Abstract
Cancer predisposition syndromes increase the incidence of tumors during childhood and are associated with significant morbidity and mortality. Imaging is paramount for ensuring early detection of neoplasms, impacting therapeutic interventions and potentially improving outcome. While conventional imaging techniques involve considerable exposure to ionizing radiation, whole-body MRI is a radiation-free modality that allows continuous imaging of the entire body and has increasingly gained relevance in the surveillance, diagnosis, staging and monitoring of pediatric patients with cancer predisposition syndromes. Nevertheless, widespread implementation of whole-body MRI faces several challenges as a screening tool. Some of these challenges include developing clinical indications, variability in protocol specifications, image interpretation as well as coding and billing practices. These factors impact disease management, patient and family experience and research collaborations. In this discussion we review the aforementioned special considerations and the potential direction that might help overcome these challenges and promote more widespread use of whole-body MRI in children with cancer predisposition syndromes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cancer predisposition syndromes include various malignancies in which a mode of inheritance has been established, with specific genetic defects described in most cases [1]. Cancer predisposition syndromes underlie a large proportion of solid tumors presenting during childhood, with 39% of cases presenting diagnostic or potentially relevant mutations on whole-exome sequencing [2]. The presentation of cancer predisposition syndromes varies from discovery in asymptomatic children in whom genetic testing was performed for a pertinent family history or from incidental imaging findings, to clinical manifestations stemming from tumor complications [3]. Early identification of cancer predisposition syndromes facilitates timely therapeutic intervention, potentially improving outcomes [4]. Thus imaging plays an increasingly relevant role in surveillance, staging and monitoring of infants, children and adolescents with cancer predisposition syndromes (Table 1; [4,5,6,7,8,9,10,11,12]).
Because of the concern for long-term effects of radiation exposure early in life, imaging techniques with negligible or nonexistent radiation doses are highly desirable in the pediatric population [13, 14]. Whole-body MRI provides extensive anatomical coverage and excellent soft-tissue contrast without exposure to ionizing radiation, prompting its advancement to a mainstay technique in pediatric oncology [15,16,17,18]. A normal whole-body MRI almost excludes the presence of detectable tumors, with a negative predictive value of 100% and a sensitivity and specificity for tumor detection of 100% and 94%, respectively [19]. Whole-body MRI is recommended for surveillance in various cancer predisposition syndromes (Table 1) and has been described in detail [20].
Nonetheless, the high sensitivity of whole-body MRI might reveal incidental lesions (false positives), that prompt unnecessary additional imaging and procedures, with a negative impact on the patient and family experience as well as increase in cost. Many young children undergoing whole-body MRI require sedation/general anesthesia because of the length of the study. Moreover, several factors hinder the standardization of a whole-body MRI protocol, deterring consensus among institutions and research collaborations. Additionally, special attention must be given to adequate interpretation of whole-body MRI, which determines cancer predisposition syndrome risk assessment, and coding practices, which underscore legal and billing compliance.
In this review we discuss these particular challenges of whole-body MRI in pediatric patients with cancer predisposition syndromes. A discussion of all cancer predisposition syndromes is beyond the scope of this article and has been covered in detail in a recent publication [21]. This discussion will focus on Li–Fraumeni syndrome because it is the most common cancer predisposition syndrome and one that is representative of current challenges with whole-body MRI.
Whole-body magnetic resonance imaging as a surveillance tool: Li–Fraumeni syndrome
Whole-body MRI has been recommended for surveillance in one of the most prevalent cancer predisposition syndromes, Li–Fraumeni syndrome [5]. Li–Fraumeni syndrome is an aggressive cancer predisposition syndrome caused by mutations in TP53, one of the prototypical tumor suppressor genes. P53, nicknamed “guardian of the genome,” is a transcription factor that controls the expression of multiple genes that govern the processes of deoxyribonucleic acid (DNA) repair and apoptosis. More than 250 pathogenic variants have been described, and there is great heterogeneity with regard to cancer type and age of onset. The highest cancer risk is associated with missense mutations in the deoxyribonucleic acid (DNA)-binding domain of P53. Genetic modifiers (e.g., DNA methylation, telomere length, polymorphism in MDM2) have been shown to affect the Li–Fraumeni syndrome phenotype. The hallmark cancers, both benign and malignant, for which children with Li–Fraumeni syndrome are at risk, include soft-tissue sarcomas, osteosarcomas, premenopausal breast cancer, adrenocortical carcinoma, leukemia and central nervous system tumors [22], as well as tumors of the kidney [8]. Imaging surveillance is lifelong, and the recommended regimen changes at age 18. Radiography and CT should be kept at a minimum. Whole-body MRI is ideal as a surveillance tool in this population because of its propensity for a multiplicity of neoplasms with multiorgan involvement. Figures 1 and 2 illustrate Li–Fraumeni syndrome cases with normal whole-body MRI and solid tumor imaging findings, respectively.
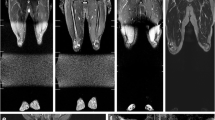
Routine surveillance in a 9-year-old boy with Li–Fraumeni syndrome. a, b Coronal (a) T2-weighted and (b) T1-weighted whole-body MRI, performed as part of annual surveillance and follow-up, shows no evidence of soft-tissue masses or lymphadenopathy. Note that a limitation of the whole-body MRI is incomplete evaluation of the extremities. The boy was diagnosed at 3 years of age through genetic testing because of significant family history, with positive TP53 mutations present in the boy’s father and brother. He undergoes surveillance with annual brain MRI, annual whole-body MR and abdominal ultrasounds every 3–4 months. To date, surveillance has remained unremarkable, without clinical or imaging findings suggesting neoplasm
Neurofibromatosis type 1 and Li–Fraumeni syndrome in a 16-month-old girl with underlying P53 mutation. Whole-body MRI was performed after abdominal ultrasound showed a suprarenal mass. a Coronal short tau inversion recovery (STIR) image shows a well-circumscribed 2.4-cm mass in the left suprarenal/para-aortic region (arrows), without dependent atelectasis in the lung. b Axial T2-weighted BLADE image shows the homogeneous suprarenal mass (arrowheads) with mild rightward aortic displacement. Histopathology was consistent with anaplastic embryonal rhabdomyosarcoma
Considerations as a screening test
With the advances in genetic testing, more families and children are being diagnosed with cancer predisposition syndromes. The goal of imaging screening is to detect cancer early so as to facilitate early treatment, potentially minimizing morbidity and improving outcome. A few essential characteristics of a screening test for this population need to be considered, such as safety, feasibility, invasiveness, cost, availability and diagnostic performance including the rate of false-positives and -negatives [23]. Recently published pediatric studies by Villani et al. [4, 24] in 2011 and 2016 have shown that a formal screening protocol including whole-body MRI with biochemical tests improved outcome in asymptomatic Li–Fraumeni syndrome subjects. Costs and benefits inherent to a noninvasive screening tool such as whole-body MRI need to be carefully weighed against the risks of not detecting a cancer earlier than would be expected without screening. Although the detection rate of new tumors in children by whole-body MRI is low at approximately 7% based on a recent meta-analysis [25], a low false-negative rate with high negative predictive value might be clinically useful in excluding malignancy. Excluding a tumor on a whole-body MRI is also valuable and reduces anxiety for a patient and family.
Overall, whole-body MRI is feasible, safe and becoming more widespread in children as part of screening regimen in pediatric practices [19, 21, 25, 26].
Sedation and anesthesia
Children younger than 7 years usually require sedation/anesthesia for completing a whole-body MRI because of fear and anxiety, intolerance of the length of exam and a need to reduce motion artifacts. Although sedation is generally safe, there is developing evidence that exposure to anesthesia in young children might have deleterious long-term cognitive and behavioral effects [27]. Therefore the need for sedation/anesthesia to perform a whole-body MRI is another consideration when weighing the risks and benefits for this type of study. Logistically sedation/anesthesia cases entail preparation prior to the appointment and more steps to daily workflow in a busy MRI practice.
The advent of newer and faster MRI sequences might thereby decrease the need for sedation and anesthesia. Some of the faster MRI techniques yielding high-qualty imaging include a combination of compressed sensing, parallel imaging and radial imaging. Many pediatric imaging providers also utilize a feed-and-swaddle method for infants requiring whole-body MRI studies to avoid sedation.
Parental and patient anxiety
Anxiety and stress surround whole-body MRI screening of children with cancer predisposition syndromes, and this is referred to as “scanxiety” [28]. Parental and patient fears and anxiety stem from two main sources: knowing there is a high chance of the child developing or having a cancer, and concern for potential false-positive findings on whole-body MRI screening examinations, which can escalate the psychological anxiety in families. It is important that the patient and families have consistent, timely communication regarding the results of the imaging studies, a thorough discussion of the appropriate interval between screening studies, and how to handle positive findings seen on whole-body MRI. Still, Malkin and colleagues [28] reported that patients and families strongly favor screening with whole-body MRI over no screening at all.
Whole-body magnetic resonance imaging in perspective
Whole-body MRI has become a more widespread method of imaging children with cancer predisposition syndromes in North America over the last several years because of its whole-body coverage, excellent contrast resolution and a one-stop evaluation of the soft tissues, solid organs and bone marrow. Schooler and colleagues [29] reported in 2018 the practice patterns of whole-body MRI utilization across North America based on a member survey by the Society for Pediatric Radiology (SPR). The results of this survey show that whole-body MRI was only being utilized for the past 6 years in academic centers and private practice groups affiliated with academic centers [29]. One of the most common indications (75% of the respondents) for using whole-body MRI was for cancer predisposition syndrome screening [29]. Positron emission tomography (PET)/MRI is a promising new modality; however it is not widely available, requires use of ionizing radiation for the PET component and is accompanied by additional cost. Functional imaging using PET radiotracers is reserved for children with known tumors for detecting metastatic disease and synchronous tumors and for preoperative planning. Because of the necessity for ongoing surveillance, imaging studies with ionizing radiation should be used on a case-by-case basis in the setting of prior disease. Studies investigating the reduction of radiotracer dose are ongoing and might provide great benefit to children with known disease followed by further surveillance [30].
Imaging protocols
The site performing whole-body MRI scans must have the appropriate hardware available, technologists familiar with scanning children, and radiologists with expertise in whole-body MRI interpretation. Considerations for an optimal scan include high-resolution and fast sequences to decrease scan time and reduce motion. Whole-body MRI consists of sequential stations integrated as a single large field-of-view (FOV) image by bringing each segment of the body to the magnet isocenter. Images are fused together by reconstruction software [31]. This can be best achieved using a combination of a moving table and multichannel coils. The term whole-body MRI is commonly used to describe head-to-foot or vertex-to-heel imaging. As proposed by Greer et al. [22], unless otherwise specified, whole-body MRI should refer to head-to-foot coverage, whereas chest, abdomen and pelvis whole-body MRI and neck, chest, abdomen and pelvis whole-body MRI might be used to denote lesser coverage. These localized approaches can be implemented to reduce scanning time in cancer predisposition syndromes that benefit from imaging of specific areas, such as hereditary paraganglioma–pheochromocytoma syndrome.
There is no established consensus for a standardized pediatric whole-body protocol that provides high diagnostic accuracy while achieving time efficiency. Nevertheless, general acquisition parameters including signal, noise and time should be optimized in all whole-body MRI protocols. In general, increasing signal and decreasing noise improves image quality, and these are determined by the strength of the magnetic field, the surface coiled used and the voxel size. Optimization of sequences must be ongoing as new sequences become available using compressed sensing, parallel imaging and non-cartesian sequences as outlined in a recent publication [21].
For tumor assessment, field strengths of 1.5 tesla (T) or 3 T have been shown to be comparable [32]. Surface coils, on the other hand, are useful in minimizing noise but can bring comfort concerns for agitated or non-cooperative children. Beyond technical factors to reduce scan time such as reducing the number of excitations and increasing the slice thickness, total scan time also depends on patient set-up time, time between sequences, and total number of sequences added. Modifying these factors might be of more benefit because technical changes can decrease time at the expense of image quality.
The coronal plane enables extensive investigation of the axial skeleton in less time than the axial plane [33] (Fig. 3). Coronal short tau inversion recovery (STIR) is a fluid-sensitive sequence widely used in whole-body MRI for cancer predisposition syndromes because of its consistent fat saturation and adequate representation of bone marrow and solid organ lesions. STIR imaging is the main workhorse sequence, with most pathological tissues resulting in high signal intensity [34]. Coronal T1 sequences are useful for assessing bone marrow and have shown utility in detecting metastatic lesions and assessing tumor response after radiation [35]. Nevertheless the axial plane is more sensitive for detecting lymphadenopathy, and sequences in this plane are frequently performed despite the longer acquisition time [18] (Fig. 4).
Coronal MRI in a 17-year-old girl who presented with resistant hypertension and elevated urine catecholamines. a–d Whole-body MR performed from vertex to toes shows large right adrenal mass compatible with pheochromocytoma (arrowhead) on coronal (a) T1-weighted, (b) T2-weighted, (c) diffusion-weighted and (d) inverted diffusion-weighted imaging. Histopathology was consistent with pheochromocytoma. The girl was to have hereditary paraganglioma–pheochromocytoma genetic testing
MRI in a 19-year-old man with Li–Fraumeni syndrome, status post hemi-pelvectomy and amputation because of a history of left pelvis and leg alveolar rhabdomyosarcoma at age 2. The man presented with a new liver nodule detected on whole-body MRI. a, b Axial (a) and coronal (b) short tau inversion recovery (STIR) whole-body MR images show a high-signal lesion in the right lobe of the liver (arrow). c Coronal T1-weighted image shows a hypointense lesions in both proximal femur and proximal tibia (arrows). The proximal tibia lesion was unchanged from multiple prior studies and considered benign. A subsequent contrast-enhanced dedicated liver MRI was performed using an intravenous hepatocyte-specific gadolinium contrast agent. d Axial T1-weighted pre-contrast MR image reveals a hypointense liver lesion (arrow) corresponding to the abnormality on the whole-body MRI. e, f Post-contrast axial arterial-phase image (e) shows early enhancement of the lesion, and axial T1-weighted portal-venous image (f) shows no enhancement of the focal liver (arrow). g Delayed axial T1-weighted hepatocyte-phase axial image shows intense increased enhancement of the normal liver but no enhancement of the focal liver lesion (arrow), suggesting a metastatic focus. The single liver abnormality on the whole-body MRI led to additional contrast-enhanced MRI of the abdomen. The value of the additional imaging helped to confirm the metastatic liver lesion but also revealed several other liver lesions not seen on the whole-body MRI screening exam. Metastatic adenocarcinoma was confirmed by CT-guided biopsy of the liver lesion and further colonoscopy revealed a sigmoid colon adenocarcinoma
The indicated timing/frequency for whole-body MRI varies according to cancer predisposition syndrome. Monsalve et al. [1] advocated for annual whole-body MRI in Li–Fraumeni syndrome for early detection of rhabdomyosarcoma and osteosarcoma. Additionally, different imaging modalities can be performed in conjunction with whole-body MRI depending on the associated tumor (e.g., brain MR in Li–Fraumeni syndrome to assess for central nervous system neoplasms). The multimodality “Toronto protocol,” based on a prospective 11-year follow-up of children and adults with Li–Fraumeni syndrome, recommends annual brain MRI in addition to whole-body MRI, with the first brain MRI performed with contrast agent. If the initial brain MRI is negative, subsequent exams are performed without contrast agent. If there is a questionable finding, contrast agent is recommended at follow-up [5, 25]. Similarly, the majority of centers do not use intravenous contrast agent for whole-body MRI to avoid any of the potential concerns related to gadolinium-based contrast media. Should an abnormality be seen on initial screening whole-body MRI examination, the child would return for a contrast-enhanced MRI on that particular region.
Functional sequences, namely diffusion-weighted imaging (DWI), are being increasingly employed in whole-body MRI. DWI is performed in the axial plane with multiple b values (0–1,000 s/mm2). DWI has been described to improve conspicuity of lesions and might detect lesions not otherwise seen on standard sequences. Whole-body DWI with background suppression has also been employed to mimic PET scans. In this manner DWI sequences are acquired in the axial plane from head to toes and reformatted in the coronal plane, and images are inverted to give the appearance of a PET scan (Fig. 5). There are pitfalls in DWI sequences because motion and mis-registration can certainly result in overestimation of disease with more false-positives. There are not sufficient data to distinguish between benign and malignant tumors using quantitative analysis with apparent diffusion coefficient (ADC) values.
The addition of sequences extends the total duration of the scan. It is important to note that technical modifications often reduce time at the expense of image quality, so a balance between adequate diagnostic information and imaging time should be pursued. Reducing imaging time improves compliance in children, thereby reducing voluntary motion artifacts and need for sedation or general anesthesia. Child-life specialists and distraction techniques using audio or video capabilities can also improve children’s compliance.
Achieving one standard whole-body MRI protocol for all cancer predisposition syndromes is challenging, considering the variable clinical presentation of cancer predisposition syndromes and their associations with different types of tumors in different locations as well as variable staffing, reader expertise, hardware and workflow practices among institutions. Clarifying terminology to describe the anatomical area encompassing whole-body MRI and establishing a standardized set of baseline sequences might foster future multicenter collaboration.
Image interpretation
Interpretation of whole-body MRI studies involves several steps to ensure accurate and valuable reports. These steps include meticulous review of the images, risk-stratification of abnormalities, structured reporting and direct communication with providers. Any positive findings on whole-body MRI screening studies invoke apprehension in patients and families. Interpretation of these studies needs to be performed by radiologists with experience in whole-body MRI.
In a 2015 publication, we suggested using a risk-stratification approach for abnormalities identified on whole-body MRI scans [19]. This methodical approach categorizes each positive abnormality as a low-, moderate- or high-risk tumoral lesion. Each lesion should be interpreted in conjunction with history, physical exam and laboratory results. For example, intra/periarticular bone marrow edema might be a result of trauma or a recent procedures rather than an ominous sign of malignancy and can be categorized as low-risk. Not all increased signal in the bone marrow is indicative of tumor development and familiarity with patterns of hematopoietic marrow across normal development is essential. Placing findings into the context of clinical information from the referring oncologist can avoid unnecessary additional imaging and invasive tests.
Standardized reporting (Table 2) can also help reduce errors, provide guidance for the interpreting radiologist and give a clear communication to providers. A family-centered approach with correct verbiage most effectively conveys the next steps in the child’s care.
Coding and billing of whole-body magnetic resonance imaging in the United States
Because whole-body MRI can consist of variable anatomical coverage and detail, guidance regarding appropriate coding and billing practices varies with the specific type of study performed by an individual practice. The recent survey of SPR members highlighted the heterogeneity of billing practices regarding whole-body MRI in pediatric radiology, with over half of respondents using separate codes for the chest, abdomen and pelvis, and approximately one-third of respondents using an unlisted MRI code [29].
No Current Procedural Terminology (CPT) code exists to report whole-body MRI performance specifically. Whole-body MRI for non-targeted evaluation (e.g., cancer predisposition syndrome screening) of most or the entire body is generally most accurately reported using an unlisted CPT code (76498, Unlisted magnetic resonance procedure [e.g., diagnostic, interventional]) [36]. When dedicated evaluation of specific regions is performed for a reason meeting criteria for medical necessity, this type of study might be better described using the appropriate CPT codes for the anatomical components imaged. Alternatively, studies performed for specific evaluation of the bone marrow might be reported in some circumstances using a bone marrow MRI code (77084, Magnetic resonance [e.g., proton] imaging, bone marrow blood supply), although this code is often not covered by insurers [36].
The distinction between these coding approaches is nuanced depending on the study ordered by the referring clinician, anatomical coverage included, technical parameters and insurer policies [37]. Accurate coding of imaging studies performed is essential to ensure legal compliance and avoid patients’ families receiving unexpected bills [36]. Therefore radiologists are strongly advised to review whole-body MRI coding practices with billing and coding professionals in advance of study performance. Use of an unlisted MRI code often receives reimbursement for medically appropriate indications but generally requires prior authorization in consultation with individual insurers to avoid nonpayment.
As the practice of whole-body MRI evolves, billing practices might also change, and pediatric radiologists should advocate for payer coverage of whole-body MRI for children who might benefit from these examinations such as in the case of cancer predisposition syndromes.
Conclusion
Whole-body MRI has become a formal part of the diagnostic workup of children with cancer predisposition syndromes. Whole-body MRI has gained widespread recognition and support as an essential noninvasive, radiation-free diagnostic tool from oncologists, geneticists and patient families. Techniques for improving whole-body MRI with faster sequences and utilization of DWI as well as billing practices in the United States are continuously evolving. Our review highlights some of the special considerations important in making whole-body MRI successful in a pediatric oncology practice.
References
Monsalve J, Kapur J, Malkin D, Babyn PS (2011) Imaging of cancer predisposition syndromes in children. Radiographics 31:263–280
Parsons DW, Roy A, Yang Y et al (2016) Diagnostic yield of clinical tumor and germline whole-exome sequencing for children with solid tumors. JAMA Oncol 2:616–624
Ripperger T, Bielack SS, Borkhardt A et al (2017) Childhood cancer predisposition syndromes — a concise review and recommendations by the Cancer predisposition working group of the Society for Pediatric Oncology and Hematology. Am J Med Genet A 173:1017–1037
Villani A, Shore A, Wasserman JD et al (2016) Biochemical and imaging surveillance in germline TP53 mutation carriers with Li-Fraumeni syndrome: 11 year follow-up of a prospective observational study. Lancet Oncol 17:1295–1305
Kratz CP, Achatz MI, Brugieres L et al (2017) Cancer screening recommendations for individuals with Li-Fraumeni syndrome. Clin Cancer Res 23:e38–e45
Kamihara J, Bourdeaut F, Foulkes WD et al (2017) Retinoblastoma and neuroblastoma predisposition and surveillance. Clin Cancer Res 23:e98–e106
Friedman DN, Lis E, Sklar CA et al (2014) Whole-body magnetic resonance imaging (WB-MRI) as surveillance for subsequent malignancies in survivors of hereditary retinoblastoma: a pilot study. Pediatr Blood Cancer 61:1440–1444
Evans DGR, Salvador H, Chang VY et al (2017) Cancer and central nervous system tumor surveillance in pediatric neurofibromatosis 1. Clin Cancer Res 23:e46–e53
Evans DGR, Salvador H, Chang VY et al (2017) Cancer and central nervous system tumor surveillance in pediatric neurofibromatosis 2 and related disorders. Clin Cancer Res 23:e54–e61
Rednam SP, Erez A, Druker H et al (2017) Von Hippel-Lindau and hereditary pheochromocytoma/paraganglioma syndromes: clinical features, genetics, and surveillance recommendations in childhood. Clin Cancer Res 23:e68–e75
Jasperson KW, Kohlmann W, Gammon A et al (2014) Role of rapid sequence whole-body MRI screening in SDH-associated hereditary paraganglioma families. Familial Cancer 13:257–265
Tabori U, Hansford JR, Achatz MI et al (2017) Clinical management and tumor surveillance recommendations of inherited mismatch repair deficiency in childhood. Clin Cancer Res 23:e32–e37
Hricak H, Brenner DJ, Adelstein SJ et al (2011) Managing radiation use in medical imaging: a multifaceted challenge. Radiology 258:889–905
Brady Z, Ramanauskas F, Cain TM, Johnston PN (2012) Assessment of paediatric CT dose indicators for the purpose of optimisation. Br J Radiol 85:1488–1498
Smith EA, Dillman JR (2016) Current role of body MRI in pediatric oncology. Pediatr Radiol 46:873–880
Nievelstein RA, Littooij AS (2016) Whole-body MRI in paediatric oncology. Radiol Med 121:442–453
Atkin KL, Ditchfield MR (2014) The role of whole-body MRI in pediatric oncology. J Pediatr Hematol Oncol 36:342–352
Chavhan GB, Babyn PS (2011) Whole-body MR imaging in children: principles, technique, current applications, and future directions. Radiographics 31:1757–1772
Anupindi SA, Bedoya MA, Lindell RB et al (2015) Diagnostic performance of whole-body MRI as a tool for cancer screening in children with genetic cancer-predisposing conditions. AJR Am J Roentgenol 205:400–408
Greer MC (2018) Imaging of cancer predisposition syndromes. Pediatr Radiol 48:1364–1375
Gottumukkala RV, Gee MS, Hampilos PJ, Greer MC (2019) Current and emerging roles of whole-body MRI in evaluation of pediatric cancer patients. Radiographics 39:516–534
Greer MC, Voss SD, States LJ (2017) Pediatric cancer predisposition imaging: focus on whole-body MRI. Clin Cancer Res 23:e6–e13
Anupindi SA (2019) Imaging of children with cancer predisposition. In: Voss S (ed) Imaging in pediatric oncology. Springer International Publishing, Basel
Villani A, Tabori U, Schiffman J et al (2011) Biochemical and imaging surveillance in germline TP53 mutation carriers with Li-Fraumeni syndrome: a prospective observational study. Lancet Oncol 12:559–567
Ballinger ML, Best A, Mai PL et al (2017) Baseline surveillance in Li-Fraumeni syndrome using whole-body magnetic resonance imaging: a meta-analysis. JAMA Oncol 3:1634–1639
Ballinger ML, Ferris NJ, Moodie K et al (2017) Surveillance in germline TP53 mutation carriers utilizing whole-body magnetic resonance imaging. JAMA Oncol 3:1735–1736
Vutskits L, Davidson A (2017) Update on developmental anesthesia neurotoxicity. Curr Opin Anaesthesiol 30:337–342
Malkin D, Nichols KE, Schiffman JD et al (2017) The future of surveillance in the context of cancer predisposition: through the murky looking glass. Clin Cancer Res 23:e133–e137
Schooler GR, Davis JT, Daldrup-Link HE, Frush DP (2018) Current utilization and procedural practices in pediatric whole-body MRI. Pediatr Radiol 48:1101–1107
Kornaczewski ER, Pointon OP, Burgess JR (2016) Utility of FDG-PET imaging in screening for succinate dehydrogenase B and D mutation-related lesions. Clin Endocrinol 85:172–179
Lauenstein TC, Freudenberg LS, Goehde SC et al (2002) Whole-body MRI using a rolling table platform for the detection of bone metastases. Eur Radiol 12:2091–2099
Mohan S, Moineddin R, Chavhan GB (2015) Pediatric whole-body magnetic resonance imaging: intra-individual comparison of technical quality, artifacts, and fixed structure visibility at 1.5 and 3 T. Indian J Radiol Imaging 25:353–358
Schmidt G, Dinter D, Reiser MF, Schoenberg SO (2010) The uses and limitations of whole-body magnetic resonance imaging. Dtsch Arztebl Int 107:383–389
Kellenberger CJ, Epelman M, Miller SF, Babyn PS (2004) Fast STIR whole-body MR imaging in children. Radiographics 24:1317–1330
Krohmer S, Sorge I, Krausse A et al (2010) Whole-body MRI for primary evaluation of malignant disease in children. Eur J Radiol 74:256–261
Chung CY, Alson MD, Duszak R Jr, Degnan AJ (2018) From imaging to reimbursement: what the pediatric radiologist needs to know about health care payers, documentation, coding and billing. Pediatr Radiol 48:904–914
Degnan AJ, Alson MD, Duszak R Jr (2019) Variability in billing practices for whole-body magnetic resonance imaging. Pediatr Radiol 49:153
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saade-Lemus, S., Degnan, A.J., Acord, M.R. et al. Whole-body magnetic resonance imaging of pediatric cancer predisposition syndromes: special considerations, challenges and perspective. Pediatr Radiol 49, 1506–1515 (2019). https://doi.org/10.1007/s00247-019-04431-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-019-04431-3