Abstract
Introduction
Aromatase inhibitors (AIs) are increasingly used in children and adolescents to augment adult height. The aim of this study was to investigate the effects AIs have on cardiac morphology, functions and their relation to several metabolic parameters in adolescent boys.
Methods
Three groups matched for sex (boys, n = 67), age (median age 13.5 years), weight, height, body mass index, and puberty stages were enrolled: (i) Group 1: 23 patients using AIs (only AI (n = 6) or in combination with growth hormone (GH) (n = 17)) for at least 6 months; (ii) Group 2: 22 patients using only GH, and (iii) Group 3: 22 healthy boys. Two-dimensional, M-mode conventional Doppler and tissue Doppler examinations of the left ventricle (LV) were performed. Bioelectrical bioimpedance analyses was conducted and follicle-stimulating hormone, luteinizing hormone, total testosterone, lipid, and hemogram parameters were obtained.
Results
Patients in Group 1 had significantly higher serum total testosterone (p < 0.001) and hemoglobin (p < 0.001) levels, fat free mass (p = 0.005), LV mass (LVM) (p = 0.002), as well as increased LV posterior wall diameter (LVPWD) (p = 0.002), interventricular septum diameter (IVSD) (p = 0.019), and myocardial systolic wave velocity (Sm) (p = 0.020) compared to the two other control groups. No significant differences were observed in terms of diastolic and systolic functions and lipid profiles (p > 0.05). There were positive correlations between total testosterone, hemoglobin levels, LVM, LVPWD and IVSD (p < 0.05).
Conclusion
Increased LVM, LVPWD, IVSD and Sm of patients receiving AI therapy in comparison to the control groups, and the significant correlations of these parameters with total testosterone and hemoglobin levels were determined as potential side effects of AIs. These findings emphasize the need of routine cardiac follow-up in patients using AIs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aromatase is a microsomal cytochrome enzyme that catalyzes the aromatization of androgens from estrogens in numerous tissues, such as the ovaries, placenta, brain, fat, and bone [1, 2]. The aromatase enzyme regulates the testosterone-to-estrogen balance, preserves bone mineral structure and maturation, and aids in the maintenance of normal body composition [3]. Loss of function of aromatase causes increased testosterone levels with delayed epiphyseal closure and tall stature, whereas an excess of the enzyme may be associated with elevated estrogen levels and accelerated bone maturation, resulting in short stature [1].
Aromatase inhibitors (AIs) bind irreversibly (type I, steroidal) or reversibly (type II, non-steroidal) to aromatase enzyme to block its activities [2]. They inhibit the conversion of testosterone to 17-β estradiol, resulting in an increase in serum total testosterone levels, and a subsequent increase in 5-α-dihydrotestosterone (DHT), both of which act through androgen receptors [4]. They were initially introduced for the treatment of estrogen-receptor-positive breast cancer, but have since been used off-label for a variety of conditions; including short stature, gynecomastia, both precocious and delayed puberty (pubertal induction), congenital adrenal hyperplasia, aromatase excess syndrome, Peutz-Jeghers syndrome, and testotoxicosis [5, 6]. The non-steroidal reversible inhibitors, anastrozole and letrozole, are often prescribed to adolescent boys with short stature or precocious puberty, with the goal of minimizing the estrogens’ effects on growth by slowing bone maturation [7]. They are commonly used with growth hormone (GH) in order to increase the predicted adult height of adolescents [3, 7].
Since AIs have not been approved by FDA for use in children, there is no pharmacological data available on the optimal dosages, hence the dosage recommendations for adults with potentially maximum effects are being used [3]. Despite high efficacy, there are several reports on the side effects of AIs. Increased testosterone-to-estrogen ratios have been associated with increase in hemoglobin levels, abnormalities in lipid metabolism, decrease in bone mineral density, derangements in bone maturation, and infertility [3, 6, 8, 9]. Fatal cardiovascular problems, including ischemic heart disease, arrhythmias, venous thromboembolism, heart failure, angina, and stroke have been linked to AI treatment among large cohorts of women with breast cancer [10]. However, its adverse effects on cardiovascular system in children and adolescents is yet to be elucidated [11,12,13].
In light of the mounting evidence on the potential risks of these drugs in women with breast cancer [11,12,13], detailed cardiovascular examinations in adolescents using these treatments are needed to ensure their safety in the pediatric group [14]. In this study, we aimed to compare the cardiac morphology and functions and metabolic states of children who had been using AIs for at least six months to those of healthy boys of comparable age, body mass index, and puberty stage. Since AIs are commonly prescribed with GH, we also compared these children to boys with similar characteristics, who had been treated with GH in order to ensure their safety.
Materials and Methods
Patients and Study Design
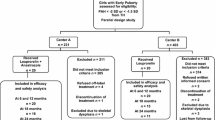
This retrospective observational case-control study was conducted at our pediatric endocrinology and cardiology clinics between January 2021 to January 2023. The study group comprised of 23 boys who had been treated with AI (anastrozole or letrozole; one tablet per day) for at least 6 months (Group I, only AI-treated (n = 6) and both AI- and GH-treated (n = 17)) aged ≥ 9 and < 16 years with bone ages ≤ 14 ½ years who were in puberty and sex- and age-matched boys using only GH (Group II, n = 22) and healthy subjects (Group III, n = 22) with normal puberty served as controls. Individuals with hypertension were not included in the study. Systolic blood pressure and diastolic blood pressure were measured twice at the right arm after a 10 min rest in the supine position using a calibrated sphygmomanometer. Hypertension was defined when blood pressure was ≥ 95th percentile for age, sex, and height on ≥ 3 occasions in children < 13 years of age and ≥ 130/80 for adolescents ≥ 13 years of age [15]. Two patients with conduction rhythm abnormalities (Wolf Parkinson White syndrome (n = 1, Group 2) and complete atrioventricular block (n = 1, Group 2) and one patient (licensed athletic trainer, Group 1) with athlete’s heart) were not included in the study. Individuals with obesity (body mass index (BMI) standard deviation (SD) scores ≥ 2), genetic anomalies, scoliosis, chronic diseases (such as diabetes mellitus or celiac disease), low birth weight, or neoplasia were also excluded from the study. Data on age, gender, anthropometry, body composition, cardiac measurements, and biochemical analysis were collected. All measurements and examinations, including anthropometric evaluations, Pulsed Tissue Doppler Echocardiography, bioelectrical impedance analysis and laboratory evaluations were performed in the morning after an overnight fasting.
AI treatment was prescribed to patients with pubertal gynecomastia (n = 2) and to patients (n = 21) for height gain (GH deficiency (n = 17), idiopathic short stature with rapid pubertal progression (n = 3), and constitutional pubertal delay and growth (n = 1)) [3, 7]. GH deficiency was defined in the evidence of either; short stature or growth deceleration of velocity < 25% of corresponding chronological age with serum peak GH concentration less than 7 ng/ml in two different GH stimulation tests (i.e., clonidine, insulin tolerance test, and levodopa) [16]. Each child with GH deficiency received 25–35 mcg/kg/day of rhGH [17].
Data Collection
Anthropometric Measurements
Participants were weighted with light clothes and without shoes. Height was measured with a sensitivity of 0.1 cm, using a Harpenden stadiometer (cm). Weight was measured using a scale with a sensitivity of 0.1 kg (kg). BMI was calculated as weight per height (kg/m2). The respective SD scores were calculated according to Turkish standards [18]. The following clinical parameters were recorded: age (years); gender; pubertal status [according to Tanner [19]; bone age [calculated according to Greulich and Pyle Atlas [20]], and weight (kg), height (cm), BMI (kg/m2) and the respective SD scores.
Cardiac Evaluation
Conventional Two-dimensional Doppler Echocardiography
The same pediatric cardiologist (YDA), under the supervision of MK, performed all M-mode and two-dimensional echocardiograms and Doppler analyses on all subjects using a commercially accessible device (IE 33, Philips Ultrasound system, Bothell, Washington, USA; equipped with a S 5 − 1 standard sector probe) [21]. Before imaging, all individuals rested for 10 min and echocardiographic examinations were conducted in left lateral decubiti’s position.
Two-dimensional parasternal long-axis and short-axis views and apical two-chamber and four-chamber images were acquired using standard transducer positions. The following parameters were measured: (i) Left ventricle (LV) morphological parameters (interventricular septum (IVS) thickness, LV posterior wall diameter, LV end diastolic diameter (LVEDD), LV end-systolic diameter (LVESD)), (ii) systolic parameters (LV ejection fraction, per cent fractional LV shortening, ejection time (ET)) and (iii) diastolic parameters (Mitral early diastolic velocity (peak E), late diastolic velocity (peak A)).
Indicative of diastolic function, the E/A ratio, was computed by dividing peak E by peak A. Peak A and peak E transmitral peak flow velocities were measured using pulsed wave Doppler in an apical four-chamber view. LV mass (grams) was measured using M-mode echocardiography [22]. Devereux’s formula was used to determine LVM index, by which LV mass was indexed to height raised to allometric power of 2.7 (grams/meters2.7) [22, 23]. Mitral DT was measured in milliseconds. The LV dimensions were reported in centimeters, and z-scores were computed using published normal values [24]. The fraction of shortening was computed as (LVEDD - LVESD)/LVEDD. Myocardial performance index (MPI), demonstrating overall ventricular (systolic and diastolic) function, was calculated by measuring mitral inflow Doppler tracings (interval a = isovolumic relaxation time (IVRT) + isovolumic contraction time (IVCT) + ET and LV outflow (interval b = ET). Interval ‘a’ defined the time interval between two consecutive mitral inflow velocity tracings (from cessation to onset of mitral inflow). The LV outflow velocity trace (ET, or interval ‘b’) was acquired from apical 5-chamber view with the Doppler sample volume positioned just below the aortic valve. MPI was calculated using the method MPI = a-b/b [25]. All of these time intervals represented the average of five measurements taken during consecutive cardiac cycles [21].
Pulsed Tissue Doppler Echocardiography
To evaluate systolic and diastolic mitral annular velocities, tissue Doppler echocardiography (TDE) was used [21]. The sample volume was deposited on the septal and lateral mitral annulus locations in the apical four chamber view. Each subject’s systolic (Sm, IVCTm, ET) and diastolic (E’m, A’m, and IVRTm) velocities and intervals were recorded from both the mitral annulus and the interventricular septum. E’m/A’m ratio suggesting LV diastolic function and ventricular filling was calculated by dividing E’m by A’m. By dividing the sum of IVCT and IVRT by ET, the MPI was computed. LV diastolic function, calculated by pulsed wave Doppler-derived E-wave velocity/TDE-derived E’m velocity ratio was calculated as well.
Bioelectrical Impedance Analysis data
Bioimpedance analysis was performed using Body Composition Analyzer TANITA BC-418 MA model single frequency (50 Hz) (Tanita Corporation, Tokyo, Japan). The eight electrode bioelectrical impedance analysis was performed by the same person following the operating principles of the device [26]. Body fat (FM, kg), body fat mass percentage (FMP, %), fat free mass (FFM, kg), total body water (TBW, kg), and appendicular skeletal muscle mass (ASMM, the sum of muscle mass of four limbs, kg) were recorded. Muscle-to-fat ratio was also calculated [MFR = (ASMM (kg)/ FM (kg)] [27].
Laboratory Data
All previous biochemical and hormonal evaluations were obtained from patient files. Serum triglyceride (mg/dL), total cholesterol (mg/dL), high density lipoprotein (mg/dL), low-density lipoprotein (mg/dL) levels were recorded. Serum levels of luteinizing hormone (LH, mIU/mL), follicle-stimulating hormone (FSH, mIU/mL), total testosterone (ng/dL) and hemoglobin (g/dL) levels were also recorded.
Sample Size Calculation
An a priori power analysis was conducted using G*Power program version 3.1.9.4 for Windows to determine the minimum sample size required to test the study hypothesis [28]. There were no previous similar cross sectional analyses to inform our power calculations. Using Cohen’s standard effect sizes [29], in order to detect a large effect size (f = 0.4), the total sample size required was calculated to be 64 participants (21 in each of the three groups) to have 80% power at the 5% alpha level.
Ethical Approval
The study was approved by the local ethics committee of Dokuz Eylül University Faculty of Medicine (Ethical approval number: 2023/04–18). Written informed consents of all the parents were obtained prior to the study.
Statistical Analysis
Statistical analyses were performed using SPSS v.24 for Windows. Clinical variables were tested for normality using the Kolmogorov-Smirnov test. All variables complied with nonsymmetrical distribution. Continuous variables were expressed as median with interquartile ranges (IQR). In order to compare the three groups, Kruskal-Wallis analysis was performed and Dunn’s multiple comparison post-hoc test was used for nonparametric pairwise multiple comparisons. The associations between total testosterone, hemoglobin and cardiac parameters were performed with Spearman’s correlation. For all tests, p < 0.05 was considered statistically significant.
Results
The study included 23 boys [Group I, median (IQR) age of 13.5 (1.9) years] using AI (anastrozole (n = 16) or letrozole (n = 7)), 22 boys [Group II, median (IQR) age of 13.3 (2.7) years] using GH, and 22 healthy controls [Group III, median (IQR) age of 13 (1.5) years]. The duration of treatment for boys treated only with AI (n = 6) and both AI + GH (n = 17) were 14 (22.3) and 12 (13.5) months, respectively. Overall the duration of AI for Group I (n = 23) and GH for Group II (n = 22) groups were 12 (15) and 24 (60) months respectively. There was statistically no significant difference between the groups regarding age, puberty stages, SD scores for weight, height and BMI (p > 0.05) (Table 1). None of the participants have reported any adverse cardiac events or symptoms, including palpitations, fluttering, chest pain, shortness of breath, or dizziness. None of the participants had hypertension (Table 1) and the participants did not report to be involved in extensive physical activity.
Serum levels of FSH, LH, total testosterone, and hemoglobin were significantly higher in Group I compared to control groups (p < 0.001) (Table 1). The median (IQR) level of total testosterone was 531 (512) ng/dL, five of whom had levels greater than 1000 ng/dL in Group I (n = 23); while in the control Groups of I and II the medians were 187 (323) and 207 (308) ng/dL, respectively, with levels ranging from 9 to 694 ng/dL (Fig. 1). The median testosterone levels were similar between patients using anastrozole (n = 16) and letrozole (n = 7) (514 (319) vs. 1205 (927) ng/dL, p = 0.082).
The comparison of the levels of total testosterone (ng/dL) (Fig. 1a) and left ventricle mass (grams) (Fig. 1b) measurements between groups. The whiskers in boxes indicate minimum to maximum levels. Group I, patients using only aromatase inhibitor (n = 6) or both aromatase inhibitor + growth hormone (n = 17); Group I-A, patients using anastrozole (n = 16), Group I-L, patients using letrozole (n = 7), Group II, patients using growth hormone (n = 22); Group III, healthy controls (n = 22). ns: not statistically significant (p > 0.05); **p < 0.01; *p = 0.01
All parameters (FM, FMP, FFM, TBW, MFR, and ASMM) of bioelectrical impedance analysis were similar between Groups I and II, while patients in Group I had significantly higher FFM, TBW, and ASMM than both Groups II and III (Table 1). The levels of total testosterone were positively associated with FFM, TBW, and ASMM (Spearman’s correlation (rs) = 0.621, (rs) = 0.626, (rs) = 0.604; p < 0.001), while there was no association with FM ((rs) = 0.161, p = 0.19). FFM was positively correlated with LVEDD (rs = 0.651, p = < 0.01), LVESD (rs = 0.499 p < 0.01), IVSD (rs = 0.651, p < 0.001), LVPWD (rs = 0.674, p < 0.001) and LVM (rs = 0.815, p < 0.001), while other parameters (FS, EF, Peak E, Peak A, E/A, MPI) were not found to have any correlations.
Patients in Group I, had significantly higher LVM and LVMI compared to both groups of II and III, while IVSD and LVPWD of Group I were also higher than those in Group II (Table 2). LVM of 13% (n = 3; anastrozole (n = 2), letrozole (n = 1)) of the patients who received AI treatment were above the reference limits for age and height, while the parameters of all other patients were within the reference ranges. The median LVM were similar between patients using anastrozole and letrozole (100 (27) vs. 111 (24) grams, p = 0.133). Other parameters (LVEDD, LVESD, FS, EF, Peak E, Peak, E/A, MPI) of M-mode and Doppler echocardiography were similar in all groups (Table 2). Total testosterone levels were positively correlated with LVEDD, LVESD, IVSD, LVPWD, LVM ((rs) = 0.408, p = 0.001; (rs) = 0.335, p = 0.006; (rs) = 0.517, p < 0.001; (rs) = 0.481, p < 0.001, respectively). Total testosterone was strongly associated with Hb levels ((rs) = 0.635, p < 0.001). Hb levels were also correlated with LVEDD, IVSD, LVPWD, and LVM. ((rs) = 0.266, p = 0.03; (rs) = 0.410, p = 0.001; LVPWD (rs) = 0.429, p < 0.001; 0.475, p < 0.001). There was no correlation between the duration of treatment and total testosterone levels, LVM, and LVMI (p = 0.26, p = 0.64, p = 0.66).
The parameters (IVCTm, ET, E’m, A’m, IVRT, MPI) regarding the lateral localisations of the mitral annulus or the interventricular septum were not different between all three groups (p > 0.05, Table 3). Patients in Group I had longer Sm time than those in Group III (11.4 (2.9) vs. 9.6 (2.2) cm/s, p = 0.02). Total testosterone levels were positively correlated with Sm, MPI, IVRTm ((rs) = 0.434, p < 0.001; (rs) = 0.279, p = 0.019; (rs) = 0.305; p = 0.012), but not with IVCTm, ET, E’m, A’m.
Discussion
This study evaluates the effects of AIs by comparing the data on conventional two-dimensional and pulsed tissue Doppler echocardiography, bioelectrical bioimpedance and laboratory analysis of 67 age-matched adolescent boys with similar characteristics. The results demonstrate that adolescents on AI treatment show significantly increased levels of serum total testosterone and hemoglobin in association with significant changes in cardiac morphology in comparison to controls.
In our cohort, total testosterone levels were significantly elevated in patients using AIs, compared to those patients not receiving AIs. A quarter of patients’ testosterone levels, who were using AIs, had reached levels above 1000 ng/dL, and despite not reaching statistical significance, patients using letrozole had higher median levels in comparison to patients using anastrozole. Testosterone levels exceeding the upper limits of normal have been reported as a potential side effect of aromatase inhibitors [30, 31]. However, these exceedingly high androgen levels are particularly noteworthy, since various tissues and systems express androgen receptors; including bone, muscle, adipose tissue, reproductive, immune, neural, hematopoetic and cardiovascular systems [32]. While increased testosterone levels, and subsequently elevated DHT, both act through androgen receptors; the interactions between androgens and androgen receptors are complex with diversities in ligand-receptor reactions [32]. It has been demonstrated that increased levels of androgens may upregulate androgen receptor levels; both by increasing receptor half-lives and rates of receptor synthesis [33]. Further, there are ligand dependent and independent pathways regarding androgen and androgen receptor interactions, along with DNA binding and non-DNA binding mechanisms; each unique to different tissue types [32]. Hence, the impacts of androgens on different tissues may vary unpredictably. Given that the patients using letrozole had comparably higher median testosterone levels, anastrozole seems to be a safer treatment option for adolescent boys.
We also demonstrated increases in FSH, LH and hemoglobin levels compared to control groups. Even though the levels did not exceed the upper limits of normal, these modest increases were strongly positively correlated with testosterone levels, similar to previous studies [14, 31]. The increases in FSH and LH have not been found to be detrimental to future spermatogenesis capacity; on the contrary, Kohva et al. [34] had even suggested that it could actually improve Sertoli cell proliferation. On the other hand, erythrocytosis caused by increased testosterone levels [6], may pose risks due to potentially increased rates of thrombocytosis [35]. Therefore, the increases in hemoglobin levels should be cautiously monitored.
Our results have shown that adolescent males in Group 1 had higher FFM, TBW, and ASMM compared to control groups. Further, levels of testosterone were positively correlated with ASMM and FFM in adolescents. Several parameters in bioelectrical bioimpedance analysis have been associated with serious conditions in adults, including cardiovascular risks, mortality, coronary calcification, and heart failure, among others[36]. Although, MFR were similar between groups in our analysis, patients in Groups 1 had higher FFM and ASMM, indicating a relatively increased muscle mass compared to other groups. However, none of these patients had levels greater than the previously reported reference ranges for this age group [37]. The associations between sex hormones and muscle mass has been primarily studied in older men and women, but scarcely explored in adolescents [38,39,40]. While a meta-analysis by Sasha et al. [41] found no association between testosterone and muscle mass in older women, Auyeung et al. [42] reported that testosterone was related to both muscle mass and strength among 1489 older man. Similar to our results, a study by Xu et al. [39] reported a strong association between total testosterone and muscle strength in male adolescents.
In our study, in comparison to control groups, patients receiving AIs had a significantly higher LVM, LVMI, as well as thicker LVPWD, and IVSD. These measures were also strongly correlated with total testosterone levels. Even though, all participants in our study had normal levels of blood pressure and had reported not to be involved with extensive physical activities, it is important to note that the anatomy of heart can be influenced by blood pressure and physical activity, as shown by the correlations with LVM in previous studies [43, 44]. The effects of sex hormones on both the anatomy and functions of heart is still a matter of debate [45,46,47,48]. Testosterone and its active metabolite DHT have direct anabolic effects on myocytes, which may lead to myocardial hypertrophy [49, 50]. Oral testosterone administration was not found to have any adverse effects on cardiac functions in 16 pediatric individuals treated over a median duration of 2.5 years [51]. However, among healthy men aged 25–84 years, a negative association with testosterone levels and LVM was reported [47], while a positive association was demonstrated with RVM [46]. In contrast, Subramanya et al. [52] demonstrated among 1941 women and 2221 men aged 45–84 years, higher testosterone levels were associated with a greater increase in LVM. However, these studies investigated the relationship of sex hormones with cardiac profiles in healthy men and postmenopausal women; while a different spectrum of changes had been demonstrated with supra-physiological testosterone doses. In consistent with our findings, supra-physiological androgen levels, mostly reported in male athletes, have been linked to left ventricular hypertrophy with systolic and diastolic dysfunctions in several studies [48, 53,54,55]. Similarly, Pirgon et al.[56] demonstrated a rapid growth of LV, due to increased total testosterone levels, after human chorionic gonadotropin injection in boys with cryptorchidism. Further, at the extreme ends of the spectrum, the patients with aromatase deficiency were reported to be at risk for the development of cardiovascular diseases and clinical follow-up was recommended [57].
This is also the first study to evaluate the ventricular functions using TDE in children treated with AIs. TDE, which quantifies myocardial velocities to assess segmental and global ventricular functions [58], has been extensively studied in several areas [21, 59,60,61,62]. We showed that other than systolic lateral mitral annulus velocity wave (Sm), all TDI parameters were similar in patients using AI as compared to the other groups. Sm, which measures the peak velocity of cardiac fiber shortening in the longitudinal direction [63], was significantly higher in patients using AI, despite being within reference ranges [64]. Significant differences in motion measurements evaluated at different regions, such as lateral, posterior, anterior walls have previously been documented [65, 66]. We hypothesize, that the comparable measurements of annulus motion of IVS between the three groups can be explained by the supporting role of the normal right ventricular functions. Given that TDE is able to detect abnormalities even at subclinical stages and increased peak mitral annulus velocity has been shown to be an independent predictor of mortality, different lateral mitral Sm measurements may be noteworthy [67]. Relatively increased Sm levels could be explained in light of the positive inotropic effects of testosteron, which are due to increased and rapid rates of calcium removal [68,69,70]. In line with these findings, Wadthaison et al. [68] demonstrated in vivo that after the initial beneficial state following testosterone treatment, cardiac pump function, myocyte contractility, and cardiac myofilament functions were depressed significantly after 12 weeks of treatment. This had been postulated to be the result of a maladaptive process, occurring due to reduced cardiac Troponin-I phosphorylation, which leads to decreased myofilament function [68]. Duration of testosterone administration have also been highlighted as a risk factor for the development of cardiac fibrosis [68, 71]. AIs, which are administered for longer durations, increase testosterone levels above reference intervals, with some exceeding 1000 ng/dL, resulting in increased Sm levels; therefore, it is reasonable to assume that these adolescents may also experience these adverse effects; it would be prudent to monitor their cardiac functions for longer durations.
Endogenous testosterone has several beneficial effects on cardiovascular system, and low levels are associated with an elevated risk of cardiovascular death [72]. Despite the independent causal relationship between DHT and cardiovascular disease, stroke, and all-cause mortality [73], significant associations have not been made with total testosterone levels [74]. Due to clinical heterogeneity and variable methodology, the association of cardiovascular risks with testosterone treatment have been considered to be controversial [53, 75, 76]. While previous studies and meta-analyses have found no significant differences in testosterone related cardiovascular events, a meta-analysis by Borst et al. [77] has revealed statistically significant cardiovascular risks linked with oral testosterone forms, demonstrating that not only the serum levels, but also the routes of administration may be associated with adverse outcomes. However, it should be noted that the majority of these studies included men aged 65 to 97 years, with modestly elevated testosterone levels [73]. Therefore, the supra-physiological levels created with an oral medication by inhibiting a crucial enzyme of testosterone-estrogen balance might influence the cardiovascular system to a greater extent, and uncertain cardiovascular risks might be observed.
Study Strengths and Limitations
The main strength of our study is that, to our knowledge, it is the first to evaluate and compare all parameters related to AI use, including cardiac structure, function, clinical features, and laboratory data, in a holistic way. The homogeneity of all groups in terms of age, sex, puberty and blood pressure, all of which are potential confounding factors influencing the anatomical parameters of heart, including the LVM, increases the strength of our findings in the study. However, there were some limitations too. Given that total testosterone exists both in inactive and active bioavailable forms; the measurement of free testosterone or DHT would have been more reliable markers in our study. Further, the patients in the Group I were receiving both AI and GH treatments, hence it could be argued that it would be impossible to designate whether AIs alone had caused these alterations or they were the consequences of the synergistic adverse effects of both treatments on heart. Moreover, we speculated that the statistical significance was not attained between patients using letrozole and anastrozole, due to small numbers of patients in each subgroup. Homogenous studies with greater patient numbers comparing the effects of these drugs separately are needed. Finally, despite the statistically significant difference between several anatomical features, the findings should be interpreted carefully, since parameters such as LVMI were below the thresholds designated for a pathological increase. On the other hand, the probability that they might actually reach such high levels after longer terms of use cannot be ruled out among these pediatric cases using off-label medications; hence longer follow-up periods in future studies are warranted. Therefore, we have planned to monitor all our patients using AIs prospectively periodically for longer durations to document the reversibility of the changes in cardiac morphology, the progression of the increase in LVM, the correlation of these changes with respect to drug administration and dosage, and the occurrence of further cardiac events.
Conclusion
This is the first study to date evaluating the cardiac morphology and functions of adolescent boys using AIs. In this study, we found that (i) LVM, LVMI, LVPWD and IVSD were significantly higher in the patient group receiving AI therapy compared to the control groups and that (ii) these parameters were significantly correlated with serum total testosterone and hemoglobin levels. These significant differences and correlations were evaluated as potential side effects of AIs. Patients receiving AI should be regularly monitored for potential long-term cardiovascular morbidity and reversibility of the existing condition.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Miller WL, Auchus RJ (2011) The Molecular Biology, Biochemistry, and physiology of human steroidogenesis and its Disorders. Endocr Rev 32:81–151. https://doi.org/10.1210/er.2010-0013
Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A (2009) History of aromatase: Saga of an important Biological Mediator and Therapeutic Target. Endocr Rev 30:343–375. https://doi.org/10.1210/er.2008-0016
Wit JM (2021) Should skeletal maturation be manipulated for Extra Height Gain? Front Endocrinol (Lausanne) 12:812196. https://doi.org/10.3389/fendo.2021.812196
Rana K, Davey RA, Zajac JD (2014) Human androgen deficiency: insights gained from androgen receptor knockout mouse models. Asian J Androl 16:169–177. https://doi.org/10.4103/1008-682x.122590
Hero M, Varimo T, Raivio T (2020) Aromatase inhibitors in puberty. Curr Opin Endocr Metabolic Res 14:37–41. https://doi.org/10.1016/j.coemr.2020.04.001
Wit JM, Hero M, Nunez SB (2012) Aromatase inhibitors in pediatrics. Nat Reviews Endocrinol 8:135–147. https://doi.org/10.1038/nrendo.2011.161
Mauras N, Gonzalez de Pijem L, Hsiang HY, Desrosiers P, Rapaport R, Schwartz ID et al (2008) Anastrozole increases predicted adult height of short adolescent males treated with growth hormone: a Randomized, Placebo-Controlled, Multicenter Trial for one to three years. J Clin Endocrinol Metabolism 93:823–831. https://doi.org/10.1210/jc.2007-1559
Hero M, Ankarberg-Lindgren C, Taskinen MR, Dunkel L (2006) Blockade of oestrogen biosynthesis in peripubertal boys: effects on lipid metabolism, insulin sensitivity, and body composition. Eur J Endocrinol 155:453–460. https://doi.org/10.1530/eje.1.02226
Hero M, Wickman S, Hanhijärvi R, Siimes MA, Dunkel L (2005) Pubertal upregulation of erythropoiesis in boys is determined primarily by androgen. J Pediatr 146:245–252. https://doi.org/10.1016/j.jpeds.2004.09.002
Sund M, Garcia-Argibay M, Garmo H, Ahlgren J, Wennstig AK, Fredriksson I et al (2021) Aromatase inhibitors use and risk for cardiovascular disease in breast cancer patients: a population-based cohort study. Breast 59:157–164. https://doi.org/10.1016/j.breast.2021.07.004
Boszkiewicz K, Piwowar A, Petryszyn P (2022) Aromatase inhibitors and risk of Metabolic and Cardiovascular adverse Effects in breast Cancer Patients-A systematic review and Meta-analysis. J Clin Med 11. https://doi.org/10.3390/jcm11113133
Sun JC, Sun ZF, He CJ, Zhai CL, Qian G (2022) Association between Aromatase inhibitors and myocardial infarction morbidity in women with breast Cancer: a Meta-analysis of Observational Studies. Cancer Control 29:10732748221132512. https://doi.org/10.1177/10732748221132512
Yu Q, Xu Y, Yu E, Zheng Z (2022) Risk of cardiovascular disease in breast cancer patients receiving aromatase inhibitors vs. tamoxifen: a systematic review and meta-analysis. J Clin Pharm Ther 47:575–587. https://doi.org/10.1111/jcpt.13598
Dutta D, Singla R, Surana V, Sharma M (2022) Efficacy and safety of Letrozole in the management of constitutional Delay in Growth and Puberty: a systematic review and Meta-analysis. J Clin Res Pediatr Endocrinol 14:131–144. https://doi.org/10.4274/jcrpe.galenos.2021.2021.0169
Lande MB, Batisky DL (2019) New American Academy of Pediatrics Hypertension Guideline. Hypertension 73:31–32. https://doi.org/10.1161/HYPERTENSIONAHA.118.11819
Hage C, Gan H-W, Ibba A, Patti G, Dattani M, Loche S et al (2021) Advances in differential diagnosis and management of growth hormone deficiency in children. Nat Reviews Endocrinol 17:608–624. https://doi.org/10.1038/s41574-021-00539-5
Collett-Solberg PF, Ambler G, Backeljauw PF, Bidlingmaier M, Biller BMK, Boguszewski MCS et al (2019) Diagnosis, Genetics, and therapy of short stature in children: a growth hormone Research Society International Perspective. Horm Res Paediatr 92:1–14. https://doi.org/10.1159/000502231
Demir K, Konakci E, Ozkaya G, Kasap Demir B, Ozen S, Aydin M et al (2020) New features for Child Metrics: further growth references and blood pressure calculations. J Clin Res Pediatr Endocrinol 12:125–129. https://doi.org/10.4274/jcrpe.galenos.2019.2019.0127
Tanner JM, Whitehouse RH (1976) Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child 51:170–179. https://doi.org/10.1136/adc.51.3.170
Greulich WW, Pyle SI (1959) Radiographic atlas of skeletal development of the hand and wrist. Stanford university press
Çatli G, Kir M, Anik A, Yilmaz N, Böber E, Abaci A (2015) The effect of L-thyroxine treatment on left ventricular functions in children with subclinical hypothyroidism. Arch Dis Child 100:130–137. https://doi.org/10.1136/archdischild-2014-306381
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16:233–270. https://doi.org/10.1093/ehjci/jev014
Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I et al (1986) Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 57:450–458. https://doi.org/10.1016/0002-9149(86)90771-x
Kampmann C, Wiethoff CM, Wenzel A, Stolz G, Betancor M, Wippermann CF et al (2000) Normal values of M mode echocardiographic measurements of more than 2000 healthy infants and children in central Europe. Heart 83:667–672. https://doi.org/10.1136/heart.83.6.667
Murphy GS, Marymont JH, Szokol JW, Avram MJ, Vender JS (2007) Correlation of the myocardial performance index with conventional echocardiographic indices of systolic and diastolic function: a study in cardiac surgical patients. Echocardiography 24:26–33. https://doi.org/10.1111/j.1540-8175.2007.00346.x
Kurtoglu S, Mazicioglu MM, Ozturk A, Hatipoglu N, Cicek B, Ustunbas HB (2010) Body fat reference curves for healthy turkish children and adolescents. Eur J Pediatr 169:1329–1335. https://doi.org/10.1007/s00431-010-1225-4
McCarthy HD, Samani-Radia D, Jebb SA, Prentice AM (2014) Skeletal muscle mass reference curves for children and adolescents. Pediatr Obes 9:249–259. https://doi.org/10.1111/j.2047-6310.2013.00168.x
Faul F, Erdfelder E, Buchner A, Lang A-G (2009) Statistical power analyses using G* power 3.1: tests for correlation and regression analyses. Behav Res Methods 41:1149–1160
Cohen J (2013) Statistical power analysis for the behavioral sciences. Routledge
Salehpour S, Alipour P, Razzaghy-Azar M, Ardeshirpour L, Shamshiri A, Monfared MF et al (2010) A double-blind, placebo-controlled comparison of letrozole to oxandrolone effects upon growth and puberty of children with constitutional delay of puberty and idiopathic short stature. Horm Res Paediatr 74:428–435. https://doi.org/10.1159/000315482
Hero M, Wickman S, Dunkel L (2006) Treatment with the aromatase inhibitor letrozole during adolescence increases near-final height in boys with constitutional delay of puberty. Clin Endocrinol 64:510–513. https://doi.org/10.1111/j.1365-2265.2006.02499.x
Davey RA, Grossmann M (2016) Androgen receptor structure, function and Biology: from bench to Bedside. Clin Biochem Rev 37:3–15
Syms AJ, Norris JS, Panko WB, Smith RG (1985) Mechanism of androgen-receptor augmentation. Analysis of receptor synthesis and degradation by the density-shift technique. J Biol Chem 260:455–461
Kohva E, Varimo T, Huopio H, Tenhola S, Voutilainen R, Toppari J et al (2020) Anti-Müllerian hormone and letrozole levels in boys with constitutional delay of growth and puberty treated with letrozole or testosterone. Hum Reprod 35:257–264. https://doi.org/10.1093/humrep/dez231
Diaz-Thomas A, Shulman D (2010) Use of aromatase inhibitors in children and adolescents: what’s new? Curr Opin Pediatr 22:501–507. https://doi.org/10.1097/MOP.0b013e32833ab888
Popiolek-Kalisz J, Szczygiel K (2023) Bioelectrical Impedance Analysis and Body Composition in Cardiovascular Diseases. Curr Probl Cardiol 48:101911. https://doi.org/10.1016/j.cpcardiol.2023.101911
Gultekin T, Dasgupta P, Koca Ozer B (2014) Segmental bioelectrical impedance analysis in children aged 7–18 years living in Ankara-Turkey: age and sex difference in the measures of adiposity. Papers on Anthropology 23:23. https://doi.org/10.12697/poa.2014.23.2.02
Almeida-Neto PF, de Matos DG, Pinto VCM, Dantas PMS, Cesario TM, da Silva LF et al (2020) Can the neuromuscular performance of young athletes be influenced by hormone levels and different stages of Puberty? Int J Environ Res Public Health 17. https://doi.org/10.3390/ijerph17165637
Xu Y, Wen Z, Deng K, Li R, Yu Q, Xiao SM (2021) Relationships of sex hormones with muscle mass and muscle strength in male adolescents at different stages of puberty. PLoS ONE 16:e0260521. https://doi.org/10.1371/journal.pone.0260521
Hou WW, Tse MA, Lam TH, Leung GM, Schooling CM (2015) Adolescent testosterone, muscle mass and glucose metabolism: evidence from the ‘Children of 1997’ birth cohort in Hong Kong. Diabet Med 32:505–512. https://doi.org/10.1111/dme.12602
Taylor S, Islam RM, Bell RJ, Hemachandra C, Davis SR (2023) Endogenous testosterone concentrations and muscle mass, strength and performance in women, a systematic review of observational studies. Clin Endocrinol (Oxf) 98:587–602. https://doi.org/10.1111/cen.14874
Auyeung TW, Lee JS, Kwok T, Leung J, Ohlsson C, Vandenput L et al (2011) Testosterone but not estradiol level is positively related to muscle strength and physical performance independent of muscle mass: a cross-sectional study in 1489 older men. Eur J Endocrinol 164:811–817. https://doi.org/10.1530/eje-10-0952
Joseph G, Marott JL, Biering-Sørensen T, Johansen MN, Saevereid HA, Nielsen G et al (2020) Level of physical activity, left ventricular Mass, Hypertension, and prognosis. Hypertension 75:693–701. https://doi.org/10.1161/HYPERTENSIONAHA.119.14287
Lovic D, Narayan P, Pittaras A, Faselis C, Doumas M, Kokkinos P (2017) Left ventricular hypertrophy in athletes and hypertensive patients. J Clin Hypertens (Greenwich) 19:413–417. https://doi.org/10.1111/jch.12977
Yeo Y, Park SW, Lee SC, Song YM (2022) The relationship between serum sex hormone and cardiac echocardiographic findings in healthy men. Sci Rep 12:12787. https://doi.org/10.1038/s41598-022-17101-6
Ventetuolo CE, Ouyang P, Bluemke DA, Tandri H, Barr RG, Bagiella E et al (2011) Sex hormones are associated with right ventricular structure and function: the MESA-right ventricle study. Am J Respir Crit Care Med 183:659–667. https://doi.org/10.1164/rccm.201007-1027OC
Svartberg J, von Mühlen D, Schirmer H, Barrett-Connor E, Sundfjord J, Jorde R (2004) Association of endogenous testosterone with blood pressure and left ventricular mass in men. The Tromsø Study. Eur J Endocrinol 150:65–71. https://doi.org/10.1530/eje.0.1500065
Čulić V (2015) Androgens in cardiac fibrosis and other cardiovascular mechanisms. Int J Cardiol 179:190–192. https://doi.org/10.1016/j.ijcard.2014.11.079
Schafstedde M, Nordmeyer S (2023) The role of androgens in pressure overload myocardial hypertrophy. Front Endocrinol 14. https://doi.org/10.3389/fendo.2023.1112892
Marsh JD, Lehmann MH, Ritchie RH, Gwathmey JK, Green GE, Schiebinger RJ (1998) Androgen receptors mediate hypertrophy in cardiac myocytes. Circulation 98:256–261. https://doi.org/10.1161/01.cir.98.3.256
Lee SL, Lim A, Munns C, Simm PJ, Zacharin M (2020) Effect of Testosterone treatment for delayed puberty in Duchenne muscular dystrophy. Horm Res Paediatr 93:108–118. https://doi.org/10.1159/000508290
Subramanya V, Zhao D, Ouyang P, Lima JA, Vaidya D, Ndumele CE et al (2018) Sex hormone levels and change in left ventricular structure among men and post-menopausal women: the multi-ethnic study of atherosclerosis (MESA). Maturitas 108:37–44. https://doi.org/10.1016/j.maturitas.2017.11.006
Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM et al (2010) Adverse events associated with testosterone administration. N Engl J Med 363:109–122. https://doi.org/10.1056/NEJMoa1000485
D’Andrea A, Caso P, Salerno G, Scarafile R, De Corato G, Mita C et al (2007) Left ventricular early myocardial dysfunction after chronic misuse of anabolic androgenic steroids: a Doppler myocardial and strain imaging analysis. Br J Sports Med 41:149–155. https://doi.org/10.1136/bjsm.2006.030171
Smit DL, Voogel AJ, den Heijer M, de Ronde W (2021) Anabolic androgenic steroids induce reversible left ventricular hypertrophy and Cardiac Dysfunction. Echocardiography results of the HAARLEM Study. Front Reprod Health 3:732318. https://doi.org/10.3389/frph.2021.732318
Pirgon O, Atabek ME, Oran B, Suleymanoglu S, Meral C (2009) Treatment with human chorionic gonadotropin induces left ventricular mass in cryptorchid boys. J Pediatr Endocrinol Metab 22:449–454. https://doi.org/10.1515/jpem.2009.22.5.449
Maffei L, Murata Y, Rochira V, Tubert G, Aranda C, Vazquez M et al (2004) Dysmetabolic syndrome in a man with a Novel mutation of the Aromatase Gene: Effects of Testosterone, Alendronate, and Estradiol Treatment. J Clin Endocrinol Metabolism 89:61–70. https://doi.org/10.1210/jc.2003-030313
McDicken WN, Moran CM, Hoskins PR, Monaghan MJ, Sutherland GR (1996) New technology in echocardiography II: imaging techniques. Heart 75:9–16. https://doi.org/10.1136/hrt.75.6_suppl_2.9
Zeybek C, Yalcin Y, Erdem A, Polat TB, Aktuglu-Zeybek AC, Bayoglu V et al (2007) Tissue doppler echocardiographic assessment of cardiac function in children with bronchial asthma. Pediatr Int 49:911–917. https://doi.org/10.1111/j.1442-200X.2007.02486.x
von der Born J, Baberowski S, Memaran N, Grams L, Homeyer D, Borchert-Mörlins B et al (2022) Impact of sex and obesity on echocardiographic parameters in children and adolescents. Pediatr Cardiol 43:1502–1516. https://doi.org/10.1007/s00246-022-02876-2
Bressieux-Degueldre S, Fenton M, Dominguez T, Burch M (2022) Exploring the possible impact of echocardiographic diastolic function parameters on Outcome in Paediatric Dilated Cardiomyopathy. Child (Basel) 9. https://doi.org/10.3390/children9101500
Isa Tafreshi R, Radgoodarzi M, Arjmandi Rafsanjani K, Soheilipour F (2022) Subclinical left ventricular dysfunction in children and adolescence with Thalassemia Intermedia. Front Pediatr 10:774528. https://doi.org/10.3389/fped.2022.774528
Kaye AD, Hoover JM, Baber SR, Ibrahim IN, Fields AM (2004) The effects of load on systolic mitral annular velocity by tissue doppler imaging. Anesth Analg 99:758–763. https://doi.org/10.1213/01.ane.0000131972.99804.28
Koestenberger M, Nagel B, Ravekes W, Avian A, Cvirn G, Rehak T et al (2014) Reference values of the mitral annular peak systolic velocity (sm) in 690 healthy Pediatric patients, calculation of Z-Score values, and comparison to the mitral annular plane systolic excursion (MAPSE). Echocardiography 31:1122–1130. https://doi.org/10.1111/echo.12541
Sharif D, Sharif-Rasslan A, Shahla C (2011) Mitral annular systolic velocities predict left ventricular wall motion abnormality during dobutamine stress Echocardiography. Cardiol Res 2:16–26. https://doi.org/10.4021/cr14w
Borges MCC, Colombo RCR, Gonçalves JGF, Ferreira JdO, Franchini KG (2006) Longitudinal mitral Annulus velocities are reduced in hypertensive subjects with or without left ventricle hypertrophy. Hypertension 47:854–860. https://doi.org/10.1161/01.HYP.0000216123.57284.b0
Weng L, Liu YT, Du B, Zhou JF, Guo XX, Peng JM et al (2012) The prognostic value of left ventricular systolic function measured by tissue doppler imaging in septic shock. Crit Care 16:R71. https://doi.org/10.1186/cc11328
Wadthaisong M, Witayavanitkul N, Bupha-Intr T, Wattanapermpool J, de Tombe PP (2019) Chronic high-dose testosterone treatment: impact on rat cardiac contractile biology. Physiol Rep 7:e14192. https://doi.org/10.14814/phy2.14192
Golden KL, Marsh JD, Jiang Y, Moulden J (2005) Acute actions of testosterone on contractile function of isolated rat ventricular myocytes. Eur J Endocrinol 152:479–483. https://doi.org/10.1530/eje.1.01845
Gagliano-Juca T, Basaria S (2019) Testosterone replacement therapy and cardiovascular risk. Nat Rev Cardiol 16:555–574. https://doi.org/10.1038/s41569-019-0211-4
Pirompol P, Teekabut V, Weerachatyanukul W, Bupha-Intr T, Wattanapermpool J (2016) Supra-physiological dose of testosterone induces pathological cardiac hypertrophy. J Endocrinol 229:13–23. https://doi.org/10.1530/joe-15-0506
Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA (2011) Clinical review: endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab 96:3007–3019. https://doi.org/10.1210/jc.2011-1137
Shores MM, Biggs ML, Arnold AM, Smith NL, Longstreth WT Jr, Kizer JR et al (2014) Testosterone, dihydrotestosterone, and incident cardiovascular disease and mortality in the cardiovascular health study. J Clin Endocrinol Metab 99:2061–2068. https://doi.org/10.1210/jc.2013-3576
Thirumalai A, Anawalt BD (2022) Relationships between endogenous and exogenous testosterone and cardiovascular disease in men. Rev Endocr Metab Disord 23:1305–1322. https://doi.org/10.1007/s11154-022-09752-7
Onasanya O, Iyer G, Lucas E, Lin D, Singh S, Alexander GC (2016) Association between exogenous testosterone and cardiovascular events: an overview of systematic reviews. Lancet Diabetes Endocrinol 4:943–956. https://doi.org/10.1016/s2213-8587(16)30215-7
Auerbach JM, Khera M (2022) Testosterone replacement therapy and cardiovascular disease. Int J Impot Res 34:685–690. https://doi.org/10.1038/s41443-021-00516-6
Borst SE, Shuster JJ, Zou B, Ye F, Jia H, Wokhlu A et al (2014) Cardiovascular risks and elevation of serum DHT vary by route of testosterone administration: a systematic review and meta-analysis. BMC Med 12:211. https://doi.org/10.1186/s12916-014-0211-5
Funding
No funding was received in this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Conceptualization: [Ayhan Abacı]; Methodology: [Ayhan Abacı]; Material preparation, data collection and analysis were performed by [Özge Besci], [Yağmur Damla Akçura]; Writing - original draft preparation: [Özge Besci]; Writing - review and editing: [Korcan Demir, Ayhan Abacı, Ece Böber, Mustafa Kır] All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Statement of Ethics
The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. All participants’ parents or legal guardians have given their written informed consent.
Study Approval Statement
This study protocol was reviewed and approved by [Dokuz Eylül University], approval number [2023/04–18].
Consent to Participate Statement
All participants’ parents or legal guardians have given their written informed consent to participate in the study.
Conflict of Interest
The authors have no conflicts of interest to declare.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Besci, Ö., Akçura, Y.D., Acinikli, K.Y. et al. Aromatase Inhibitors May Increase the Risk of Cardiometabolic Complications in Adolescent Boys. Pediatr Cardiol 45, 228–239 (2024). https://doi.org/10.1007/s00246-023-03260-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-023-03260-4