Abstract
The current study was to report our initial experiences of fetal pulmonary valvuloplasty (FPV) for fetuses with pulmonary atresia with intact ventricular septum (PA/IVS) and critical pulmonary stenosis (CPS), including case selection, technical feasibility, and the effects of FPV on utero and postnatal outcome. Two fetuses with PA/IVS and three fetuses with CPS were enrolled between September 2016 and April 2018. All fetuses were with concomitant severe right ventricular dysplasia and growth arrest. Parameters of right cardiac development and hemodynamics, including tricuspid/mitral annulus ratio (TV/MV), right ventricle/left ventricle long-axis ratio (RV/LV), tricuspid valve inflow duration/cardiac cycle ratio (TVI/CC), degree of tricuspid regurgitation (TR), and blood flow direction of arterial duct and ductus venosus, were evaluated using echocardiogram. FPV was performed trans-abdominally under ultrasound guidance. Echocardiogram was performed post-FPV and every 2–4 weeks thereafter until delivery. The median gestational age at the time of FPV was 28 weeks. From technical perspective, pulmonary balloon valvuloplasty was successfully performed and the opening of pulmonary valve was improved in all fetuses in 2–4 weeks. However, progressive restenosis was observed in four fetuses with gestation advancing, and re-atresia occurred in two PA/IVS fetuses at 36th and 37th weeks’ gestation, respectively. The growth trajectories of TV/MV, RV/LV, and TVI/CC were improved in the 1st week after FPV and then slowed down along with pulmonary valve restenosis. All fetuses were born alive and underwent postnatal interventions, including pulmonary balloon valvuloplasty in three fetuses and surgical procedures in two fetuses. During follow-up, three fetuses turned to be biventricular, one became one and a half ventricular at 1-year old, and one died of neonatal infection. Although pulmonary valve restenosis might occur as gestation advancing, FPV seems to be a safe and feasible procedure to improve the growth trajectories of right heart for fetuses with PA/IVS and CPS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Both pulmonary atresia with intact ventricular septum (PA/IVS) and critical pulmonary stenosis (CPS) are associated with increased risks of morbidity and mortality for fetuses and neonates. The prevalence of PA/IVS and CPS are approximately 5% and 1–3%, respectively [1]. PA/IVS and CPS are morphologically heterogeneous lesions which are characterized by varied dimensions of right ventricle (RV) and tricuspid valve (TV), ranging from normal size to varied degrees of hypoplasia. In addition, PA/IVS and CPS are commonly associated with coronary circulation anomalies. Notably, a substantial proportion of fetuses with CPS will progress to a complete atresia, and some with PA/IVS will worsen during fetal life [2, 3].
Previous studies have demonstrated that fetal pulmonary valvuloplasty (FPV) was a feasible approach. FPV could promote right heart growth and improve fetal hemodynamics, which were beneficial for biventricular circulation [4,5,6,7,8,9]. Compared to Europe and US, the prevalence of CPS is slightly higher in Asian populations [10]. Herein, we report our initial experiences of FPV for fetuses with PA/IVS and CPS, including case selection, technical feasibility, and the effects of FPV on utero and postnatal outcome in China.
Methods
Cases Selection
Between September 2016 and April 2018, five fetuses underwent FPV in our center. Two cases were diagnosed as PA/IVS and three cases were CPS with severe right ventricular dysplasia. Parameters of right ventricle development and hemodynamics, including tricuspid/mitral annulus ratio (TV/MV), right ventricle/left ventricle long-axis ratio (RV/LV), tricuspid valve inflow duration/cardiac cycle ratio (TVI/CC), degree and velocity of tricuspid regurgitation (TR), blood flow direction of arterial duct, and ductus venosus, were evaluated using echocardiogram every 2–4 weeks. All fetal echocardiogram was performed by the same experienced pediatric cardiologist (CCP).
The criteria, which were recommended by Tulzer et al. in 2016, were used in the current study to select fetuses for FPV [11]. The inclusion criteria were (1) membranous PA or CPS with intact ventricular septum, (2) an identifiable small RV (defined as RV < LV) with the TR jet velocity \(\ge\) 2.5 m/s, (3) RV growth arrest for 3–4 weeks, and (4) retrograde flow within ductus arteriosus. The exclusion criteria were (1) muscular atresia of RV outflow tract obstruction, (2) severe TR with low velocity, and (3) presence of large RV sinusoid.
According to prior report, [12] the following parameters, including TV/MV < 0.7, RV/LV < 0.6, TVI/CC < 0.315, and the presence of RV sinusoids, were used to evaluate the postnatal outcome. For example, if three of these four criteria were fulfilled, then the sensitivity and specificity to predict a nonbiventricular outcome would be 100% and 75%, respectively.
The study was approved by the Guangdong Provincial People’s Hospital Ethics Committee (Yue Medical Ethics 2016 NO. 10), and written informed consents were obtained from the parents of the fetuses before FPV procedure.
Fetal Pulmonary Valvuloplasty
FPV procedure was performed trans-abdominally under ultrasound guidance (Fig. 1a–d) as previously described [11]. The mothers underwent general anesthesia during the operations. An 18- or 19-gage needle (Cook) and a 4-mm coronary balloon catheter (Boston Scientific) were used for pulmonary valve puncture and dilatation. Technical success was defined as successful passage and inflation of a dilated balloon across the pulmonary valve. Specifically, pulmonary valve was measured the day before FPV in RV outflow tract view. Echocardiogram was performed to evaluate the improvement of antegrade flow. Fetal complications, including pericardial effusion and bradycardia, during procedure were recorded. Pericardiocentesis was performed immediately if hemodynamic compromise occurred. If persistent bradycardia developed, epinephrine was administered into fetal left heart or was injected via maternal vein in case of emergency. Specifically, 0.1 mg of epinephrine was intravenously infused to the mother, and 0.01 mg/kg for fetus (body weight of the fetus was estimated using fetal echocardiogram the day before FPV). If technical failures or severe complication occurred during the first attempt, the procedure would discontinue, and second attempt was performed in the next day.
Post-FPV Follow-Up and Postnatal Outcome
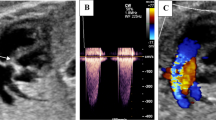
After the procedure, the mother was hospitalized for 2–3 days. The fetus was assessed using fetal echocardiogram immediately after FPV and the day before discharge. The fetuses were follow-up at our center. Fetal echocardiogram was performed to evaluate the opening of pulmonary valve, right ventricle growth (Fig. 2a–d), and hemodynamics status every 2–4 weeks until delivery. After delivery, all fetuses were managed as neonates with PA/IVS or CPS.
Results
Fetal Pulmonary Valvuloplasty
Among the five cases, one case was PA/IVS, which matched the criteria for the univentricular outcome completely, and the other four cases partially matched the criteria as mentioned above [12]. Seven FPV procedures were performed in the five cases. Three cases were at 28 weeks’ gestational age and two were at 30 weeks’ gestational age. From technical perspective, pulmonary balloon valvuloplasty were performed successfully in these five cases. The balloon-to-pulmonary valve ratio was 0.77–0.82. There were no fetal deaths during procedure. One case with large pericardial effusion underwent pericardiocentesis, and hemodynamic status become stable immediately. Small pericardial effusion in the other four fetuses resolved automatically after 3–5 days. Five cases developed persistent severe bradycardia. Four fetuses were treated with maternal intravenous infusion and fetal intra-cardiac injection of epinephrine, and the other one was only treated with maternal intravenous infusion of epinephrine (Table 1). No complications occurred to the mothers.
FPV Effects on Utero
All cases were follow-up at our center. Parameters of RV development and hemodynamics before and after FPV (1–4 weeks and 5–8 weeks) are shown in Table 2. Improvement of pulmonary valve opening was observed in all cases at 1–4 weeks post-FPV. However, restenosis was detected in four fetuses with gestation advancing, and re-atresia occurred in two PA/IVS cases (case 1 and case 4) at 36 weeks’ and 37 weeks’ gestation, respectively. The improvement of TV/MV, RV/LV, and TVI/CC occurred in the first 1–4 weeks post-FPV and then slowed down as pulmonary valve restenosis developed. The growth trajectories of TV/MV, RV/LV, and TVI/CC are illustrated in Fig. 3a–c. The hemodynamics were stable during the intrauterine course. The direction of blood flow through the arterial duct changed to bidirectional in three cases, and the ductus venosus flow were improved in two cases (Table 2).
Postnatal Outcome
All fetuses were born alive and underwent postnatal interventions, including pulmonary balloon valvuloplasty in 3 fetuses and surgical procedures in 2 fetuses. The duration of follow-up ranged from 0.42 to 2.00 years. Postnatal procedures and outcome are listed in Table 1. In specific, two PA/IVS fetuses developed pulmonary valve re-atresia and underwent pulmonary valve commissurotomy and modified Blalock-Taussig shunt at the 8th day (case 1) and the 32nd day (case 4), respectively. Case 1, that fulfilled the criteria for a univentricular outcome, had a bidirectional Glenn shunt and became one and a half ventricular circulation at 1 year old due to small RV and TV dysplasia. Case 4 died at the 37th day due to neonatal infection and hemodynamic instability despite with an appropriate RV size and underwent an emergency postnatal surgery. The other three fetuses underwent transcutaneous pulmonary balloon valvuloplasty in neonatal period and became biventricles.
Discussion
Since the first report of fetal cardiac intervention in 1991, more than 50 FPV have already been performed for the management of PA/IVS and CPS. Accumulating evidence has shown that restoring blood flow improves RV development, [13,14,15] justifying the utilization of fetal cardiac intervention for the treatment of PA/IVS and CPS. However, considering the potential risk associated with FPV, two essential points need to be addressed: first, selecting appropriate cases that may benefit most from FPV; second, considering the feasibility, safety, and outcomes of FPV so as to balance the risks and benefits of each individual case.
Case Selection
The criteria of selection for FPV remain controversial. It is reasonable to perform FPV for PA/IVS and CPS that is complicated by severe right heart failure during the second trimester because FPV can improve fetal hydrops and avoid fetal demise. Although most of PA/IVS and CPS fetuses do not progress to significant fetal hydrops in compensatory era, FPV remains an important therapeutic strategy during gestational period because postnatal intervention is less likely to improve fetuses’ growth [16]. Prior animal experiments have shown that increased pre- and post-load leads to cardiomyocytes proliferation and hyperplasia [17,18,19]. RV grows corresponding to body size growth after neonatal procedure, and therefore, FPV is an important approach to improve prenatal RV growth and avoid postnatal univentricular outcome.
In the absence of a large RV sinusoid fistulae or a muscular atresia of RV outflow tract, RV and TV sizes at birth are the major determinants of biventricular outcome. A “hypoplastic” but still “salvageable” RV structure is an important determinant to consider FPV intervention at the second trimester. Fetuses with an unidentifiable RV and coronary anomalies are “unsalvageable,” which is unable to restore biventricular circulation regardless of prenatal intervention. On the other hand, fetuses with an appropriate RV size can be reserved for intervention after birth. Notably, the above two conditions are not good candidates for FPV. Between these two extreme conditions are fetuses with an identifiable but varied RV hypoplasia. This group includes potential candidates for FPV. Several studies have reported the prediction of a univentricular outcome in fetuses with PA/IVS and CPS [6, 12, 20,21,22]. Due to lack of effective evaluation of Z-score in China, we adopted a frequently used four-criteria scoring system to predict the postnatal outcome, [12] which includes TV/MV ratio < 0.7, RV/LV length ratio < 0.6, TVI/CC < 31.5%, and presence of RV sinusoids. After excluding the cases with large RV sinusoid, ideal candidates for FPV should match the other three criteria to justify treatment. In the current study, one fetus with PA/IVS matched the criteria for a univentricular outcome, and the other four fetuses were partially matched the criteria. Fetal echocardiogram was performed to closely monitor RV growth. If there was without any increase in RV size over a period of 2–4 weeks, then a spontaneous reversal is unlikely and FPV should be considered to open the pulmonary valve.
Technical Considerations
Although experience with FPV is still limited to few case reports and small case series, prior studies have shown that FPV in the mid-gestation is technically feasible [4,5,6, 9, 23]. The technical success rate (70–80%) is approximated to that of fetal aortic valvuloplasty [5, 6]. In 2018, the Children’s Heart Center in Linz reported the largest series of 35 attempted FPV in 23 fetuses [23]. Seventeen cases underwent successful procedure, and no procedure-related fetal deaths and maternal complication occurred. However, a hypoplastic RV with a narrow, short, and curved segment below the valve makes FPV a technically challenging procedure. The fetal position and an experienced team in coordinating imaging, needling, catheter manipulation, and managing complications are the key factors influencing the success rate.
During the procedure, the most common fetal complications are severe bradycardia and pericardial effusion. However, timely and proper therapy can effectively avoid intrauterine fetal death. It has been demonstrated that it is safer to dilate pulmonary valve than aortic valve, which is attributed to the lower risk of compromising coronary artery when dilating pulmonary valve [6]. FPV was performed successfully in the five cases. Among these cases, severe bradycardia occurred when the needle was puncturing the RV outflow track or when balloon catheter was dilating pulmonary valve. Epinephrine administered via maternal peripheral vein could increase fetal heart rate. The fetuses with persistent bradycardia were injected epinephrine via maternal peripheral vein under anesthesiologist monitoring, and one fetus recovered instantly before cardiac puncture for intra-cardiac epinephrine injection. Large pericardial effusion with sign of hemodynamic compromise occurred in one fetus, which was resolved immediately with pericardiocentesis. Small pericardial effusion in four fetuses resolved automatically 3–5 days later.
Outcome
Previous studies have shown that pulmonary valve developed restenosis after FPV with gestation advancing [23]. In the current study, we also observed that four fetuses developed restenosis. The potential explanation was that we used a relatively small balloon to dilate pulmonary valve (balloon/pulmonary valve diameter ratio 0.77–0.82). Notably, balloon size should be at least 10–20% larger than the valve diameter [11]. However, with the current available equipment, the largest size of balloon is 4 mm, which is compatible to an 18G needle. Large needles will increase the risk amniotic membranes and fetal heart injury, resulting in bradycardia, thrombus, and pericardial effusions. The other potential explanation might be due to low RV stroke volume, which was associated with hypertrophic, restrictive RV and dysplastic TV. TV dysplasia with tricuspid stenosis might have contributed to pulmonary valve re-atresia and slow RV improvement in Case 1. In such case, although FPV and postnatal procedure were performed successfully, severe inflow obstruction might still limit RV filling and pulmonary forward flow, which is important for RV growth. Therefore, besides measuring TV annulus, it is important to evaluate the presence of thickened and fusional TV leaflets, restricted TV opening and TV inflow duration. Pulmonary valve restenosis might be unavoidable as pregnancy advancing. However, a less decompression of RV post-FPV treatment might be able to improve hemodynamics [23]. Furthermore, restenosis mainly occurred in near-term pregnancy, and FPV can still improve the growth trajectories of fetal RV structure in the early weeks and benefit some fetuses with PA/IVS and CPS. Meanwhile, in the current study, the growth trajectories of TV/MV, RV/LV, and TVI/CC were improved in the early weeks although these get slowed down gradually along with restenosis, indicating that reduction of ventricular pressure is able to improve ventricular filling, compliance, and growth.
Fetal cardiac intervention should be considered as a palliative therapy. It was unable to eliminate the gradient across the pulmonary valve, especially in the last gestation stage, and normal RV size could not be achieved despite successful FPV. One or more additional postnatal procedures are required for the management of the defect after birth. In the current study, all five fetuses underwent postnatal transcatheter or surgical interventions. Two fetuses with pulmonary valve re-atresia underwent surgical procedures after birth, and among them, one with TV stenosis became one and a half ventricular circulation at 1 year old, and the other one with a well-sized RV died of neonatal infection. Three fetuses underwent transcutaneous pulmonary balloon valvuloplasty in the neonatal period and became biventricular.
Limitation
This study is limited by its small sample size and nonrandomized design. In addition, several issues need to be improved before FPV can be routinely applied to fetuses with pulmonary atresia and stenosis. For example, better understanding the nature history of PA/IVS or CPS could help us unify the case selection criteria. In order to minimize risks and optimize technical success, improvements in needles, balloon, and imaging would be essential. In addition, as we have mentioned that the largest size of balloon is 4 mm, which is comparable to an 18G needle. If a larger balloon was available, a better outcome might be possible. There was no control group in the current study, and therefore, the findings of current analysis were subjected to various biases. Tricuspid valve annulus z-score data were not available in the current study which is a major limitation. Last but not the least, since the body habitus of pregnant women in China is likely to be generally different to that of those in the West, where most of the previous reports of FPV originate, findings of the current study might not be extrapolated to other population groups. For example, due to the differences in body size, the diagnostic criteria using echocardiographic indexes might be different between the Chinese and western populations. In addition, the sizes of technical instruments used during FPV might also be different due to differences in the body size of pregnant women, which might also result in differences in the outcomes.
Conclusion
In conclusion, this is the first case series from China. Although pulmonary valve restenosis occurs with pregnancy advancing, FPV still improves the growth trajectories of fetal RV structure in the early weeks. With appropriate case selection and the advancement of technical skills and instrument, FPV remains a feasible and safe therapeutic strategy for fetuses with PA/IVS and CPS.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- FPV:
-
Fetal pulmonary valvuloplasty
- PA/IVS:
-
Pulmonary atresia with intact ventricular septum (PA/IVS)
- CPS:
-
Critical pulmonary stenosis
- TV:
-
Tricuspid valve
- MV:
-
Mitral valve
- RV:
-
Right ventricle
- LV:
-
Left ventricle
- PV:
-
Pulmonary valve
- AV:
-
Aortic valve
- TV/MV:
-
Tricuspid/mitral annulus ratio
- RV/LV:
-
Right ventricle/left ventricle long-axis ratio
- TVI/CC:
-
Tricuspid valve inflow duration/cardiac cycle ratio
- TR:
-
Tricuspid regurgitation
- AD:
-
Arterial duct
- DV:
-
Ductus venosus
- Sv:
-
Severe
- Rv:
-
Reversed
- Mx:
-
Mixed
- Ab:
-
Absent
- At:
-
Antegrade
References
Todros T, Paladini D, Chiappa E et al (2003) Pulmonary stenosis and atresia with intact ventricular septum during prenatal life. Ultrasound Obstet Gynecol 21(3):228–233
Rice MJ, McDonald RW, Reller MD (1993) Progressive pulmonary stenosis in the fetus: two case reports. Am J Perinatol 10(6):424–427
Todros T, Presbitero P, Gaglioti P, Demarie D (1988) Pulmonary stenosis with intact ventricular septum: documentation of development of the lesion echocardiographically during fetal life. Int J Cardiol 19(3):355–362
Tulzer G, Arzt W, Franklin RC, Loughna PV, Mair R, Gardiner HM (2002) Fetal pulmonary valvuloplasty for critical pulmonary stenosis or atresia with intact septum. Lancet 360(9345):1567–1568
Tworetzky W, McElhinney DB, Marx GR et al (2009) In utero valvuloplasty for pulmonary atresia with hypoplastic right ventricle: techniques and outcomes. Pediatrics 124(3):e510–e518
Gómez ME, Herraiz I, Mendoza A, Galindo A (2012) Fetal intervention in right outflow tract obstructive disease: selection of candidates and results. Cardiol Res Pract 2012:592403
Pedra SF, Peralta CF, Carlos Augusto Cardoso P (2013) Future directions of fetal interventions in congenital heart disease. Interv Cardiol Clin 2(1):1–10
Moon-Grady AJ, Morris SA, Belfort M et al (2015) International fetal cardiac intervention registry: a worldwide collaborative description and preliminary outcomes. J Am Coll Cardiol 66(4):388–399
Galindo A, Gutiérrez-Larraya F, Velasco JM, de la Fuente P (2006) Pulmonary balloon valvuloplasty in a fetus with critical pulmonary stenosis/atresia with intact ventricular septum and heart failure. Fetal Diagn Ther 21(1):100–104
Mitchell B, Mhlongo M (2018) The diagnosis and management of congenital pulmonary valve stenosis. SA Heart 15:36–45
Tulzer G, Butera G (2016) Fetal and hybrid procedures in congenital heart diseases. Springer International Publishing, Cham, pp 84–92
Roman KS, Fouron JC, Nii M, Smallhorn JF, Chaturvedi R, Jaeggi ET (2007) Determinants of outcome in fetal pulmonary valve stenosis or atresia with intact ventricular septum. Am J Cardiol 99(5):699–703
Hanséus K, Björkhem G, Lundström NR, Laurin S (1991) Cross-sectional echocardiographic measurements of right ventricular size and growth in patients with pulmonary atresia and intact ventricular septum. Pediatr Cardiol 12(3):135–142
Tworetzky W, Wilkins-Haug L, Jennings RW et al (2004) Balloon dilation of severe aortic stenosis in the fetus: potential for prevention of hypoplastic left heart syndrome: candidate selection, technique, and results of successful intervention. Circulation 110(15):2125–2131
Tulzer G, Gardiner H (2006) Cardiac interventions in the fetus: potential for right-sided lesions. Fetal interventions in right heart disease. Prog Pediatric Cardiol 22(1):79–83
Ovaert C, Qureshi SA, Rosenthal E, Baker EJ, Tynan M (1998) Growth of the right ventricle after successful transcatheter pulmonary valvotomy in neonates and infants with pulmonary atresia and intact ventricular septum. J Thorac Cardiovasc Surg 115(5):1055–1062
Clark EB, Hu N, Frommelt P, Vandekieft GK, Dummett JL, Tomanek RJ (1989) Effect of increased pressure on ventricular growth in stage 21 chick embryos. Am J Physiol 257(1 Pt 2):H55-61
Saiki Y, Konig A, Waddell J, Rebeyka IM (1997) Hemodynamic alteration by fetal surgery accelerates myocyte proliferation in fetal guinea pig hearts. Surgery 122(2):412–419
deAlmeida A, McQuinn T, Sedmera D (2007) Increased ventricular preload is compensated by myocyte proliferation in normal and hypoplastic fetal chick left ventricle. Circ Res 100(9):1363–1370
Salvin JW, McElhinney DB, Colan SD et al (2006) Fetal tricuspid valve size and growth as predictors of outcome in pulmonary atresia with intact ventricular septum. Pediatrics 118(2):e415–e420
Wohlmuth C, Tulzer G, Arzt W, Gitter R, Wertaschnigg D (2014) Maternal aspects of fetal cardiac intervention. Ultrasound Obstet Gynecol 44(5):532–537
Gardiner HM, Belmar C, Tulzer G et al (2008) Morphologic and functional predictors of eventual circulation in the fetus with pulmonary atresia or critical pulmonary stenosis with intact septum. J Am Coll Cardiol 51(13):1299–1308
Tulzer A, Arzt W, Gitter R et al (2018) Immediate effects and outcome of in-utero pulmonary valvuloplasty in fetuses with pulmonary atresia with intact ventricular septum or critical pulmonary stenosis. Ultrasound Obstet Gynecol 52(2):230–237
Acknowledgements
We appreciate very much for our participants and the nurses who helped to take care of our participants. Appreciate for Dr. Anping Cai in helping revising the paper.
Funding
The current study was supported by the National Key Research and Development Program (2018YFC1002600), Guangdong Project of Science and Technology (2017A070701013, 2017B090904034, 2017B030314109).
Author information
Authors and Affiliations
Contributions
The manuscript was drafted by PCC, PW, ZCB, and critically revised by all authors. The ideal of the work was conceived by LYF, ZJ. FPV procedure was performed by ZZW, ZX, HFZ, SYX, and WS. Access to data was provided by PCC and PW. All author read and agree for the manuscript to be published.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical Approval
The study was approved by Guangdong General Hospital Ethics Committee (Yue Medical Ethics 2016 NO. 10).
Informed consent
Written informed consents were obtained from parents of the patient before FPV procedure.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
#Chengcheng Pang and Chengbin Zhou are co-first author.
Rights and permissions
About this article
Cite this article
Pang, C., Zhou, C., Zhang, Z. et al. Fetal Pulmonary Valvuloplasty in Fetuses with Right Ventricular Outflow Tract Obstructive Disease: Experience and Outcome of the First Five Cases in China. Pediatr Cardiol 42, 340–348 (2021). https://doi.org/10.1007/s00246-020-02488-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-020-02488-8