Abstract
Morphological abnormalities of the internal acoustic canal (IAC), albeit rare, are sometimes associated with hearing loss in children. We present an illustration of the spectrum of IAC abnormalities together with a brief review of the embryology and anatomy of the IAC and the techniques used when imaging the petrous temporal bone. This review focuses on morphological abnormalities of the IAC together with their clinical implications and impact on clinical management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The association between hearing loss and temporal bone abnormalities was first postulated by Mondini in 1791. Subsequent studies showed that as many as 40% of patients with unilateral sensorineural hearing loss were found to have structural inner ear anomalies on temporal bone imaging [1]. Internal acoustic canal (IAC) abnormalities are themselves rare, forming only 12% of all congenital temporal bone abnormalities. The detection of IAC anomalies in children with potentially treatable hearing loss is vital for clinical management that in turn is crucial for language and cognitive development.
The aim of this article is to illustrate a full complement of morphological IAC abnormalities and to briefly review the embryology and anatomy of the IAC and the techniques utilized when imaging the petrous temporal bone.
Embryology
The fetal development of the inner ear structures is a finely tuned cascade of events. The axons of the vestibulocochlear nerve ganglion neurons that develop in the 3rd week of fetal life by a process of delamination from the otocyst migrate toward the inner ear and the brainstem [2, 3]. This process of migration is sustained by neurotrophic factors (most notably neurotrophic factor and neurotrophin 3), which are expressed by the otocyst [4]. The cochlear ganglion axons reach the brainstem by the 5th– 6th week of fetal development and they in turn connect to the cochlear nucleus by the 16th week of gestation [4]. The formation of the cartilaginous IAC in the 9th gestational week is postulated to relate to the migrating vestibulocochlear nerve axons [3, 4]. Glastonbury et al. [5] hypothesized that the calibre of the IAC is related to the volume of the migrating vestibulocochlear nerve fibres during fetal life. Morphologically, abnormal IACs frequently feature as part of a syndrome; we hypothesize that, in these cases, IAC anomalies are likely the result of a generalized skull base developmental anomaly rather than a stand-alone focal embryonal insult.
Imaging of the inner ear structures
High-resolution CT and MRI are utilized as complementary modalities to assess the petrous temporal bone and the inner ear structures. High-resolution CT of the petrous temporal bone achieves minute bony detail while MRI is invaluable in assessing the fluid-filled structures of the inner ear.
In our institution, high-resolution CT scans of the petrous temporal bones are acquired with a 128-slice CT scanner (Somatom Definition Flash; Siemens Healthcare, Forchheim, Germany). The following scan parameters are used: collimation of 16 × 0.3 mm, pitch of 0.85, 1 s rotation time and a tube power of 120 kV. Following acquisition of the images using a very sharp bony algorithm, images are reconstructed with a 0.5 mm slice thickness and a reconstruction interval of 0.3 mm. The images are transferred to a separate workstation for post-processing in a coronal and parasagittal oblique plane for both inner ears separately. Using these parameters in our institution, the average CTDIvol dose index (CTDIvol) is 28.63 mGy and the average dose length product (DLP) is 145 mGy∙cm. In our centre, non-sedated feed-and-wrap scans are performed on infants younger than 12 months of age, sedation is utilized in the 1- to 2-year age bracket and general anaesthesia is administered for children in the 2- to 4-year age group. A mock CT scan with play therapy is trialed in children ages 4–6 years and generally children older than 6 years are fully cooperative with no sedation required for the scan.
MRI assessment of the inner ear for patients with sensorineural hearing loss routinely includes assessment of the brain for exclusion of intraparenchymal abnormalities with a limited screening sequence protocol. Dedicated evaluation of the inner ear structures is performed through the acquisition of heavily T2-weighted high-resolution axial CISS (constructive interference in steady state) images that are reconstructed into coronal and sagittal oblique planes with a slice thickness of 0.5 mm. The CISS sequence is a gradient echo sequence with two true FISP (fast imaging with steady-state precession) sequences acquired with differing radiofrequency pulses that are then combined to result in heavily T2-weighted high-resolution 3-D images. CISS sequences provide excellent contrast resolution for evaluating the cranial nerves. The MRI parameters utilized in the acquisition of this scan are: TR of 11.2 ms, TE of 5.6 ms and a flip angle of 70°. The overall average acquisition time for an MRI scan of the inner ear is 20–25 min including 4 min for the CISS images. Infants younger than 3 months of age are scanned in line with a non-sedated, feed-and-wrap and beanbag procedure. Otherwise, a similar patient preparation algorithm as that used for CT is used for children older than 3 months.
Normal anatomy and morphology of the IAC
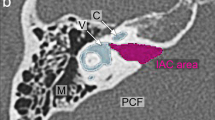
The IAC is a smooth, narrow, cylindrical (less commonly ovoid) canal that extends from the internal acoustic meatus (porus acousticus) at the cerebellopontine angle medially to the fundus adjacent to the labyrinth within the petrous temporal bone laterally [6]. The canals emanating from the fundus of the IAC transmit the branches of the vestibulocochlear nerves: the cochlear nerve canal leading to the modiolus and cochlea, the lateral ampullary nerve canal (for the nerve to the horizontal semicircular canal), the singular canal (for the nerve to the posterior semicircular canal), the canal for the labyrinthine segment of the facial nerve and the vestibule (Fig. 1). On multiplanar MRI image acquisition, the membranous labyrinth and the contents of the IAC (the facial nerve, vestibular and cochlear nerves and their branches) are clearly visible (Fig. 2).
Axial high-resolution CT belonging to 6-year old boy. a a section through the level of the horizontal semicircular canal demonstrates the fundus as the lateral aspect of the internal acoustic canal (IAC) (asterisk) and the internal acoustic meatus on the intracranial aspect of the IAC (double asterisks). Structures shown at this level are the labyrinthine segment of the facial nerve (thin arrow), the lateral ampullary nerve canal (thick arrow) and the vestibule (x). b The modiolus (asterisk), cochlear aperture (arrowheads) and the singular canal (arrow) can be seen at the more inferior level of the cochlea
CISS (constructive interference steady state) sequence MRI scan belonging to a 6-year old boy. a An axial image superiorly within the internal acoustic canal demonstrates the facial nerve (thin arrow) and the superior vestibular nerve (thick arrow). b An axial image at a mid-internal acoustic canal level demonstrates the cochlear nerve (thin arrow) and the vestibular nerve (thick arrow). c A parasagittal oblique shows the facial nerve (thin arrow) anterosuperiorly, the cochlear nerve anteroinferiorly (thick arrow) and the bifurcation of the vestibular nerve into the superior vestibular nerve posterosuperiorly (asterisk) and the inferior vestibular nerve posteroinferiorly (double asterisks). A anterior, P posterior
The morphology and dimensions of the IAC are generally assessed on a subjective basis in everyday clinical practice. Reference levels (in the form of a range of normality for each dimension) derived from morphometric analysis of the IAC on high-resolution CT imaging, are available, however; this numerical data is especially important in preoperative planning purposes [7]. The range of normality for IAC dimensions was found to relate to gender (all dimensions were found to be overall larger in boys than girls) and racial variability. The greatest reported inter-individual variability is in the length of the IAC, partly because of variations in which this dimension is measured and partly because the medial portion of the IAC keeps growing until a child is 10 years old (causing variations in the younger and older than 10 age groups) [7]. Intra-individual variability between the two IACs of all three dimensions is also common but frequently small with an accepted discrepancy of 1–2 mm [7].
Drawing a line 2 mm inside the posterior lip of the internal acoustic meatus to the anterior wall perpendicular to a line drawn through the mid canal allows the width to be measured on an axial plane (Fig. 3). A normal IAC width is quoted as 2–8 mm (5.02–5.9 mm being the mean quoted in large studies) [6–8]. The clinical importance of this measurement lies in the case of stenotic IACs that measure <2 mm. By choosing the point at which the calibre is at its largest, a line drawn through the mid canal on a coronal section allows the height of the IAC to be measured (Fig. 3). Also on the coronal plane, the length of the IAC is measured by drawing a line parallel to the axis of the IAC from the falciform crest to the midpoint in between the superior and inferior lip of the internal acoustic meatus (Fig. 3). The upper limit for the height of the IAC is taken to be 8 mm while the length of the IAC generally measures approximately 10 mm [6, 7]. The normal IAC angles 60–65° anteriorly from an axis drawn along the posterior petrous edge intersecting with a line drawn along the posterior wall of the IAC with the apex of this angle being medial to the IAC (Fig. 3) [9].
High-resolution petrous temporal bone CT belonging to a 6-year old boy. a An axial image demonstrates the measurement of the width (bidirectional arrows) of the internal acoustic canal. b A coronal image demonstrates the measurement of the height (bidirectional arrows) and the length of the internal acoustic canal-line. c An axial plane for measurement of the internal acoustic canal angulation (lines)
Pictorial review
IAC anomalies can be classified into three broad categories: abnormalities of calibre, length and angulation and duplication (Fig. 4). Abnormalities of calibre include stenotic, widened or dilated canals or bulbous deformities. Abnormalities of length and angulation include foreshortening, tortuous or posteriorly angulated canals. Duplicated IACs are classified as a third separate entity of IAC abnormalities. A spectrum of abnormalities exists in which morphological abnormalities of the IAC can be found together.
IAC stenosis
Stenosis of the IAC makes up 12% of all congenital temporal bone malformations [3, 7]. The most important clinical implication of stenotic IACs (<2 mm in diameter) is their association with aplastic or hypoplastic cochlear nerves (Figs. 5 and 6) [7]. Hypoplastic cochlear nerves are generally defined as those with a diameter smaller than that of the ipsilateral vestibulocochlear nerve on an oblique sagittal reconstruction of the internal acoustic meatus; the term aplastic is used when the nerve is not identified [3]. One must keep in mind that there are reports of patients who were found to have diminished hearing on clinical examination and bilateral cochlear nerve aplasia on MRI who at surgery were found to have cochlear nerve fibres that were either too fine to be identified on MRI or nerves that were intermingled with facial or vestibular nerve fibres [3]. McClay et al. [7] reported that IAC stenosis was statistically more common in patients with a diagnosis of sensorineural hearing loss (particularly in syndromic patients, Fig. 7) as compared to patients who were not found to have sensorineural hearing loss [3]. There are often multiple ear anomalies that are present in tandem; more severe grades of the cochlear aplasia and common cavity malformation spectrum were found in patients with stenosis of the fundus of the internal acoustic meatus than in patients with incomplete partition type II and vestibular semicircular canal malformations [3].
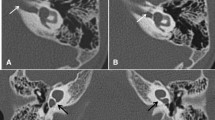
High-resolution petrous temporal bone CT of a 30-month-old girl with bilateral sensorineural hearing loss who had cross-sectional imaging prior to consideration for a cochlear implant. Axial (a) and (b) coronal images demonstrates internal acoustic canal stenosis (arrows) with a canal measuring 1 mm in calibre
High-resolution heavily weighted CISS (constructive interference steady state) MRI sequence of a 5-year-old girl known to have profound bilateral sensorineural hearing loss. a Axial image demonstrates internal acoustic canal stenosis (arrow). b Parasagittal image demonstrates the normal appearance of the internal acoustic canal containing four nerves. c Parasagittal image demonstrates absence of the cochlear nerve, which should be located in an anteroinferior location (arrow) on this projection. A anterior, P posterior
High-resolution petrous temporal bone CT of a 3-year-old boy known to have osteopetrosis who presented with a 2-week history of bilateral facial nerve palsy. Axial (a) and (b) coronal images show a case of internal acoustic canal stenosis (arrows) in a patient with osteopetrosis. c Axial high-resolution CISS (constructive interference steady state) MRI image in the same patient demonstrates paucity of cerebrospinal fluid in the stenotic internal acoustic canal (arrows)
The cochlear nerve canal (the lateral branch of the IAC that transmits the cochlear nerve to the cochlea) is another canal that may demonstrate stenosis or atresia (Fig. 8). Various studies that have attempted to correlate cochlear nerve canal calibre with cochlear nerve hypoplasia have quoted calibres ranging from 1.4 to 1.7 mm as being representative of a stenotic cochlear nerve canal [3].
Axial high-resolution petrous temporal bone CT of a 7-month-old boy who had profound congenital hearing loss and who was undergoing investigations prior to consideration for a cochlear implant. The CT image demonstrates cochlear nerve canal stenosis (arrows) and a thickened modiolus (asterisk) that is replaced by bone
Cochlear nerve aplasia as an associated finding with internal acoustic meatus/cochlear nerve canal stenosis is vital to identify since it is a contraindication for cochlear implantation in patients with severe to profound sensorineural hearing loss [3]. Instead, auditory brainstem implantation is now being considered as a management option for this patient cohort [4]. Patients with cochlear nerve hypoplasia may still benefit from a cochlear implant; however, results are not as good as in those patients with a normal cochlear nerve diameter [3].
IAC widening
IACs can assume fusiform widening of the IAC or a more bulbous configuration with a focal area of widening in the middle third of the canal (Figs. 9, 10 and 11). A widened IAC is frequently found in syndromic patients; the most commonly associated syndromes being Goldenhar syndrome, CHARGE syndrome, Apert syndrome and Patau syndrome [10].
Imaging of a 3-year-old boy with bilateral sensorineural hearing loss who was undergoing imaging prior to consideration for a cochlear implant. a Axial high-resolution CT image of the petrous temporal bone shows a widened and foreshortened internal acoustic canal (bidirectional arrows). In the top right corner, an example of a normal-calibre internal acoustic canal for comparison b Axial heavily T2-weighted image shows the bilateral internal acoustic meatus in the same patient
Imaging of a 3-year-old boy with bilateral sensorineural hearing loss who was being considered for cochlear implantation. a Axial high-resolution petrous temporal bone CT image shows a widened internal acoustic canal in a bulbous configuration (bidirectional arrows). b Parasagittal heavily T2-weighted sequence in the same patient demonstrates the internal acoustic canal as a capacious cerebrospinal fluid-filled cavity as compared to c a normal calibre internal acoustic canal
Axial high-resolution petrous temporal bone CT image of a 3-year-old boy with good hearing who was found to have an opaque lesion behind the left tympanic membrane. The image demonstrates bilateral widened and posteriorly angulated (angled lines to the right internal acoustic canal) internal acoustic canals. Double-ended arrow: left internal acoustic canal
McClay et al. [7] and other authors have widely reported no significant association between an isolated finding of a widened IAC (which is frequently but not always associated with foreshortening and tortuosity of the IAC) and sensorineural hearing loss. Weinberg et al. [11] and Tomura et al. [12] go as far as hypothesizing that a widened IAC that is not associated with sensorineural hearing loss is, in fact, a normal anatomical variant. The clinical importance of noting a widened IAC is that it features as one of the constellation of findings in the rare condition of X-linked stapes gusher. This condition presents clinically as mixed hearing loss in boys. In these cases, the bulbous IAC is associated with incomplete separation of the fundus of the IAC from the basal turn of the cochlea (lateral fundus partial dehiscence) [13]. In this condition, increased communication between the inner ear and the IAC with resultant disruption of the normal pressure gradients causes incremental damage to the cochlear nerve that contributes to the sensorineural limb of the hearing loss whilst stapes footplate fixation causes the conductive component of the hearing loss [13, 14]. The recognition of X-linked stapes gusher is vital in the avoidance of the complication of perilymphatic stapes gushing -- the heavy flow of perilymph and cerebrospinal fluid that occurs after surgical removal of the stapedial footplate. This complication is characterised clinically by worsening hearing loss and dizziness post stapedectomy [15]. In patients with predominantly conductive hearing loss, usage of an external hearing aid is an option whereas patients with a more pronounced sensorineural hearing loss are treated with cochlear implantation (with particular attention so as not to advance the electrode too far centrally into the IAC) [13]. We have not identified any proof of association with sensorineural hearing loss of an isolated finding of a normal-diameter foreshortened, tortuous or posteriorly angulated IAC.
Duplicated IAC
Duplication of the IAC is an extremely rare developmental anomaly of the IAC. The duplicated IAC is seen to originate from the cerebellopontine angle with variable extension towards the inner ear structures. The more anterosuperior of the two canals is representative of the facial nerve canal while the more posteroinferior canal represents the redundant vestibulocochlear nerve component (Fig. 12). Both canals are of soft-tissue density on CT imaging and are generally of smaller calibre than the normal IAC. As was noted previously, an absent vestibulocochlear nerve is often demonstrated on MRI in the stenotic vestibulocochlear component of the IAC (Fig. 12).
Imaging of an 18-month-old girl with bilateral deafness who was being assessed for suitability for cochlear implantation. a Axial high-resolution CT image shows a duplicated internal acoustic canal as an anterior facial nerve canal (thin arrow) and a posterior, stenotic vestibulocochlear nerve canal (thick arrow). b Axial heavily T2-weighted MRI sequence in the same patient shows a single nerve, the facial nerve (arrow), entering the internal acoustic canal at the internal acoustic meatus traversing the facial nerve canal and no vestibulocochlear nerve entering the stenotic vestibulocochlear nerve canal
The clinical significance of a duplicated IAC is the association of the stenotic vestibulocochlear nerve canal with congenital sensorineural hearing loss secondary to cochlear nerve hypoplasia/aplasia.
Conclusion
There is a broad spectrum of abnormalities associated with IAC, some of which have a high frequency of association with sensorineural hearing loss. High-resolution CT imaging of the petrous temporal bone complemented by MRI imaging of the internal acoustic meatus are invaluable in characterizing the constellation of features that are often present in these patients. Recognition by radiologists of these abnormalities and knowledge of this association and their clinical implications is crucial in guiding the clinical management in this cohort of patients.
References
Song JJ, Choi HG, Seung HO et al (2009) Unilateral sensorineural hearing loss in children: the importance of temporal bone computed tomography and audiometric follow-up. Otol Neurotol 30:604–608
Moore JK, Linthicum FHJ (2007) The human auditory system: a timeline of development. Int J Audiol 46:460–478
Li Y, Yang J, Liu J et al (2014) Restudy of malformations of the internal auditory meatus, cochlear nerve canal and cochlear nerve. Eur Arch Otorhinolaryngol. doi:10.1007/s00405-00014-02951-00404
Huang BY, Roche JP, Buchman CA et al (2010) Brain stem and inner ear abnormalities in children with auditory neuropathy spectrum disorder and cochlear nerve deficiency. AJNR Am J Neuroradiol 31:1972–1979
Glastonbury CM, Davidson HC, Harnsberger HR et al (2002) Imaging findings of cochlear nerve deficiency. AJNR Am J Neuroradiol 23:635–643
Marques SR, Ajzen S, D’Ippolito G et al (2012) Morphometric analysis of the internal auditory canal by computed tomography imaging. Iran J Radiol 9:71–78
McClay JE, Tandy R, Grundfast K et al (2002) Major and minor temporal bone abnormalities in children with and without congenital sensorineural hearing loss. Arch Otolaryngol Head Neck Surg 128:664–671
Day JD, Kellogg JX, Fukushima T et al (1994) Microsurgical anatomy of the inner surface of the petrous bone: neuroradiological and morphometric analysis as an adjunct to the retrosigmoid transmeatal approach. Neurosurgery 34:1003–1008
Kesser BW, Raghavan P, Mukherjee S et al (2010) Duplication of the internal auditory canal: radiographic imaging case of the month. Otol Neurotol 31:1352–1353
Bisdas S, Lenarz M, Lenarz T et al (2006) The abnormally dilated internal auditory canal: a non-specific finding or a distinctive pathologic entity. J Neuroradiol 33:269–280
Weinberg PE, Kim KS, Gore RM (1981) Unilateral enlargement of the internal auditory canal: a developmental variant. Surg Neurol 15:39–42
Tomura N, Sashi R, Koboyashi M et al (1995) Normal variations of the temporal bone on high resolution CT: their incidence and clinical significance. Clin Radiol 50:144–148
Kumar G, Castillo M, Buchman CA (2003) X-linked stapes gusher: CT findings in one patient. AJNR Am J Neuroradiol 24:1130–1132
Birman CS, Gibson W (1999) Hearing loss associated with large internal auditory meatus: a report of five pediatric cases. J Laryngol Otol 113:1015–1019
Byron Snow J, Wackym PA, Ballenger JJ (2009) Ballenger’s otorhinolaryngology: head and neck surgery, 17th edn. BC Decker Inc., London, p 295
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Chetcuti, K., Kumbla, S. The internal acoustic canal - another review area in paediatric sensorineural hearing loss. Pediatr Radiol 46, 562–569 (2016). https://doi.org/10.1007/s00247-015-3496-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-015-3496-4