Abstract
Langerhans cell histiocytosis (LCH) with involvement of the gastrointestinal tract is rare and typically identified in patients with systemic disease. We describe a 16-month-old girl who initially presented with bilious vomiting, failure to thrive and a rash. An upper gastrointestinal (GI) examination revealed loss of normal mucosal fold pattern and luminal narrowing within the duodenum, prompting endoscopic biopsy. Langerhans cell histiocytosis of the digestive tract was confirmed by histopathology. A skeletal survey and skin biopsy identified other systemic lesions. Although uncommon, it is important to consider LCH in the differential diagnosis for gastrointestinal symptoms of unclear origin, especially when seen with concurrent rash. Findings of gastrointestinal involvement on upper GI examination include loss of normal mucosal fold pattern and luminal narrowing in the few published case reports.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Langerhans cell histiocytosis (LCH) is a disease of unknown etiology characterized by a proliferation of atypical Langerhans cells. Involvement of the gastrointestinal tract is rare and typically identified in patients with severe systemic disease. We present an unusual case of a young child with LCH who first presented for medical evaluation due to gastrointestinal symptoms and abnormalities found on an upper upper gastrointestinal (GI) examination.
Case report
A 16-month-old girl presented to our gastroenterology clinic with a 4-month history of worsening emesis and 6 months of poor weight gain. She was born at term via an uncomplicated vaginal delivery. Soon after her first birthday, the child began vomiting bilious and gastric contents daily, which gradually increased to 2 to 5 times per day. The onset of vomiting roughly corresponded with a switch from formula to whole cow’s milk. The child was prescribed a trial of soy milk by her primary care physician, which resulted in diarrhea that resolved with removal of soy milk. In addition, she had a 1-year history of seborrheic dermatitis and an eczematous inguinal rash that initially resolved with topical steroids but returned shortly after steroids were discontinued. On presentation to our institution, she was having one to three bowel movements per day and no grossly abnormal stools were reported. She was noted to have decreased activity, stamina and oral intake.
On physical examination, the child was thin with diffuse muscle wasting. Her weight was 8.4 kg (10th percentile) and height 76.5 cm (25th percentile). Diffusely dry skin with scattered eczematous lesions, seborrheic dermatitis of the scalp and an eczematous inguinal rash were present. Her abdomen was slightly distended with increased tympany. No organomegaly or perianal lesions were noted. Initial laboratory studies showed mild leukocytosis with a white blood cell count of 14.5 (normal=6.0-14.0 K/uL), thrombocytosis with a platelet count of 906 K/uL (normal=150-450 K/uL), a low total protein of 5.0 g/dl (normal=5.6-7.2 g/dL), a low albumin of 2.1 g/dL (normal=3.8–5.4 g/dL) and elevated alkaline phosphatase of 466 IU/L (normal=110–320 IU/L).
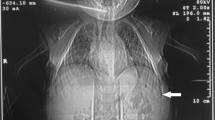
Given the presentation of bilious emesis, there was clinical concern for malrotation and midgut volvulus, which prompted an upper GI examination. The upper GI exam showed a normal course of the duodenum and a normal position of the duodenal-jejunal junction, but the duodenum was narrowed with a loss of the normal fold pattern throughout its length (Fig. 1). The proximal jejunum also showed a loss of the normal fold pattern, but a small bowel follow-through demonstrated a normal appearance of the remainder of the jejunum and ileum.
A 16-month-old with bilious emesis and duodenal narrowing. Frontal (a) and lateral (b) fluoroscopic images after administration of barium by mouth show a normal course of the duodenal sweep, but the duodenum is narrowed throughout its course (arrows). Also, there is loss of the normal fold pattern throughout the duodenum
An upper endoscopy revealed mucosal swelling in the stomach with gastric antral erosions. There was a large circumferential ulcer in the proximal duodenum and proximal duodenal narrowing beyond which the endoscope was unable to be passed (Fig. 2). Colonoscopy showed areas of nodular mucosa with interspersed white patches and increased vascularity (Fig. 2), which are indicative of inflammation.
Endoscopic images of the duodenum and colon at 16 months of age show ulceration, nodular mucosa and prominent vasculature. a There is narrowing in the proximal duodenum (arrowheads), beyond which the scope could not be passed. Additionally, there is a large circumferential ulcer in the duodenum (arrows), with a central crater (asterisk). Images of the sigmoid colon show diffusely nodular mucosa (b) and areas of white patches (black arrows) and diffusely prominent vasculature (white arrows) (c) indicative of inflammation
Biopsies obtained during the upper endoscopy and colonoscopy were sent for pathological evaluation. The duodenal biopsy showed chronic inflammation with ulcers. Biopsies from the colonoscopy showed diffuse involvement from the terminal ileum to sigmoid colon (Fig. 3). The mucosa showed distorted crypts and expanded lamina propria with a top-heavy infiltrate composed of medium-sized mononuclear cells along with mixed inflammatory cells including lymphocytes, eosinophils, plasma cells and neutrophils. The infiltrating mononuclear cells had moderate pale cytoplasm and nuclei with indentations and grooves consistent with Langerhans cells. Immunohistochemistry for CD1a (cluster of differentiation 1a) demonstrated crisp membranous positivity. These findings are diagnostic for Langerhans cell histiocytosis. A shave biopsy from the abdomen showed skin involvement by Langerhans cell histiocytosis. A skeletal survey showed a single lytic lesion in the right fifth digit proximal phalanx consistent with osseous involvement of LCH (Fig. 4).
Microphotographs of the colonic biopsy. The sigmoid colon shows focal surface epithelial cell sloughing and reactive changes. The crypt architecture is preserved overall. The lamina propria is expanded by infiltration of mononuclear cells with abundant pale cytoplasm and oval to curved nuclei with occasional grooves and indentations consistent with Langerhans cells (arrows). Accompanying mixed inflammatory cell infiltrate including eosinophils (arrowheads) and neutrophils is noted. Hematoxylin and eosin, original magnification x200. Immunohistochemistry for CD1a (inset) shows membranous positivity of the infiltrating Langerhans cells
This child received treatment according to the LCH III protocol, initially receiving methylprednisolone and vinblastine with improvement of her symptoms [1]. After initiation of therapy, the child resumed a regular diet and demonstrated catch-up growth. A follow-up upper GI examination 3 months after the initial study showed persistent but improved narrowing of the second portion of the duodenum (Fig. 5). A repeat skeletal survey, 1 year after the initial survey, showed resolution of the fifth digit proximal phalanx lesion and no other osseous lesions.
Improvement of the luminal narrowing of the duodenum after treatment. Upper GI examination performed 3 months after the initial exam in Fig. 1 shows persistent narrowing within the second portion of the duodenum (solid arrow) but improvement of the narrowing throughout the remainder of the duodenum (dashed arrows)
Discussion
LCH is a proliferation of dendritic cells of unknown etiology that can be limited. LCH can be limited to a single organ system or involve multiple organ systems. Single system disease typically affects bone, but may also affect the skin or lungs, and may be unifocal or multifocal. Multisystem disease often produces pulmonary and osteolytic lesions along with hepatomegaly and splenomegaly. Definitive diagnosis is established by a pathological examination of the affected tissue [2]. Treatment for LCH is based on protocol and currently and involves steroids and vinblastine with response determining duration of therapy [1].
Involvement of the digestive tract is a rare manifestation of LCH most commonly seen in infants. Clinical findings of diarrhea, constipation, hematochezia, vomiting, protein-losing enteropathy, perianal lesions and even intestinal perforation may characterize GI involvement [3, 4]. The clinical symptom of bilious emesis raising concern for malrotation and midgut volvulus as seen in the child we present here is very unusual. Endoscopic findings of nodular mucosa and inflammation as seen in our patient have been reported [4]. In this child, the duodenal ulceration likely developed secondary to stasis of gastric and intestinal contents in the duodenum proximal to the duodenal narrowing. The majority of patients with gastrointestinal involvement of LCH first present with rash [5]. Importantly, digestive lesions in LCH may indicate severe systemic disease and demand aggressive work-up and treatment.
Two cases of LCH have been reported in the literature in which imaging identified digestive tract involvement. Patel et al. [5] demonstrated narrowing of the small bowel and loss of the normal mucosal fold pattern from the third segment of the duodenum to the jejunum on an upper GI examination with water-soluble contrast. Damry et al. [6] showed an abnormal jejunal fold pattern and jejunal narrowing on an upper GI examination with barium. These are similar to the radiologic findings seen in the case we present here. Luminal narrowing and the loss of the normal fold pattern on an upper GI examination are nonspecific findings with a differential diagnosis, which includes infectious enteritis, inflammatory bowel disease (particularly Crohn disease) and ischemic enteritis. Celiac disease can also lead to loss of the normal fold pattern, particularly within the jejunum, but typically does not lead to the degree of luminal narrowing that was seen in this child. Additionally, the distal bowel findings (increased number and thickness of bowel folds in the ileum) that are characteristic of celiac disease were not present on the small bowel follow-through performed on this child.
The importance of the radiographic work-up in LCH is well-established for identifying lesions within the skeleton, solid organs (such as liver and spleen) and lymph nodes [7, 8]. Although rare, digestive tract involvement in LCH may present as changes in the small bowel mucosa as well as luminal narrowing on upper GI examination. LCH should be considered in children with these findings who have other characteristic lesions of this disorder. Suspicion should ultimately lead to biopsy of the most accessible lesion.
References
Minkov M (2011) Multisystem Langerhans cell histiocytosis in children: current treatment and future directions. Paediatr Drugs 13:75–86
Satter EK, High WA (2008) Langerhans cell histiocytosis: a review of the current recommendations of the histiocyte society. Pediatr Dermatol 25:291–295
Yadav S, Kharya G, Mohan N et al (2010) Langerhans cell histiocytosis with digestive tract involvement. Pediatr Blood Cancer 55:748–753
Hait E, Liang M, Degar B et al (2006) Gastrointestinal tract involvement in Langerhans cell histiocytosis: case report and literature review. Pediatrics 118:e1593–e1598
Patel BJ, Chippindale AJ, Gupta SC (1991) Small bowel histiocytosis-X. Clin Radiol 44:62–63
Damry N, Hottat N, Azzi N et al (2000) Unusual findings in two cases of Langerhans cell histiocytosis. Pediatr Radiol 30:196–199
Khung S, Budzik JF, Amzallag-Bellenger E et al (2013) Skeletal involvement in Langerhans cell histiocytosis. Insights Imaging 4:569–579
de Souza Maciel Rocha Horvat N, Coelho CR, Roza LC et al (2015) Spectrum of abdominal imaging findings in histiocytic disorders. Abdom Imaging 40:2738–2746
Acknowledgments
The authors thank Dr. Ronald Jaffe, Department of Pathology, Children’s Hospital of Pittsburgh, for confirming the pathological diagnosis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Zei, M., Meyers, A.B., Boyd, K.P. et al. Langerhans cell histiocytosis of the digestive tract identified on an upper gastrointestinal examination. Pediatr Radiol 46, 1341–1344 (2016). https://doi.org/10.1007/s00247-016-3558-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-016-3558-2