochemical parameters are reassuring. However, it is understandable that closer surveillance of these lesions may reassure clinicians and patients.
Central image review
A central review of all focal liver lesions within a specialist paediatric hepatobiliary centre should be performed to determine the likely pathology. If imaging is from an outside institution, the decision of the specialist centre should be based on the confidence of diagnosis from supplied imaging. Where the diagnosis is uncertain, repeat baseline imaging should be performed (usually at, but not limited to, the specialist centre). This will usually take the form of standard US. If the lesion is solitary, easily visualised and the patient is otherwise asymptomatic, then contrast-enhanced US should be attempted.
If this option is not viable or there is persisting doubt, then MR with contrast (hepatobiliary-specific contrast agents if available) is advised. If the diagnosis still cannot be established, tissue biopsy is advised. If percutaneous tissue biopsy is not feasible due to lesion size or location, and there are no red flag features (malignant imaging features, rapid growth, raised AFP, lymphadenopathy, concern for relapsed previous malignancy), then consider imaging follow-up at intervals using the most appropriate baseline modality (US or MR). Otherwise, a more invasive approach may be required, i.e. open biopsy.
An important point to note is that our data show that in 11 patients who underwent both CT and MR, similar morphological characteristics and enhancement patterns of the lesions (arterial, portal venous) were seen. None of the patients received hepatobiliary-specific contrast agents for their MR. Therefore, if a patient has had good quality CT imaging and there is little diagnostic uncertainty, there is likely little value in simply confirming with MR. If a CT has been performed and diagnostic uncertainty persists, an MR with hepatobiliary-specific contrast agent (where available) is recommended, as this will add valuable information about the hepatocellular nature of the lesion. We do not advocate the routine use of CT for the characterisation of FNH due to the associated dose factors and the lack of information provided in the delayed phases compared to MR.
Imaging strategy
Given the experience and imaging findings gathered in this cohort of patients, we propose the following imaging strategy (Fig. 4) for initial diagnosis, confirmation and follow-up:
-
1)
Central review of outside imaging from referring centres.
-
2)
If a good quality contrast-enhanced US, CT or MRI has been performed in an outside institute, and the lesion is incidental, asymptomatic and not associated with any concerning clinical or radiologic features, a confident diagnosis can be made and routine follow-up can occur. You may wish to consider performing an US even if the diagnosis is certain to provide a baseline for future follow-up. This is helpful in incidental lesions found on cross-sectional imaging.
-
3)
Otherwise, a baseline US should be performed at the specialist centre and consideration given to contemporaneous contrast-enhanced US (if solitary and accessible).
-
4)
If the lesion(s) is not well appreciated on baseline US or is atypical/multiple, then MRI is advised. If hepatobiliary-specific contrast agents are available, their use is recommended.
-
5)
If after MR the diagnosis is still in doubt, tissue biopsy should be considered. If this is not feasible, then imaging follow-up with the best baseline modality is advised (US or MRI). Follow-up contrast-enhanced US is not routinely recommended.
-
6)
The frequency of clinical and imaging follow-up will vary depending on the level of clinical suspicion, diagnostic doubt, the age of the patient, the clinical context and the size of the lesion. We would advocate an initial clinico-radiologic follow-up at 3 and 12 months, then at 12- to 24-month intervals if stable. As a minimum, the imaging at 3 and 12 months should take place at the specialist centre. Greater frequency of scanning can be used if there is diagnostic uncertainty.
-
7)
The radiologic follow-up after the initial year can take place in the patient’s local institute if this is feasible. This is to reduce patient travel time and decrease imaging waiting times at specialist centres. The follow-up imaging should still be reviewed centrally and we advocate follow-up until the patient is 16 years old, at which point the child transitions to adult services.
Proposed algorithm for diagnosis and follow-up of focal nodule hyperplasia in children. Solid line = suggested pathway, dashed line = consider pathway if appropriate, or suggested pathway not practicable. † Ultrasound (US) (non-contrast) advised if feasible, otherwise, magnetic resonance imaging (MRI). *Can be undertaken in patient’s local centre with central review of images. CEUS contrast-enhanced ultrasound, HBSCA hepatobiliary specific contrast agent
Limitations
Our data are limited but reflect evolving real-world practice over this time period, with a trend of moving to non-ionising radiation modalities and the incremental improvement of US and MR image quality. For many lesions, especially in recent years, there is no histological correlate as practice has changed to a more conservative approach. Therefore, the smaller lesions are presumptive diagnoses in the clinical context of the patient and imaging findings. In the absence of histological confirmation, we cannot be certain that some lesions are not in fact other entities such as mono- or mulitacinar regenerative nodules or adenomas, for example.
Furthermore, applicability of the imaging algorithm is not universal depending on which modalities, expertise and access are available at a single or network of institutions, instead reflecting a best-practice approach. The algorithm is not specifically designed for oncology patients who may have multiple FNH-like lesions, as their follow-up is tailored by oncology input, and lesions tend to be smaller, multiple and therefore lack central scars. We have not performed a cost-analysis of this approach to imaging FNH in children.
Take-home points
-
1.
Only 35% of lesions show all the “typical” features of focal nodule hyperplasia.
-
2.
Atypical features include lack of a central scar, poor arterial enhancement, washout.
-
3.
Contrast-enhanced US and/or MRI with hepatobiliary agents should be first-line modalities for diagnosis.
-
4.
Poor arterial enhancement should prompt a search for a congenital portosystemic shunt.
-
5.
Specialist centre expertise should be sought at diagnosis and for guiding management and follow-up due to the rarity of these lesions.
Conclusion
FNH in children generate apprehension due to diagnostic uncertainty, atypical imaging appearances compared to the classically described adult lesions and propensity to grow. Predisposing factors for FNH development should be sought in every case. Specifically, in tumours with poor arterial enhancement, a concerted effort to find and exclude a congenital portosystemic shunt is strongly recommended. Given the higher anxiety surrounding focal liver lesions in young children, a robust imaging strategy is required to give confidence to patients, families and clinicians alike when following up this entity. This can be achieved broadly by being aware of the differences between adult and paediatric FNH imaging appearances, biological behaviour and by using a stepwise multi-modality approach, which allows for personalisation of care for individual patients.
References
Reymond D, Plasckes J, Luthy A et al (1995) Focal nodular hyperplasia of the liver in children: review of follow-up and outcome. J Ped Surg 30:1590–1593
Chiorean L, Cui XW, Tannapfel A et al (2015) Benign liver tumors in pediatric patients - Review with emphasis on imaging features. World J Gastroenterol 21:8541–8561
Wanless I, Mawdsley C, Adams R (1985) On the pathogenesis of focal nodular hyperplasia of the liver. Hepatology 5:1194–1200
Kondo F (2001) Benign nodular hepatocellular lesions caused by abnormal hepatic circulation: etiological analysis and introduction of a new concept. J Gastroenterol Hepatol 16:1319–1328
Kumagi H, Masuda T, Oikawa H et al (2000) Focal nodular hyperplasia of the liver: Direct evidence of circulatory disturbances. J Gatroenterol Hepatol 15:1344–1347
Wanless I, Albrecht S, Bilbao J et al (1989) Multiple focal nodular hyperplasia of the liver with vascular malformations of various organs and neoplasia of the brain: a new syndrome. Mod Pathol 2:456–462
Marabelle A, Campagne D, Déchelotte P et al (2008) Focal nodular hyperplasia of the liver in patients previously treated for pediatric neoplastic diseases. J Pediatr Hematol Oncol 30:546–549
Benz-Bohm G, Hero B, Gossmann A et al (2010) Focal nodular hyperplasia of the liver in longterm survivors of neuroblastoma: how much diagnostic imaging is necessary? Eur J Radiol 74:e1–e5
Franchi-Abella S, Branchereau S (2013) Benign hepatocellular tumors in children: Focal nodular hyperplasia and hepatocellular adenoma. Int J Hepatol 2013:1–11
Nahm CB, Ng K, Lockie P et al (2011) Focal nodular hyperplasia — a review of myths and truths. J Gastrointest Surg 15:2275–2283
Alberti N, Frulio N, Bioulac-Sage P et al (2014) Interest of contrast-enhanced sonography to identify focal nodular hyperplasia with sinusoidal dilatation. Diagn Interv Imaging 95:77–83
Weinberg AG, Finegold MJ (1983) Primary hepatic tumors of childhood. Hum Pathol 14:512–537
Lautz T, Tantemsapya N, Dzakovic A, Superina R (2010) Focal nodular hyperplasia in children: clinical features and current management practice. J Ped Surg 45:1797–1803
Cherqui D, Rahmouni A, Charlotte F et al (1995) Management of focal nodular hyperplasia and hepatocellular adenoma in young women: a series of 41 patients with clinical, radiological, and pathological correlations. Hepatology 22:1674–1681
D’Halluin V, Vilgrain V, Pelletier G et al (2001) Evolution naturelle de l'hyperplasie nodulaire focale [Natural history of focal nodular hyperplasia. A retrospective study of 44 cases]. Gastroenterol Clin Biol 25:1008–1010
Rahili A, Cai J, Trastour C et al (2005) Spontaneous rupture and hemorrhage of hepatic focal nodular hyperplasia in lobus caudatus. J Hepatobiliary Pancreat Surg 12:138–142
Koch N, Gintzburger D, Seelentag W et al (2006) Rupture of hepatic focal nodular hyperplasia. About two cases. Ann Chir 131:279–282
Ortega G, Price M, Choo S et al (2013) Multidisciplinary management of focal nodular hyperplasia in children: experience with 10 cases. JAMA Surg 148:1068–1070
Navarro AP, Gomez D, Lamb CM et al (2014) Focal nodular hyperplasia: a review of current indications for and outcomes of hepatic resection. HPB 16:503–511
Bröker MEE, Klompenhouwer AJ, Gaspersz MP et al (2018) Growth of focal nodular hyperplasia is not a reason for surgical intervention, but patients should be referred to a tertiary referral centre. World J Surg 42:1506–1513
Schooler GR, Hull NC, Lee EY (2020) Hepatobiliary MRI contrast agents: Pattern recognition approach to pediatric focal hepatic lesions. AJR Am J Roentgenol 214:976–986
Broker M, Taimr P, de Vries M et al (2020) Performance of contrast-enhanced sonography versus MRI with a liver-specific contrast agent for diagnosis of hepatocellular adenoma and focal nodular hyperplasia. AJR Am J Roentgenol 214:81–89
Grieser C, Steffen IG, Seehofer D et al (2013) Histopathologically confirmed focal nodular hyperplasia of the liver: gadoxetic acid-enhanced MRI characteristics. Magn Reson Imaging 31:755–760.
Fang C, Bernardo S, Sellars ME et al (2019) Contrast-enhanced ultrasound in the diagnosis of pediatric focal nodular hyperplasia and hepatic adenoma: interobserver reliability. Pediatr Radiol 49:82–90
Wang DC, Jang H, Kim TK (2020) Characterization of indeterminate liver lesions on CT and MRI with contrast-enhanced ultrasound: what is the evidence? AJR Am J Roentgenol 214:1–10
Burgio MD, Ronot M, Salvaggio G et al (2016) Imaging of hepatic focal nodular hyperplasia: pictorial review and diagnostic strategy. Semin Ultrasound CT MRI 37:511–524
Zarfati A, Chambers G, Pio L et al (2020) Management of focal nodular hyperplasia of the liver: experience of 50 pediatric patients in a tertiary center. J Pediatr Surg 55:1885–1891
Weeda VB, Murawski M, McCabe AJ et al (2013) Fibrolamellar variant of hepatocellular carcinoma does not have a better survival than conventional hepatocellular carcinoma – Results and treatment recommendations from the Childhood Liver Tumour Strategy Group (SIOPEL) experience. Eur J Cancer 49:2698–2704
Nguyen B, Flejou J, Terris B et al (1999) Focal nodular hyperplasia of the liver: a comprehensive pathologic study of 305 lesions and recognition of new histologic forms. Am J Surg Pathol 23:1141–1154
Ichikawa T, Federle MP, Grazioli L et al (1999) Fibrolamellar hepatocellular carcinoma: Imaging and pathologic findings in 31 recent cases. Radiology 213:352–361
Shelmerdine SC, Roebuck DJ, Towbin AJ, McHugh K (2016) MRI of paediatric liver tumours: How we review and report. Cancer Imaging 16:1–10
Strobel D, Seitz K, Blank W et al (2008) Contrast-enhanced ultrasound for the characterization of focal liver lesions – diagnostic accuracy in clinical practice (DEGUM multicenter trial). Ultraschall Med 29:499–505
Laugesen NG, Nolsoe CP, Rosenberg J (2017) Clinical applications of contrast-enhanced ultrasound in the pediatric work-up of focal liver lesions and blunt abdominal trauma: a systematic review. Ultrasound Int Open 3:2–7
Grazioli L, Bondioni MP, Haradome H et al (2012) Hepatocellular adenoma and focal nodular hyperplasia: value of gadoxetic acid – enhanced MR imaging in differential diagnosis. Radiology 262:520–529
McInnes MD, Hibbert RM, Inacio JR, Schnieda N (2015) Focal nodular hyperplasia and hepatocellular adenoma: accuracy of gadoxetic acid – enhanced MR imaging — a systematic review. Radiology 277:413–423
Ramírez-Fuentes C, Martí-Bonmatí L, Torregrosa A et al (2013) Variations in the size of focal nodular hyperplasia on magnetic resonance imaging. Radiologia (English Edition) 55:499–504
Kim MJ, Han SY, Baek YH et al (2014) A case of focal nodular hyperplasia with growth progression during pregnancy. Clin Mol Hepatol 20:392–397
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chambers, G., Zarfati, A., Aderotimi, T. et al. Imaging strategy for focal nodular hyperplasia in children: long-term experience from two specialist European centres. Pediatr Radiol 53, 46–56 (2023). https://doi.org/10.1007/s00247-022-05420-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-022-05420-9