Abstract
The ability to provide prompt, real-time, easily accessible and radiation-free diagnostic assessments makes ultrasound (US) one of the most versatile imaging modalities. The introduction and development of stable microbubble-based ultrasound contrast agents (UCAs) in the early 1990s improved visualization of complex vascular structures, overcoming some of the limitations of B-mode and Doppler imaging. UCAs have been used extensively in the adult population to visualize vasculature and to evaluate perfusion and blood flow dynamics in organs and lesions. Since the first observations that air bubbles within a liquid can generate a strong echogenic effect, to the early makeshift approaches with agitated saline, and later to the development of industrially produced and federally approved UCAs, these agents have evolved to become both clinically and commercially viable. Perhaps the most exciting potential of UCAs is being uncovered by current research that explores the use of these agents for molecular imaging and therapeutic applications. As contrast-enhanced ultrasound (CEUS) becomes more widely available, it is important for pediatric radiologists to understand the physics of the interaction between the US signal and the microbubbles in order to properly utilize them for the highest level of diagnostic imaging and interventions. In this article we introduce the composition of UCAs and the physics of their behavior in US, and we offer a brief history of their development over the last decades.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of contrast agents in clinical radiology is well-known. Imaging modalities like radiography, computed tomography (CT), magnetic resonance imaging (MRI) and nuclear medicine examinations routinely rely on contrast agents to enhance the contrast of tissues or fluid compartments in the human body and to promote preferential uptake between normal and diseased tissues.
Image display on B-mode and Doppler ultrasound (US) is based on the interaction of sound energy and tissues or interfaces with different acoustic properties. The amplitude of reflected energy is used to generate B-mode US images, and the frequency shifts in the backscattered US provide information related to moving targets such as blood flow in both large and small vessels [1, 2]. However, Doppler US methods require a certain amount of moving particles (e.g., red blood cells) and a certain flow velocity to produce the necessary frequency shift of the transmitted sound pulse. This might impair visualization of blood flow in cases of low blood volume or very slow blood flow that cannot be depicted with Doppler techniques.
For many years, no substances were available that could be administered alongside US to improve imaging of blood flow. The initial lack of push for a US contrast agent (UCA) might be attributable to the inherent high contrast between blood and adjacent tissues on US images and the noninvasive evaluation of vasculature on color Doppler US. The concept of using contrast agents in combination with US to improve visualization of blood flow emerged in 1968 in the field of clinical echocardiography. This was because of the chance observation that the intracardiac administration of agitated saline produced strong echoes that were detectable by US [3]. These initial efforts to explore the use of contrast agents for US were largely based on various available contrast materials or other substances that were hand-agitated so that air could form bubbles within liquids. However, these big air bubbles, called macrobubbles, were unstable and dissolved rapidly [4, 5].
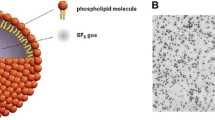
The real breakthrough occurred in the early 1990s with the industrial development of UCAs that were composed of stabilized air-filled microbubbles; in the early 2000s these were replaced by stabilized gas-filled microbubbles, which are currently used [6,7,8,9,10,11,12]. As the name suggests, the size of the microbubbles is in the range of microns, varying between 1μm and 10 μm in diameter. This size, similar to that of red blood cells, allows the microbubbles to freely circulate through the vascular system while remaining confined by the vascular endothelium. Apart from their small size, the key properties of microbubbles are the flexible composition and the large difference in acoustic impedance at the interface between their gas core and the surrounding tissues. Each microbubble is composed of a minimally diffusible gas, encapsulated within a shell that prevents diffusion of the gas. The flexibility of their shell enables them to resist the acoustic pressure of the incident US wave and the physiological pressures developed within the bloodstream. The acoustic impedance mismatch at the interface between the gas core and the surrounding tissues results in the reflection of almost all of the transmitted acoustic energy and the production of a strong backscatter signal (up to 30 dB) that distinguishes the microbubbles from the surrounding tissues [8, 9, 13, 14].
The continued evolution of UCA technology has offered new insight into the composition and kinetics of UCA microbubbles and their detection within tissues, informing routine clinical decision-making and advanced applications. In addition to augmenting a growing number of diagnostic imaging applications, microbubbles are being increasingly explored for use in the exciting new field of molecular imaging and therapeutic applications. This can be achieved by modification of the physical composition either of the shell or of the gas core of the microbubbles, enabling them to attach to cell types of interest (e.g., endothelial or cancerous cells) or certain disease processes (e.g., inflammation) [15, 16].
It is crucial for those performing pediatric US to understand the basic physical principles of UCAs and the fundamentals of their interaction with the US field in order to maximize the diagnostic capabilities of contrast-enhanced US (CEUS). The aim of this article is to support that understanding by presenting the evolution of UCA microbubbles over the last three decades. From the first makeshift approaches to the development of the currently used UCAs, we discuss the landmark developments on the path toward effective clinical use of UCAs. We describe the basic physico-chemical properties of UCAs and the fundamentals of their interaction with the US wave. Finally, we introduce the future trends in the development of microbubbles. It is important to note that UCAs were originally produced only for intravenous applications and, thus, the pre-clinical evaluations primarily targeted this route of administration. Other routes like the intravesical one were established much later, after UCAs were already in clinical use.
Properties of an optimal ultrasound contrast agent
The ideal characteristics of an optimal microbubble-based UCA that have propelled the development and the ongoing evolution of contrast agents can be summarized in the following points [17]:
-
(1)
Size: To enhance visualization of blood flow the microbubbles should behave as intravascular agents. That means that they should be small enough that they can freely circulate through the systemic and pulmonary vasculature, and large enough that they do not cross the vascular endothelium. To achieve this, the microbubbles should be of a similar size as the red blood cells, which are confined within the vascular compartment unless there is active bleeding.
-
(2)
Stability: The microbubbles must be stable enough to survive multiple undisturbed passages through the pulmonary and systemic vasculature, and to resist acoustic pressure changes when insonated.
-
(3)
Duration: The microbubbles should persist long enough to allow sufficient time for diagnostic imaging.
-
(4)
Toxicity: All materials comprising the microbubbles (gas and shell components) should be biologically inert and have low or ideally no toxicity.
-
(5)
Handling: The microbubbles must be easy to store and ship and easy to introduce into the vascular system.
First attempts to use contrast agents with ultrasound
As mentioned, the idea of using contrast agents in combination with US technology to improve visualization of blood flow first occurred in the field of clinical echocardiography. In 1968, following the observation of Dr. Claude Joyner that the intracardiac injection of normal saline resulted in striking enhancement of the echo signals (unpublished), Gramiak and Shah [3] published the first milestone study on contrast echocardiography for evaluation of the aortic root. In this study, Motion-mode (M-mode) echocardiogram was used to study the echo signals produced at the aortic root by the motion of the valve cusps during systole, as an indicator of the severity of aortic stenosis. To validate the M-mode findings, 10–20 mL of normal saline was injected through a catheter located within the aorta in a supravalvular position. A “cloud of echoes” within the aortic lumen was sonographically detected by M-mode. These echoes were thought to arise from the free air bubbles that were produced by the rapid injection of normal saline and were subsequently washed away during the ventricular systole by the movement of the non-opacified blood that was contained within the left ventricle [3].
Following this study, Gramiak et al. [5] used intracardiac injections of different contrast materials, varying from physiological substances such as normal saline and the patient’s own blood, to previously marketed dyes that were already approved for intra-arterial injection such as indocyanine green solution and 5% dextrose in water. These substances were injected at rapid rates through a catheter placed in the right heart, transseptally in the left heart, or retrograde in the ascending aorta. The authors noted that of these substances, the injection of indocyanine green produced the most intense US echoes [5]. This suggested that the small volume of foam contained in the indocyanine dye might have caused entrapment of miniature bubbles that caused the contrast effect.
Bove et al. [18] attributed the formation of bubbles at the tips of injection catheters to a localized pressure drop that induced cavitation events (i.e. formation of bubbles) within liquids. Further investigations by Kremkau et al. [19] using in vitro injections of different fluids in a plastic pressure chamber under US monitoring showed that miniature bubbles were actually formed at the catheter tip as the result of injection through a small-bore catheter. In subsequent years, several other investigators used microbubbles to study parts of the cardiovascular system. These studies showed that the rapid injection of almost any liquid through a small-bore catheter would produce this contrast effect [4, 5, 20].
However, these initial approaches resulted in the formation of free (not encapsulated) air bubbles. The fundamental limitations of these air bubbles produced by agitation was their relatively large size, non-reproducible concentration and inhomogeneous distribution, and instability. The surface tension at the interface between the air core and the surrounding liquid forced the bubbles to spontaneously dissolve within seconds.
Early attempts to improve the stability and longevity of microbubbles involved high viscosity materials, such as suspensions of gelatin and collagen, but at the expense of reduced echogenicity [4, 21]. Later, it was found empirically that sonication of a small amount of the patient’s blood added to the saline acted as a surfactant and improved the stability and effectiveness of the agitated saline as a contrast agent [22,23,24]. These early investigations were responsible for the discovery of what is commonly known today as stabilized UCA.
First-generation ultrasound contrast agents
In the hope of improving the stability of microbubbles and thus increasing the duration of contrast enhancement, continued research with these materials led to the breakthrough invention of encapsulated air microbubbles, which sparked the development of industrially manufactured first-generation UCAs. Several substances were tested for their ability to stabilize the air microbubbles against rapid dissolution, including proteins, phospholipids and biodegradable synthetic polymers [7, 13].
Among the most commonly used first-generation UCAs were Albunex (Molecular Biosystems Inc., San Diego, CA), Echovist (Schering AG, Berlin, Germany) and Levovist (Schering). Although these first-generation air-filled UCAs have been replaced by second-generation gas-filled UCAs and have been discontinued from the market, for historical reasons we briefly present their basic properties and diagnostic potential.
In 1993, Albunex became the first transpulmonary UCA to be approved by the FDA; it was approved for clinical use in echocardiography. Albunex consisted of an isotonic suspension of air-filled microbubbles encapsulated within a shell of denatured human serum albumin that was produced by sonication [23, 25]. These microbubbles had a reproducible small size, mean diameter of approximately 3.8 μm with a standard deviation of 2.5 μm, and were able to pass through the pulmonary capillaries; however, they provided stable enhancement only for a very limited time, usually less than 1 min [26].
Earlier, in 1991, Echovist had been introduced in Europe as the first commercially available UCA. It consisted of a suspension of galactose microparticle granules that formed the matrix to which air microbubbles could adhere, and this controlled the uniformity of their size [22]. However, the mean diameter of Echovist microbubbles was about 15 μm and they dissolved shortly after mixing with the blood. This rendered Echovist unable to pass the pulmonary barrier and limited its contrast effect to the venous system and the right heart cavities [27, 28]. Outside the cardiovascular system, Echovist was used as an intracavitary contrast agent for sonographic demonstration of the uterine cavity and to assess the patency of the fallopian tubes.
Levovist, the transpulmonary derivative of Echovist, was the first contrast agent for general clinical use, introduced in Europe in 1995. Similar to Echovist, Levovist consisted of a suspension of galactose microcrystals, with the addition of a minute amount of fatty acid (palmitic acid) that acted as a surfactant, making the microbubbles smaller, and thus more stable. Therefore, unlike its predecessor Echovist, Levovist was able to pass the lung capillaries and prolong the contrast effect to last for 1–4 min after an intravenous injection [6, 22, 28]. The US signal increased by approximately 20 dB and could be detected on B-mode imaging in the heart and major vessels [22].
Despite the major advances in the development of these first-generation UCAs, their limitations were still restrictive, including their instability, inability to sufficiently pass multiple times through the lung capillaries, and failure to provide sustained enhancement for longer durations. These agents’ primary drawback was the fact that they contained air, which was highly diffusible and rapidly soluble in blood, resulting in limited US examination time [7].
Second-generation ultrasound contrast agents
Further research with a focus on the size and stability of microbubbles led to the development of the second generation of UCAs. In the newer iteration of these agents, the contained air was replaced by inert gases of higher molecular weight such as perfluorocarbons or sulfur hexafluoride. These gases have low solubility in blood, and they further improved the stability and echogenic effect of the microbubbles.
A number of second-generation UCAs are commercially available, including Definity/Luminity (Lantheus Medical Imaging, North Billerica, MA), SonoVue/Lumason (Bracco Imaging SpA, Milan, Italy, and Bracco Diagnostics Inc., Monroe Township, NJ, respectively), Optison (GE Healthcare, Princeton, NJ) and Sonazoid (GE Healthcare, Oslo, Norway).
Definity/Luminity
Generic name: Perflutren lipid microsphere.
Chemical name: 1,1,1,2,2,3,3,3-octafluoropropane.
Commercial availability: Definity has been marketed in the USA since July 2001. In Europe it has been marketed under the name Luminity since 2006.
Current FDA-approved indications: Adult echocardiography.
Packaging: Definity/Luminity is supplied as a 2-mL glass vial containing clear liquid with a headspace of octafluoropropane.
Composition: Definity/Luminity is composed of a perflutren gas (octafluoropropane) and a bi-layer shell of phospholipids. The perflutren gas core has an empirical formula of C3F8 and molecular weight of 188 g/mol. The shell is composed of a blend of three phospholipids, namely DPPC (1,2-dipalmitoyl-sn-glycero-3-phosphocholine), DPPA (1,2-dipalmitoyl-sn-glycero-3-phosphate, sodium salt) and DPPE-MPEG5000 (1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-methoxy-polyethylene-glycol). DPPA and DPPC are major constituents of human cell membranes [29].
Reconstitution: The product is prepared by emulsification, e.g., by applying high-energy mechanical shear achieved by means of a mechanical shaking device (VIALMIX, Lantheus Medical Imaging Inc., North Billerica, MA) for 45 s.
Microbubbles: After activation, each 1 mL contains 1.2 × 1010 perflutren lipid microspheres and about 150 μL/mL (1.1 mg/mL) octafluoropropane. The diameter range of microspheres is 1.1–3.3 μm (maximum 20 μm), with 98% being less than 10 μm [29].
Administration: Withdrawn from the vial and administered without dilution for intravenous use.
Biodistribution: Pure intravascular agent.
Metabolism: The perflutren gas component is rapidly cleared from the systemic circulation via the lungs. Perflutren concentrations in blood were shown to decline in a mono-exponential fashion with a mean half-life of 1.3 min in healthy subjects and 1.9 min in subjects with chronic obstructive pulmonary disease. The phospholipid shell is broken down and distributed into the endogenous lipid pools in the body (e.g., in the liver) and is metabolized to free fatty acids [29].
Optison
Generic name: Perflutren protein-type A microspheres.
Chemical name: 1,1,1,2,2,3,3,3-perflutren.
Commercial availability: Optison has been marketed in the USA since January 1998 and in Europe since May 1998.
Current FDA-approved indications: Adult echocardiography.
Packaging: Optison is supplied as a 3-mL glass vial, which contains clear liquid with a white layer on top.
Composition: Optison consists of microbubbles with a perflutren gas core and a shell of denatured human albumin. Perflutren gas has an empirical formula of C3F8 and a molecular weight of 188 g/mol. It is an insoluble, chemically inert, and biologically non-toxic gas. The thin shell of denatured human albumin is responsible for these microspheres’ stability [30]. Human albumin is derived from large pools of human plasma. Optison is produced by the sonication of a heated human albumin solution in the presence of perflutren. Heating is necessary prior to sonication to denature the albumin and facilitate encapsulation [31]. During sonication the liquid/gas mixture is treated with high intensity US at a frequency of 20 kHz [24].
Reconstitution: Optison is reconstituted by gentle hand agitation just before use.
Microbubbles: Each 1 mL of reconstituted Optison contains approximately 5.0–8.0 × 108 microspheres, with a mean diameter of 3.0–4.5 μm (maximum 32 μm), with 95% of microspheres being less than 10 μm [30].
Administration: Withdrawn from the vial and administered without dilution for intravenous use.
Biodistribution: Pure intravascular agent.
Metabolism: When injected into the bloodstream, the perflutren gas component of Optison microsphere is not metabolized but is excreted intact by the lungs with the expired air. The pulmonary elimination half-life of perflutren occurs within minutes, with a mean ± standard deviation (SD) half-life of 1.3±0.69 min. The albumin component of the Optison microsphere is handled by the normal metabolic routes [30].
Lumason/SonoVue
Generic name: Sulfur hexafluoride lipid-type A microspheres.
Chemical name: Sulphur hexafluoride (SF6).
Commercial availability: SonoVue was first marketed in Europe in 2001 and in China in 2004. In 2014 it became commercially available in the USA under the name Lumason.
Current FDA-approved indications: Adult and pediatric echocardiography, adult and pediatric focal liver lesions, pediatric intravesical administration for assessment of vesicoureteral reflux.
Packaging: SonoVue/Lumason is supplied as a kit that contains one SonoVue/Lumason vial, a mini spike, and a syringe prefilled with the diluent (5 mL sodium chloride 0.9%). The vial contains a white lyophilized powder, and the headspace is filled with sulfur hexafluoride.
Composition: SonoVue/Lumason is composed of a sulfur hexafluoride gas core and a shell of phospholipids with tiny amounts of palmitic acid that act as a stabilizer. The sulfur hexafluoride gas (SF6) has a molecular weight of 145.9 g/mol. The high molecular weight of the gas and its low solubility in water and blood make these microbubbles sufficiently resistant to pressure. The shell of SonoVue/Lumason is a monolayer of phospholipids consisting of 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) and 1,2-dipalmitoyl-sn-glycero-3-phospho-rac-glycerol sodium (DPPG-Na) with palmitic acid as a stabilizer [32].
Reconstitution: SonoVue/Lumason suspension is formulated by adding the diluent into the vial followed by vigorous hand agitation.
Microbubbles: Each 1 mL of reconstituted SonoVue/Lumason contains approximately 1.5–5.6×108 microspheres, with a mean diameter of 1.5–2.5 μm (maximum 32 μm), with 99% of microspheres being equal to or less than 10 μm [32].
Administration: Withdrawn from the vial and administered without dilution for intravenous use.
Biodistribution: Pure intravascular agent.
Metabolism: Sulfur hexafluoride is an innocuous gas that undergoes little or no biotransformation. Following intravenous administration, it is rapidly eliminated via the lungs; 98% of the injected dose is detected in exhaled air within 2 min. The phospholipids of the microbubble shell are natural components of cell membranes that are metabolized in the liver by endogenous phospholipid pathways. Following administration of SonoVue/Lumason, 40–50% of the injected dose is cleared after the first minute [32].
Sonazoid
Generic name: Perfluorobutane microbubbles.
Chemical name: 1,1,1,2,2,3,3,4,4,4-decafluorobutane.
Commercial availability: Sonazoid has been marketed in several Asian countries beginning in 2006. These include Japan (2006), South Korea (2012), Taiwan (2017) and China (2018). In Europe it has been marketed only in Norway since 2014 [33]. Currently Sonazoid is not marketed in the USA.
FDA-approved indications: None (approved for liver imaging in the above-mentioned countries plus for breast imaging in Japan).
Packaging: Single vial with 16 μL of Sonazoid for injection together with a single 2-mL bottle of injection-use water for dissolution and one Chemoprotect spike for use to inject the water into the vial.
Composition: Sonazoid is made up of perfluorobutane gas microbubbles encapsulated in a lipid shell. The perfluorobutane gas has high molecular weight of 238.03 g/mol. The outer shell is a monomolecular membrane of hydrogenated egg yolk phosphatidyl serine (HEPS).
Reconstitution: Sonazoid is reconstituted before use with 2 mL of sterile water that is administered through a supplied vented filter spike followed by manual mixing for 1 min.
Microbubbles: After reconstitution, the product appears as a milky white, homogeneous suspension, containing approximately 1 × 109 microspheres/mL with a diameter range of 2–3 μm.
Biodistribution: In vitro studies showed that up to 99% of Sonazoid can be phagocytosed by Kupffer cells. This means that Sonazoid, apart from being an intravascular agent, has an additional post-vascular phase known as the Kupffer phase. The Kupffer cell uptake starts approximately 1 min after administration. The enhancement observed after 1 min represents a combination of the vascular phase and the Kupffer phase. The pure Kupffer phase (also called the post-vascular phase) starts approximately 10 min after injection when the microbubbles have been eliminated from the blood pool and lasts at least 2 h [33].
Metabolism: Perfluorobutane gas is excreted through the lungs; elimination half-life is 30–45 min. The shell of the microspheres is metabolized by the liver and kidneys.
Microbubble behavior in the ultrasound field
Scatter
Ultrasound is acoustic energy that is transmitted into the body and propagated through the tissues in the form of a pressure wave. Acoustic impedance is a property of a tissue that describes the resistance to sonography waves traveling through its substance. It is defined by the density of the tissue multiplied by the speed at which waves traverse the tissue. When a US wave encounters an interface between two tissues with different acoustic impedance, part of the acoustic energy is reflected back to the transducer while the remaining energy is transmitted through the tissue. The degree to which a US wave is reflected from a given interface depends on the difference in acoustic impedance of the materials on either side of the interface [1, 2]. When interfaces occur between tissues with very different levels of acoustic impedance, such as interfaces of tissue with air or bone, they reflect almost all the incident energy transmitted by the US probe. This results in a very strong echo signal that is detected by the transducer [2]. Gas-filled microbubbles are acoustically very active, because there is a marked difference of acoustic impedance at their interface with the adjacent blood and soft tissues; hence, they are very effective contrast agents.
Resonance
Gaseous microbubbles are not just passive reflectors of the US beam. Because of their gas-filled core, microbubbles exhibit higher compressibility compared to the adjacent blood and tissues. Therefore, they undergo volumetric oscillations (resonance) in response to the incident US wave. A US wave propagates through tissues as a series of alternating pressure waves, producing compression and rarefaction of the conducting medium. Microbubbles compress during the positive pressure and expand during the negative (rarefaction) pressure in each cycle. These volumetric oscillations depend on the amplitude of the transmitted US wave, which is referred to as the acoustic pressure [14].
If the acoustic pressure is low (150 kPa or lower), the microbubbles undergo mainly linear oscillations, i.e. steady expansion and contraction, synchronously with the incident US wave. Thus, they generate a signal response that has the same frequency as that of the incident (fundamental, f0) US wave [9].
With increasing acoustic pressure, the oscillations of the microbubbles become non-linear. For medium acoustic pressures (150–300 kPa), the microbubbles expand more than they contract in each cycle. This asymmetrical oscillation of the microbubbles produces a scattered wave that is consequently distorted and contains not only the fundamental frequency (f0) of the incident US wave, but also additional frequency components known as harmonics [9]. These additional frequencies range from below the incident wave, or subharmonics (half the transmitted frequency, or f0/2) to frequencies above the incident wave, ranging from higher harmonics (i.e. n × f0, n = 2, 3, 4…) to ultra-harmonics (i.e. n/2 × f0, n = 3, 5, 7…) [9, 34]. The harmonic frequencies have decreasing intensity, but the second harmonic frequency is still strong enough to be used for diagnostic purposes. The theoretical advantage of the harmonic over the fundamental frequency is that UCA microbubbles resonate with harmonic frequencies much more than do the adjacent tissues [9, 13, 14, 35].
The majority of commercial UCA microbubbles are 1–8 μm in diameter, which results in a resonant frequency range of 2–15 MHz. Fortunately, the resonance frequencies of these microbubbles overlap with the frequency range that is commonly used in diagnostic US [9, 13]. Microbubbles that oscillate near their resonance frequency exhibit the strongest relative radial expansion and thereby produce the strongest non-linear echo response. When the frequency of the incident US wave is moving away from the resonant frequency of the microbubbles, the signal response received from the microbubbles is reduced, which reduces the enhancement. Using a US transducer that produces US waves at a given frequency (fundamental, f0) and receives a US signal of twice that frequency (second harmonic, 2f0), it is possible to show signals mainly (but not exclusively) from the contrast agent. For that reason, harmonic imaging requires the use of transducers with broad bandwidth to enable separate transmit frequencies (f0) and receive frequencies (2f0) [9].
For high acoustic pressures (above 300–600 kPa), the periodic nature of the oscillations is lost, and the bubbles undergo forceful collapse, resulting in shell rupture and release of the contained gas. This process is referred to as inertial, unstable or transient cavitation and can also produce high localized temperatures and high-velocity jets [9].
Mechanical index
The acoustic pressure interacting with the microbubbles can be adjusted by the operator through the output power setting of the US system. The changes in the output power are reflected by the mechanical index, which is the ratio of the peak negative pressure divided by the square-root of the incidence frequency, and it is displayed on the US screen [1]. Therefore, the behavior of the microbubbles in relation to the change in mechanical index is similar to that of the change in acoustic pressure for a fixed frequency. Specifically, if the mechanical index is lower than 0.2, the bubbles oscillate in a symmetrical manner, producing a stable, mostly linear signal with a center frequency equal to that of the transmitted wave. With increasing mechanical values, excitation also increases. When these values are between 0.2 and 0.6, they cause an asymmetrical microbubble resonance, where the microbubbles expand more than contract during each cycle, producing a non-linear response that contains both the fundamental and the harmonic components. Typically, if the mechanical index increases to 0.6 or higher, the vibrations become unstable and cavitation effects result in destruction of the microbubbles [8, 31].
Currently, contrast-specific imaging techniques are divided into two categories: low mechanical index techniques that minimize destruction of the microbubbles, and high mechanical index techniques that maximize microbubbles’ destruction [13].
Contrast-specific imaging techniques: from fundamental to harmonic imaging
As UCAs evolved, a parallel development of US imaging techniques took place, which aimed to differentiate the frequency components (fundamentals, subharmonics and harmonics) of the scattered US signal produced by the microbubbles and tissues.
Initial imaging approaches with first-generation UCAs were performed in fundamental imaging mode, i.e. when the US transducers emit and receive signal over the same bandwidth of the transducer. The main limitation of fundamental imaging was a relative decrease in the backscatter signal detected from the microbubbles compared to adjacent tissues and blood. As a result, a significant amount of UCA was needed to compensate for the signal loss [13].
Subsequently, fundamental imaging with UCA was completely replaced by the contrast-specific imaging techniques. The latter are based on the detection and display only of the second harmonic or subharmonic signals from the scattered US. While harmonic imaging is available on most scanners, subharmonic imaging has only recently become commercially available [36]. The advent of the second harmonic imaging marked the beginning of an exciting new period of development of several imaging strategies applied to further maximize the non-linear properties of the microbubbles. These techniques utilize low mechanical index for continuous undisrupted imaging of microbubbles. Strategies for signal processing involve not only the choice of the amplitude, phase and frequency of the transmitted US pulse, but also methods for selective filtering of detected frequencies to separate microbubble and tissue echoes [35]. These methods can be classified in two major categories: single-pulse imaging and multi-pulse imaging.
Low mechanical index techniques
Single-pulse imaging
In the early stages of harmonic development, single-pulse sequences were used for contrast imaging. In this technique, a relatively simple frequency filter centered on the transmit frequency (fundamental frequency, f0) eliminated the fundamental signal response from the tissues and preserved the second harmonic response from the microbubbles [37]. However, the bandwidth of the fundamental signal received from the tissue is broad, and it often overlaps with the bandwidth of the second harmonic frequencies received from the microbubbles. Therefore, the effect of such filtering was to reduce the bandwidth contained in the received echoes, causing low spatial resolution of the resulting image [8, 38]. This limitation is underscored by the fact that most modern US systems use transducers that generate very short pulses to improve axial resolution. However, short pulses create a very wide bandwidth. This further highlights that the overlap between the fundamental and harmonic frequencies that cannot be efficiently filtered out.
Multi-pulse sequences
Most modern US scanners are based on multi-pulse sequences, i.e. pulse inversion or pulse amplitude modulation, which produce images with high contrast-to-tissue ratios. These techniques combine the received signals from multiple US transmit pulses and utilize subtraction techniques instead of filtering to separate the linear (fundamental) from non-linear (subharmonic and harmonic) components [13]. Among the newer multi-pulse techniques, we focus on the pulse or phase inversion harmonics, pulse amplitude modulation and power-modulated techniques.
Pulse inversion harmonics
In pulse (or phase) inversion harmonics, two US pulses are sent out from the transducer in rapid succession. In this technique, the second pulse is identical but completely opposite in phase to the first pulse, i.e. 180° phase-inverted. Because the received echoes are mirror images of the transmitted pulses, the two sets of received echoes are also phase-inverted. The algorithm then adds the two sets of echoes. The result of this summation equals zero for the linear (fundamental) component of the signal that is generated by soft tissues, whereas the summation from the non-linear signal produced by the microbubbles is higher in amplitude [38]. The advantages of this technique are that it allows low mechanical index, nondestructive, continuous imaging of microbubbles, and that there is good signal-to-noise ratio with no restrictions of bandwidth [8]. However, there is a reduction in the frame rate because it is necessary to send two pulses down each transmit line rather than one, resulting in decreased temporal resolution.
Pulse amplitude modulation
The transmitted pulses are both in the same phase, but the second transmission pulse is half the amplitude of the first one. Upon reception the signals are combined such that the smaller reflection (from the second pulse) is doubled and subtracted from the first full amplitude pulse. The summation of the backscattered signals from these two transmission pulses selectively cancels the linear response from the tissue and amplifies the non-linear response from the UCA [38]. The advantage of this technique is the ability to detect very weak harmonic echoes and suppress tissue motion, allowing for real-time perfusion imaging of rapidly moving structures such as the myocardium [8].
Power-modulated pulse inversion
In this technique, the two pulses vary in both amplitude and phase. This results in a more sensitive detection of non-linear signals.
Emerging contrast-specific imaging techniques: subharmonic imaging
As discussed, the nonlinear oscillation of microbubbles generates a backscattered US signal that contains multiple frequency components ranging from subharmonics to harmonics and ultra-harmonics. Second harmonic imaging is currently the standard commercially available contrast-specific imaging technique.
Subharmonic imaging has been introduced as an alternative contrast-specific imaging technique; it utilizes the subharmonic component of the received frequencies. In subharmonic imaging, microbubbles are insonated at the fundamental frequency (f0), and echoes are received at half the fundamental frequency (f0/2). Tissues typically do not produce significant subharmonics. As a result of the high transmit frequency and the much smaller attenuation of scattered subharmonic signals, subharmonic imaging might prove to be advantageous for suppressing tissue echoes and enhancing the contrast-to-tissue ratio, particularly while scanning structures deeper in the body.
In addition, experiments have shown that under different acoustic pressure parameters, the amplitude of the subharmonic US signal component correlates with the hydrostatic pressure changes in various structures of the human body. This correlation allows for some quantitative applications. One potential quantitative application of subharmonic imaging is the subharmonic aided pressure estimation, or SHAPE, technique. This technique utilizes the subharmonic non-linear response of microbubbles to estimate noninvasively the hydrostatic pressure gradients across vascular structures in the body. Studies have shown that SHAPE is capable of tracking hydrostatic pressure changes in the ventricles of the heart, estimating portal venous pressure in the liver, as well as monitoring intratumoral pressure changes in breast tumors undergoing neoadjuvant chemotherapy [39,40,41,42]. Multi-center clinical studies are ongoing to use SHAPE as a noninvasive tool for evaluating hydrostatic pressures in different organs in children.
High mechanical index techniques: flash contrast imaging (destruction–replenishment technique)
The contrast-specific imaging modes discussed here use low acoustic outputs so that the majority of microbubbles are not destroyed. In some instances, however, intentional destruction of the microbubbles in situ is needed to assess their influx within a tissue or organ. Flash contrast imaging, also known as destruction–replenishment imaging, involves switching between high and low mechanical index techniques. In this technique, the microbubbles are initially visualized using low mechanical index. Once constant enhancement has been achieved, application of a single, or several, high mechanical index frames destroys the microbubbles. Upon microbubbles’ destruction, their gas core is instantly released and thus it is visualized only in one frame before dissolving into solution. On return to continuous low mechanical index imaging, new contrast microbubbles are visualized entering the scan plane and replenishing the destroyed ones. The time it takes for the contrast to refill the scan-plane is an indicator of blood flow [8, 9, 13].
Future directions of ultrasound contrast agents
Increasing understanding of the physical, chemical and biological behavior of microbubbles has opened an array of promising new biomedical applications in addition to their conventional use in diagnostic imaging, including molecular imaging and therapeutic applications. This section introduces the possible future directions in the evolution of UCAs.
Improving size and distribution
The current, second-generation UCAs contain microbubbles with size distribution that is manufacturer-dependent, ranging from 1μm to 10 μm. This is known as polydisperse UCA distribution. Diagnostic US systems operate at a somewhat narrow frequency transmit bandwidth, and therefore only a fraction of these bubbles resonates in response to the driving US field. Efforts are now focused on developing the next generation of microbubble agents with a narrow and more controllable size distribution [43]. In this regard, two options are available. One is to narrow the size distribution of microbubbles in the polydisperse suspension by isolating a subset of sizes so that more microbubbles resonate at the given diagnostic frequency. The second option is to directly produce monodisperse microbubbles.
Monodisperse microbubbles
Several methods have been tested for counting and sorting microbubbles, ranging from the traditional methods of a manual counting chamber (hemocytometer) and a light microscope (optical microscopy) to mechanical filtration or centrifugation [43,44,45]. More recently, these methods have included a variety of automated technologies such as laser diffraction and electro-impedance volumetric sensing. However, despite achieving size uniformity, these methods do not always achieve acoustic uniformity.
The second approach for producing monodisperse microbubbles is through direct bubble formation in a microfluidic flow-focusing device. In such a device, a gas thread is focused between two liquid flows through a constriction. Because of the capillary instability at this level, the gas thread destabilizes and releases microbubbles. The bubble size, stability and generation frequency can be accurately controlled through the pressure of the gas thread, the liquid flow rate and the lipid mixture [15].
Recent in vitro studies have shown that size-sorted microbubbles/monodisperse UCAs (i.e. containing the same size distribution throughout the entire population of microbubbles) were able to increase the contrast sensitivity as well as the scattering cross-section per bubble by almost two orders of magnitude compared to polydisperse microbubbles [15, 46]. In addition, monodisperse microbubbles reduced shadowing artifacts and improved deep tissue imaging. It is possible that the next generation of UCAs could include monodisperse microbubbles that are tuned to specific frequencies/transducers and selected based on the type of imaging being performed.
Nanodroplets
Efforts are underway to develop bubbles significantly smaller (by about 3 orders of magnitude) than those in the current generation, called nanodroplets (in the nano scale: 10−9) [47]. These nanodroplets have different physical, chemical and acoustic properties than microbubbles, but the most important distinction is their size, which allows them to extravasate [48]. If coupled with drugs, nanodroplets could potentially penetrate cancerous tissue for therapeutic drug delivery, which microbubbles are unable to do. Studies have shown that these nanodroplets circulate longer after administration and that they can be selectively activated both spatially and temporally to provide US contrast enhancement [49,50,51]. Their size is also beneficial to the rapidly advancing super-resolution imaging techniques that are better suited to imaging smaller acoustic targets [52]. Nanodroplets are an exciting new avenue of research in the field of CEUS that could shift UCAs from being a purely intravascular imaging modality to one that is also able to provide extravascular information.
Improving composition
A recent study explored the development of polymer-shelled microbubbles using elastomeric polyvinyl alcohol (PVA) shells, which enabled the microbubbles to remain stable for over a month. In contrast, the current commercially available UCAs last from a few hours to a few days [53]. The prolonged stability of these newer iterations of UCAs is particularly suited not only for diagnostic imaging but also for steady release of drugs over longer periods of time.
Molecular imaging
One of the most promising areas of research in UCA development is the field of molecular imaging, i.e. selective targeting of microbubbles to particular sites within the body. This is like radiolabeling of antibodies for radionuclide imaging or the administration of [F-18]2-fluoro-2-deoxyglucose (FDG) for positron emission tomography (PET) imaging. When microbubbles with shells composed of albumin or lipid are injected into the body, they behave as antibodies and can bind to activated cells or molecules in which the receptor antigens are expressed. Thus, microbubbles can be retained within the microcirculation of activated tissues and yet remain acoustically active so that their signal can be detected by the US transducer. This principle forms the basis for molecular imaging: the acoustic visualization of localized biological markers expressed in disease processes [16, 54].
In addition to the inherent chemical properties of the microbubble shell that promote retention of microbubbles within tissues, newer molecular imaging strategies rely on selective attachment of antibodies, peptides or other ligands to the microbubble surface that recognize disease-related antigens [55]. Early results in this area have shown promise with applications that include the targeting of microbubbles to atherosclerosis, intravascular thrombi, and sites of angiogenesis and tissue inflammation [13].
Drug and gene delivery
Ultrasound energy can be used to cause a transient increase of permeability, i.e. sonoporation, of the cell wall that enables the microbubbles to transport and deliver extracellular materials into the cell, such as drugs or genes. These materials can either be incorporated within the microbubbles or injected near the site of sonoporation [15, 16, 54]. Early research suggested that the underlying mechanism of this sonoporation was inertial cavitation, i.e. the rupture of the microbubble when it reached the particular site. However, more recent work using high-speed cameras suggests that micro-streaming phenomena with non-inertial cavitation might also be associated with sonoporation. Micro-streaming can generate high temperatures associated with inertial cavitation and might result in an increase in the fluidity of the phospholipid membranes, thus allowing the microbubbles to fuse with the cell membrane. The presence of the gas lowers the threshold for cavitation, making the drug-carrier microbubbles sensitive to US for local activation. Additionally, drug-carrier microbubbles are acoustically active and can be visualized with real-time US. In this field, several clinical trials are in progress for treatment of vascular thrombosis or vulnerable plaques with US [56,57,58,59,60].
Conclusion
Since their discovery in the late 1960s, UCAs have rapidly evolved into their current commercially and clinically approved form. The evolution continues with a number of agents being developed for research or in the pre-clinical stages. UCAs have improved the capabilities of US imaging by providing valuable information about vascularity. CEUS has been performed extensively in adults during the last two decades, and its use in pediatrics is rapidly expanding. UCAs are ideally suited for pediatrics given that they reduce or eliminate imaging studies with radiation or sedation and can be performed at the bedside. With the widespread use of CEUS, US system manufacturers have taken an interest in producing state-of-the-art imaging tools that are specifically designed for CEUS applications. Future developments in UCAs are expected to enable CEUS exploration of various diseases and pathologies from both an intra- and extra-vascular perspective.
References
Merritt CRB (2018) Physics of ultrasound. In: Rumack CM (ed) Diagnostic ultrasound, 5th edn. Elsevier, Philadelphia, pp 1–33
Martin K, Ramnarine K (2010) Physics. In: Hoskins PR, Martin K, Thrush A (eds) Diagnostic ultrasound: physics and equipment. Cambridge University Press, Cambridge
Gramiak R, Shah PM (1968) Echocardiography of the aortic root. Investig Radiol 3:356–366
Ziskin MC, Bonakdarpour A, Weinstein DP, Lynch PR (1972) Contrast agents for diagnostic ultrasound. Investig Radiol 7:500–505
Gramiak R, Shah PM, Kramer DH (1969) Ultrasound cardiography: contrast studies in anatomy and function. Radiology 92:939–948
Balen FG, Allen CM, Lees WR (1994) Ultrasound contrast agents. Clin Radiol 49:77–82
Nanda NC (1997) History of echocardiographic contrast agents. Clin Cardiol 20:17–11
Burns PN, Wilson SR (2006) Microbubble contrast for radiological imaging: 1. Principles. Ultrasound Q 22:5–13
Burns PN (2018) Contrast agents for ultrasound. In: Rumack CM (ed) Diagnostic ultrasound, 5th edn. Elsevier, Philadelphia, pp 53–73
Eisenbrey J (2019) Ultrasound contrast agents: Optison. In: Lyshchik A (ed) Specialty imaging: fundamentals of CEUS. Elsevier, Philadelphia, pp 120–123
Eisenbrey J, Hilaire L (2019) Ultrasound contrast agents: Definity. In: Lyshchik A (ed) Specialty imaging: fundamentals of CEUS. Elsevier, Philadelphia, pp 116–119
Eisenbrey J, Greis C (2019) Ultrasound contrast agents: Lumason/SonoVue. In: Lyshchik A (ed) Specialty imaging: fundamentals of CEUS. Elsevier, Philadelphia, pp 110–115
Moran CM (2011) Ultrasonic contrast agents. In: Allan PL, Baxter GM, Weston MJ (eds) Clinical ultrasound, 3rd edn. Churchill Livingstone, Edinburgh, pp 77–89
Forsberg F, Averkiou M, Eisenbrey J (2019) Physical principles of CEUS. In: Lyshchik A (ed) Specialty imaging: fundamentals of CEUS. Elsevier, Philadelphia, pp 92–97
Frinking P, Segers T, Luan Y, Tranquart F (2020) Three decades of ultrasound contrast agents: a review of the past, present and future improvements. Ultrasound Med Biol 46:892–908
Paefgen V, Doleschel D, Kiessling F (2015) Evolution of contrast agents for ultrasound imaging and ultrasound-mediated drug delivery. Front Pharmacol 6:197
Faez T, Emmer M, Kooiman K et al (2013) 20 years of ultrasound contrast agent modeling. IEEE Trans Ultrason Ferroelectr Freq Control 60:7–20
Bove AA, Adams DF, Hugh AE, Lynch PR (1968) Cavitation at catheter tips: a possible cause of air embolus. Investig Radiol 3:159–164
Kremkau FW, Gramiak R, Carstensen EL et al (1970) Ultrasonic detection of cavitation at catheter tips. Am J Roentgenol Radium Therapy, Nucl Med 110:177–183
Ophir J, Parker KJ (1989) Contrast agents in diagnostic ultrasound. Ultrasound Med Biol 15:319–333
Ophir J, Gobuty A, McWhirt RE, Maklad NF (1980) Ultrasonic backscatter from contrast producing collagen microspheres. Ultrason Imaging 2:67–77
Calliada F, Campani R, Bottinelli O et al (1998) Ultrasound contrast agents: basic principles. Eur J Radiol 27:S157–S160
Feinstein SB, Heidenreich PA, Dick CD et al (1988) Albunex: a new intravascular ultrasound contrast agent: preliminary safety and efficacy results. Circulation 78:565
Jablonski EG, Dittrich HC, Bartlett JM, Podell SB (1998) Ultrasound contrast agents: the advantage of albumin microsphere technology. In: Thompson DO, Chimenti DE (eds) Review of progress in quantitative nondestructive evaluation: volume 17A. Springer US, Boston, pp 15–22
Feinstein SB, Cheirif J, Ten Cate FJ et al (1990) Safety and efficacy of a new transpulmonary ultrasound contrast agent: initial multicenter clinical results. J Am Coll Cardiol 16:316–324
Correas JM, Bridal L, Lesavre A et al (2001) Ultrasound contrast agents: properties, principles of action, tolerance, and artifacts. Eur Radiol 11:1316–1328
Schlief R, Schurman R, Niendorf HP (1993) Basic properties and results of clinical trials of ultrasound contrast agents based on galactose. Ann Acad Med Singap 22:762–767
Schurmann R, Schlief R (1994) Saccharide-based contrast agents. Characteristics and diagnostic potential. Radiol Med 87:15–23
No authors (2011) Definity. Highlights of prescribing information. Online document. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021064s011lbl.pdf. Accessed 23 Mar 2021
GE Healthcare (2016) Optison. Highlights of prescribing information. Online document. http://www3.gehealthcare.com/~/media/documents/MarketoPDFsnogating/OPT-1H-OSLO_Optison_BK. Accessed 23 Mar 2021
Sirsi S, Borden M (2009) Microbubble compositions, properties and biomedical applications. Bubble Sci Eng Technol 1:3–17
Bracco (2020) Lumason. https://imaging.bracco.com/sites/braccoimaging.com/files/technica_sheet_pdf/us-en-2020-01-15-spc-lumason.pdf. Accessed 23 Mar 2021
Lee JY, Minami Y, Choi BI et al (2020) The AFSUMB consensus statements and recommendations for the clinical practice of contrast-enhanced ultrasound using Sonazoid. J Med Ultrasound 28:59–82
Shi WT, Forsberg F, Hall AL et al (1999) Subharmonic imaging with microbubble contrast agents: initial results. Ultrason Imaging 21:79–94
Qin S, Caskey CF, Ferrara KW (2009) Ultrasound contrast microbubbles in imaging and therapy: physical principles and engineering. Phys Med Biol 54:R27–R57
Forsberg F, Gupta I, Machado P et al (2020) Contrast-enhanced subharmonic aided pressure estimation (SHAPE) using ultrasound imaging with a focus on identifying portal hypertension. J Vis Exp. https://doi.org/10.3791/62050
Chong WK, Papadopoulou V, Dayton PA (2018) Imaging with ultrasound contrast agents: current status and future. Abdom Radiol 43:762–772
Starkoff B (2014) Ultrasound physical principles in today’s technology. Australas J Ultrasound Med 17:4–10
Dave JK, Halldorsdottir VG, Eisenbrey JR et al (2012) Noninvasive LV pressure estimation using subharmonic emissions from microbubbles. JACC Cardiovasc Imaging 5:87–92
Eisenbrey JR, Dave JK, Halldorsdottir VG et al (2013) Chronic liver disease: noninvasive subharmonic aided pressure estimation of hepatic venous pressure gradient. Radiology 268:581–588
Gupta I, Eisenbrey JR, Machado P et al (2021) Diagnosing portal hypertension with noninvasive subharmonic pressure estimates from a US contrast agent. Radiology 298:104–111
Nam K, Eisenbrey JR, Stanczak M et al (2017) Monitoring neoadjuvant chemotherapy for breast cancer by using three-dimensional subharmonic aided pressure estimation and imaging with US contrast agents: preliminary experience. Radiology 285:53–62
Sennoga CA, Yeh JSM, Alter J et al (2012) Evaluation of methods for sizing and counting of ultrasound contrast agents. Ultrasound Med Biol 38:834–845
Feshitan JA, Chen CC, Kwan JJ, Borden MA (2009) Microbubble size isolation by differential centrifugation. J Colloid Interface Sci 329:316–324
Sennoga CA, Mahue V, Loughran J et al (2010) On sizing and counting of microbubbles using optical microscopy. Ultrasound Med Biol 36:2093–2096
Segers T, de Jong N, Versluis M (2016) Uniform scattering and attenuation of acoustically sorted ultrasound contrast agents: modeling and experiments. J Acoust Soc Am 140:2506
Guvener N, Appold L, de Lorenzi F et al (2017) Recent advances in ultrasound-based diagnosis and therapy with micro- and nanometer-sized formulations. Methods 130:4–13
Kripfgans OD, Fowlkes JB, Miller DL et al (2000) Acoustic droplet vaporization for therapeutic and diagnostic applications. Ultrasound Med Biol 26:1177–1189
Matsunaga TO, Sheeran PS, Luois S et al (2012) Phase-change nanoparticles using highly volatile perfluorocarbons: toward a platform for extravascular ultrasound imaging. Theranostics 2:1185–1198
Sheeran PS, Luois S, Dayton PA, Matsunaga TO (2011) Formulation and acoustic studies of a new phase-shift agent for diagnostic and therapeutic ultrasound. Langmuir 27:10412–10420
Sheeran PS, Rojas JD, Puett C et al (2015) Contrast-enhanced ultrasound imaging and in vivo circulatory kinetics with low-boiling-point nanoscale phase-change perfluorocarbon agents. Ultrasound Med Biol 41:814–831
Zhang G, Harput S, Hu H et al (2019) Fast acoustic wave sparsely activated localization microscopy (fast-AWSALM): ultrasound super-resolution using plane-wave activation of nanodroplets. IEEE Trans Ultrason Ferroelectr Freq Control 66:1039–1046
Domenici F, Brasili F, Oddo L et al (2019) Long-term physical evolution of an elastomeric ultrasound contrast microbubble. J Colloid Interface Sci 540:185–196
Brown E, Lindner JR (2019) Ultrasound molecular imaging: principles and applications in cardiovascular medicine. Curr Cardiol Rep 21:30
Lindner JR (2004) Molecular imaging with contrast ultrasound and targeted microbubbles. J Nucl Cardiol 11:215–221
Dimcevski G, Kotopoulis S, Bjanes T et al (2016) A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J Control Release 243:172–181
Eisenbrey JR, Forsberg F, Wessner CE et al (2021) US-triggered microbubble destruction for augmenting hepatocellular carcinoma response to transarterial radioembolization: a randomized pilot clinical trial. Radiology 298:450–457
Slikkerveer J, Kleijn SA, Appelman Y et al (2012) Ultrasound enhanced prehospital thrombolysis using microbubbles infusion in patients with acute ST elevation myocardial infarction: pilot of the Sonolysis study. Ultrasound Med Biol 38:247–252
Wang Y, Li Y, Yan K et al (2018) Clinical study of ultrasound and microbubbles for enhancing chemotherapeutic sensitivity of malignant tumors in digestive system. Chin J Cancer Res 30:553–563
El Kadi S, Porter TR, van Rossum AC, Kamp O (2020) Sonothrombolysis in the ambulance for ST-elevation myocardial infarction: rationale and protocol. Neth Heart J. https://doi.org/10.1007/s12471-020-01516-9
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Eisenbrey has received grant support and speaker fees from Lantheus Medical Imaging, equipment support from Siemens, and grant and equipment support from GE Healthcare. Dr. Forsberg has received equipment support from GE Healthcare, Siemens, Canon and Butterfly; UCA support from Lantheus, GE Healthcare and Bracco; and is a consultant for Samumed and Exact Therapeutics.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sridharan, A., Eisenbrey, J.R., Forsberg, F. et al. Ultrasound contrast agents: microbubbles made simple for the pediatric radiologist. Pediatr Radiol 51, 2117–2127 (2021). https://doi.org/10.1007/s00247-021-05080-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-021-05080-1