Abstract
As survival and neuromuscular function in Duchenne muscular dystrophy (DMD) have improved with glucocorticoid (GC) therapy and ventilatory support, cardiac deaths are increasing. Little is known about risk factors for cardiac and non-cardiac causes of death in DMD. A multi-center retrospective cohort study of 408 males with DMD, followed from January 1, 2005 to December 31, 2015, was conducted to identify risk factors for death. Those dying of cardiac causes were compared to those dying of non-cardiac causes and to those alive at study end. There were 29 (7.1%) deaths at a median age of 19.5 (IQR: 16.9–24.6) years; 8 (27.6%) cardiac, and 21 non-cardiac. Those living were younger [14.9 (IQR: 11.0–19.1) years] than those dying of cardiac [18 (IQR 15.5–24) years, p = 0.03] and non-cardiac [19 (IQR: 16.5–23) years, p = 0.002] causes. GC use was lower for those dying of cardiac causes compared to those living [2/8 (25%) vs. 304/378 (80.4%), p = 0.001]. Last ejection fraction prior to death/study end was lower for those dying of cardiac causes compared to those living (37.5% ± 12.8 vs. 54.5% ± 10.8, p = 0.01) but not compared to those dying of non-cardiac causes (37.5% ± 12.8 vs. 41.2% ± 19.3, p = 0.58). In a large DMD cohort, approximately 30% of deaths were cardiac. Lack of GC use was associated with cardiac causes of death, while systolic dysfunction was associated with death from any cause. Further work is needed to ensure guideline adherence and to define optimal management of systolic dysfunction in males with DMD with hopes of extending survival.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Duchenne muscular dystrophy (DMD) is the most common form of childhood muscular dystrophy worldwide, with prevalence estimates of 4.78/100,000 males [1, 2]. The clinical course is marked by progressive skeletal muscle weakness and death in early adulthood from respiratory or cardiac failure. Median age at critical milestones includes, loss of ambulation at 11.3 years, development of cardiomyopathy at 16.7 years, initiation of non-invasive ventilation at 18 years, and death at 21.8 years [3]. Cardiac disease in DMD manifests as cardiomyopathy (CM), that progresses with age, and/or cardiac arrhythmia [4, 5]. While only 25% of boys with DMD have CM by age 6 years, cardiac involvement is nearly universal in older patients, with more than 90% of men > 18 years of age demonstrating cardiac dysfunction [6]. Survival, neuromuscular function, and quality of life in DMD are improving due to glucocorticoid (GC) treatment and advances in respiratory care, and as a result, cardiac disease is increasing as a major cause of death [7,8,9,10,11,12,13,14].
Benefits of GC therapy for DMD patients include prolonged ambulation, less spinal stabilization surgery, improved cardiopulmonary function, delayed non-invasive ventilation requirements, and increased survival and quality of life [8]. Life expectancy for DMD patients has increased over time, highlighted in several series worldwide [9,10,11,12]. Passamano et al. (Italy), assessed mortality at age 20 and 25 years for DMD patients born over several decades and found that for those born in the 1960s; 76.7% would have died by age 20 years and 86.5% by age 25 years, whereas for those born in the 1980s; 40.2% would have died by age 20 years and 50.8% by age 25 years. The authors attributed this improvement in part to nocturnal ventilation [9]. Kieny et al. (France) reported median survival of 25.8 years for DMD patients born from 1955–1969, increasing to 40.9 years for patients born after 1970 with the use of ventilatory support. By comparison, these advances in respiratory therapy yielded a proportionate increase in cardiac causes of death from 8 to 44% over the same time period (1981–2011) [12].
Few studies have evaluated risk factors for death in the DMD population in the modern era, either overall or due to cardiac causes. Cheeran et al., evaluated a cohort of 43 adult DMD patients with CM and found several poor prognostic factors with the non-surviving cohort having a lower Body Mass Index (BMI), lower aminotransferase levels, lower maximum inspiratory pressure on pulmonary function tests, and elevated cardiac biomarkers [15]. Larger, multi-center studies are lacking in this regard. Therefore, our objective was to identify risk factors for cardiac and non-cardiac causes of death in a large cohort of males with DMD followed across North America.
Methods
A retrospective cohort study was conducted at 17 pediatric hospitals (Supplemental Table 1) across North America (16 United States, 1 Canada) analyzing subjects with DMD from which clinical information acquired between January 1, 2005 and December 31, 2015 was collected. Inclusion criteria were males with the diagnosis of DMD who had ≥ 2 cardiac evaluations at their home institution. The diagnosis of DMD was determined by clinical record review. Genetic testing results were collected, but not mandatory for inclusion. Exclusion criteria were females and the diagnosis of congenital heart disease. Analysis was limited to those whose status was known at study end (alive or deceased). Subjects were censored at death or study end date. The following data were collected: age of diagnosis, age ambulation ceased, respiratory support needed, GC use, all cardiac testing, cardiac medication use, acute heart failure (HF) hospitalizations, implantation of an internal cardiac defibrillator, implantation of a ventricular assist device, heart transplantation, date of death, and cause of death. The clinical approach used to determine candidacy for advanced therapies was site specific. Cardiac testing data were acquired from study reports and not interpreted independently for this study. Data collected regarding ejection fraction (EF) included 4 chamber, biplane, and 3D methods all of which were combined for analysis purposes. Cause of death was determined by each site investigator after an extensive chart review and classified into one of the following categories: cardiac [sudden cardiac death (SCD) or progressive HF], respiratory, multi-organ failure, neurologic, other/unknown. SCD was defined as witnessed sudden death with/without documented ventricular fibrillation, death within 1 hour of new symptoms, or nocturnal deaths with no antecedent history of worsening symptoms. Each investigator obtained IRB approval at their own institution with Data Use Agreements established as needed. Study data were collected and managed using an electronic database hosted at the University of Rochester Medical Center.
Statistical Analysis
Statistical analyses were conducted using Stata 13.1 (StataCorp LP, College Station, TX). All variables were assessed for normality prior to analysis and expressed as mean ± standard deviation for parametric and median [interquartile range (IQR)] for non-parametric distributions. Group differences were tested using Student’s t test or Wilcoxon rank-sum, as appropriate; proportions by a Chi-squared test. Differences in repeated measures were assessed by Wilcoxon signed-rank test. Multiple logistic regression was used to determine if associations between the primary outcome (death) and clinical characteristics of interest were confounded by subject age. Significance was defined using a two-tailed hypothesis test with p < 0.05 for all analyses.
Results
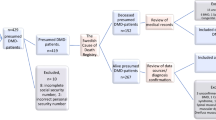
Four hundred and thirty-six male subjects with DMD were studied, of whom 28 subjects were lost to follow-up and excluded from analysis, resulting in a study cohort of 408 subjects. Excluded subjects did not differ from the remainder of the cohort by age at diagnosis or age at loss of ambulation, but were less likely to have been treated with GC (Supplemental Table 2). For the 408 subjects analyzed, the median age of DMD diagnosis was 4 (IQR: 3–6) years and the median age of those alive at study end was 14.9 (IQR 11.0–19.1) years. Three hundred and nineteen (78.2%) subjects were treated with GC, with prevalence of use greater for subjects born after 2000 (87% vs 68%, p < 0.001). Selected characteristics of subjects are outlined in Table 1. Use of cardiac medications and advanced HF therapies are summarized in Table 2. By study end, 29 subjects were deceased (7.1%) at a median age of 19.5 (IQR: 16.9–24.6) years with 8 (27.6%) deaths attributed to a cardiac cause (SCD, n = 5 and progressive HF, n = 3), 8 due to a respiratory cause, 3 due to multi-organ failure, 1 from a neurologic cause (stroke), and 9 due to an unknown/other cause. The survival probability at age of last follow-up is shown in Fig. 1. BMI was calculated at the time of last evaluation (prior to death or study end) and did not differ for those who died, median 20.7 (IQR 16.5–31.4) kg/m2 compared to those who were alive, median 21.3 (IQR 16.9–27.1) kg/m2, p = 0.85.
Kaplan–Meier curve depicting survival probability at age of last follow-up in 407 boys with Duchenne muscular dystrophy. Number at risk is the number of subjects used in the denominator to determine the proportion of subjects surviving per decade of age at study end. Shaded gray area indicates the 95% confidence interval. The starting number is 1 less than the entire cohort due to an unknown death date for one subject
Characteristics of DMD Subjects Dying of Cardiac Causes, Non-cardiac Causes, and Those Alive at Study End
The clinical characteristics of subjects dying of cardiac causes, non-cardiac causes, and those alive at study end are summarized in Table 3. Those dying of cardiac [median 18.4 (IQR: 15.9–24.8) years] and non-cardiac [19.6 (IQR: 17.5–23.5) years] causes were of similar age, p = 0.58, but were significantly older than those alive at study end [14.9 (IQR: 11.0–19.1) years], p = 0.03 and p = 0.002 respectively. The median age at loss of ambulation did not differ for those dying of cardiac [9 (IQR: 7–11) years] and non-cardiac causes [9 (IQR: 7–11) years], p = 0.68, but those dying of non-cardiac causes [9 (IQR: 7–11) years] lost ambulation at a younger age compared to those alive at study end [11 (IQR: 9–12) years], p < 0.01. The need for ventilatory support [non-invasive (CPAP/BIPAP) or invasive (tracheostomy) ventilation] was significantly greater for those dying of non-cardiac causes (80.9%) compared to those alive at study end (33.5%), p < 0.01, but not when compared to those dying of cardiac causes (50%), p = 0.16, and not when comparing those dying of cardiac causes to those alive at study end, p = 0.45. GC use (at any time during the study period) was significantly lower for those dying of cardiac causes (25%) compared to those alive at study end (80.4%), p < 0.01, but did not differ from those dying of non-cardiac causes (61.9%), p = 0.11. GC use did not differ between those dying of non-cardiac causes versus those alive at study end, p = 0.05.
The prevalence of angiotensin converting enzyme inhibitor (ACE-I) or an angiotensin receptor blocker (ARB) use during the study period was 87.5% for those dying of cardiac causes, 76.2% for those dying of non-cardiac causes, and 59.1% for those alive at study end, p = 0.09. Acute HF hospitalizations were significantly more common for those dying of both cardiac (37.5%) and non-cardiac causes (38.1%) when compared to those alive at study end (5.3%), p = 0.01 and p = 0.08, respectively, but not when compared to each other p = 0.99. The median time from first acute HF hospitalization to death was 0.3 (IQR: 0–2.6) years.
Echocardiogram
Left ventricular (LV) EF and fractional shortening (FS) measured prior to death or study end were both significantly lower for those dying of cardiac and non-cardiac causes compared to those alive at study end but neither EF nor FS differed when comparing those dying of cardiac causes to those dying of non-cardiac causes (shown in Fig. 2). Longitudinal FS data were more complete for deceased subjects (23/29); those who died had a greater decline in FS over the study period, − 6.5% (IQR: − 13% to − 2.4%) compared to those who were alive at study end, − 2.1% (IQR: − 7% to 1%), p = 0.01.
Cardiac Magnetic Resonance Imaging
Cardiac magnetic resonance imaging (MRI) data were available for only 54 subjects, therefore, the analysis was focused on those dying versus those alive at study end. Of these, 29 subjects (53.7%) demonstrated evidence of myocardial delayed enhancement (MDE). Presence of MDE on cardiac MRI did not differ significantly for those who died (4/5, 80%) compared to those alive at study end (25/49, 51%), p = 0.2. The median LV EF by MRI was significantly lower for those who died [34.7% (IQR: 23.9–38%)] compared to those alive at study end [56% (IQR: 47.8–61%)], p = 0.01. The median right ventricular EF did not differ for those who died [51.6% (IQR: 41.1–57.5%)] compared to those alive at study end [56.5% (IQR: 52–60%)], p = 0.24.
Cardiac Biomarkers
Cardiac biomarker data were limited, though, when checked the last N-terminal pro-brain natriuretic peptide (NT-proBNP) and brain natriuretic peptide (BNP) measured prior to death or study end were significantly greater for those who died compared to those alive at study end. The median NT-proBNP for deceased subjects was 1139 (IQR: 289–2437, n = 4) pg/mL versus 83 (IQR: 50–265, n = 19) pg/mL for living subjects, p = 0.04. The median BNP for deceased subjects was 154 (IQR: 21–721, n = 15) pg/mL versus 16 (IQR: 11–51, n = 74) pg/mL for living subjects, p = 0.01.
Adjusting for Subject Age
Based on the findings that subjects who died during the study period were significantly older than those alive at study end, secondary analyses using multiple regression were performed to determine if the relationships between risk factors of interest and death were confounded by subject age (Table 4). Age at loss of ambulation, absence of GC use, lower measures of cardiac function (EF and FS by echocardiogram and EF by MRI), and higher last BNP all remained associated with greater odds of death after adjusting for age.
Discussion
To our knowledge, this is the largest contemporary study investigating the relationships between clinical risk factors and cardiac and non-cardiac causes of death in boys with DMD. Approximately 30% of deaths in our series were attributed to cardiac etiologies, including both SCD and progressive HF. Lack of GC use was associated with cardiac causes of death. Younger age at loss of ambulation, absence of GC therapy, lower measures of ventricular function on last cardiac imaging, and higher BNP levels were all associated with greater odds of death from any cause.
DMD is the most common form of childhood muscular dystrophy, with cardiac involvement that is nearly universal in older patients [6]. Over the past 2 decades, with the availability of invasive and non-invasive ventilation and the use of GC, the average age at death for individuals with DMD has increased from the late teens to the late 30 s or 40 s [10,11,12]. With this, cardiac causes of death in DMD appear to be increasing, a finding that is further supported by our data [13]. Importantly, our results build upon previous studies by providing greater insight into the clinical characteristics associated with death and more specifically, both cardiac and non-cardiac causes of death in males with DMD.
The prevalence of past or current GC treatment was significantly lower for those dying of cardiac causes compared to those alive at study end and may serve as an important target for intervention. Prevalence of use was greater for subjects born after 2000 (87% vs 68%, p < 0.001) and hopefully this trend will continue as GCs have been proven to be a mainstay of treatment for DMD, associated with long-term benefits such as prolongation of ambulation, reduced need for spinal stabilization surgery, improved cardiopulmonary function, delayed need for non-invasive ventilation, and increased survival and quality of life [8]. With regards to cardiac function, there are several single-center studies demonstrating that DMD patients treated with GC had significant LV EF preservation and even reduction in all-cause mortality compared with patients not receiving GC [16,17,18,19,20]. These findings were supported on a larger scale by Barber et al. who evaluated a population-based sample of 462 boys with DMD in five surveillance sites in the United States and found that GC treatment was associated with delayed CM onset. Additionally, duration of treatment positively correlated with delayed CM onset with analysis suggesting that a boy with DMD treated for 5 years with GC might experience a 20% decrease in the likelihood of developing CM compared with untreated boys, though, optimal timing of GC initiation [early childhood (< 5 years) versus later childhood (≥ 5 years)] has been called into question and requires further study [20, 21]. Given this and that recent guidelines recommend the initiation of steroids before substantial physical decline with continued use in the non-ambulatory stage, it is important to ensure this therapy is being used for all eligible DMD patients with dose adjustment for side effects [22, 23].
LV systolic function on echocardiogram measured by EF and FS was significantly lower for those dying of both cardiac and non-cardiac causes compared to those alive at study end in our series. Furthermore, greater declines in FS were noted in subjects that died over the course of the study period. Last measured NT-proBNP and BNP levels were also higher for those who died compared to those alive at study end, which was not a surprising finding as NT-proBNP is a well-known marker of cardiac dysfunction that portends a worse prognosis in acute decompensated heart failure [24]. These finding are consistent with the work of Cheeran et al., who analyzed a cohort of 43 adult DMD patients with CM, and found several poor prognostic factors when comparing the non-surviving to the surviving cohorts including lower body mass index, maximum inspiratory pressures, and elevated cardiac biomarkers [15].
The fact that those dying of both cardiac and non-cardiac causes were more likely to have an acute HF hospitalization than those alive at study end supports the clinical significance of decreased ventricular function in this population. Ventricular dysfunction can lead to a low output state, particularly during periods of stress, and may predispose the patient to potentially life-threatening arrhythmias. This was supported by Villa et al., who analyzed 442 Holter monitors from 235 DMD patients and found that a LV EF < 35% was the only predictor of clinically significant Holter monitor findings [25]. It seems that for DMD patients, focus should be on optimization of ventricular systolic function and that further work is needed to better understand optimal preventive medical management of dystrophic CM, likely in the form of multi-center randomized controlled trials. Further, a better understanding of how end-stage disease may be augmented with use of mechanical support in addition to HF medications is needed. Ensuring that guideline recommendations are followed with regards to annual cardiac surveillance from the time of diagnosis will allow providers to identify changes in ventricular function sooner and initiation of an ACE-I or ARB by age 10 may help delay the onset and progression of CM with a tolerable side effect profile [23].
Cardiac MRI data were limited in this analysis. Though the presence of MDE was greater for those who died [4/5 (80%) versus 25/49 (51%), p = 0.2], this did not reach statistical significance likely due to small number. As guidelines suggest that cardiac MRI be used for cardiac surveillance annually starting at age ≥ 6–7 years old, more will be learned about the association of MDE and ventricular dysfunction, arrhythmia, and cardiac causes of death in the DMD population over time and this may augment treatment strategies [23].
To our knowledge, this is the largest, contemporary, multi-center study evaluating risk factors for cardiac and non-cardiac causes of death in boys with DMD. Despite these strengths, there are limitations. This study is limited with regards to small sample size, with only 29 deaths during the study period. There is a risk of misclassification bias with respect to the primary outcome of cause of death. We acknowledge the inherent challenge of determining the exact cause of death from chart review, particularly if death occurs outside of the hospital. Further, cardiopulmonary interactions are such that clarity in a primary respiratory versus primary cardiac insult as the primary cause of death can be difficult to ascertain. Interestingly, many of the cardiac risk factors that we evaluated were associated with death, irrespective of etiology. This suggests that cardiac dysfunction may have played a role in a greater number of the deaths than were classified as cardiac. Unfortunately, ambulatory cardiac monitoring (i.e., Holter monitor) data were limited precluding in-depth analysis; only 2/5 subjects suffering SCD had Holter monitoring data, neither of whom had evidence for non-sustained ventricular tachycardia. Finally, those dying of both cardiac and non-cardiac causes were significantly older than those alive at study end, an anticipated finding given that DMD is a progressive condition with deterioration demonstrated across the clinical spectrum over time. Secondary analyses that adjusted for age at study end did not alter the direction of our findings.
In conclusion, in a large, multi-center cohort of DMD patients, approximately 30% of deaths were attributable to a cardiac cause. Lack of steroid use was associated with cardiac causes of death, while LV systolic dysfunction was associated with death from any cause. Adherence to published guidelines with regards to initiation and continuation of GC as well as annual cardiac surveillance from the time of DMD diagnosis should be targets of intervention for this population. Future studies are needed to define optimal medical management of systolic dysfunction in males with DMD with hopes of extending survival.
References
Romitti PA, Zhu Y, Puzhankara S, James KA, Nabukera SK, Zamba GKD, Ciafaloni E, Cunniff C, Druschel CM, Mathews KD, Matthews DJ, Meaney FJ, Andrews JG, Caspers-Conway KM, Fox DJ, Street N, Adams MM, Bolen J, on behalf of the MD STARnet (2015) Prevalence of Duchenne and Becker muscular dystrophy in the United States. Pediatrics 135(3):513–521
Mah JK, Korngut L, Dykeman J, Day L, Prinsheim T, Jette N (2014) A systematic review and meta-analysis on the epidemiology of Duchenne and Becker muscular dystrophy. Neuromuscul Disord 24:482–491
Pandya S, James KA, Westfield C, Thomas S, Fox DJ, Ciafaloni E, Moxely RT (2018) Health profile of a cohort of adults with Duchenne muscular dystrophy. Muscle Nerve 58(2):219–223
Kermadec JM, Becane HM, Chenard A, Tertrain F, Weiss Y (1994) Prevalence of left ventricular systolic dysfunction in Duchenne muscular dystrophy: an echocardiographic study. Am Heart J 127(618–6):23
Chenard AA, Becane HM, Tertrain F, de Kermadec JM, Weiss YA (1993) Ventricular arrhythmia in Duchenne muscular dystrophy: prevalence, significance and prognosis. Neuromuscul Disord 3:201–206
Nigro G, Comi LI, Politano L et al (1990) The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int J Cardiol 26:271–277
Kim S, Campbell KA, Fox DJ, Matthews DJ, Valdez R, The MD STARnet (2015) Corticosteroid treatment in males with Duchenne muscular dystrophy: treatment duration and time to loss of ambulation. J Child Neurol 30(10):1275–1280
Moxley RT, Pandya S, Ciafaloni E, Fox DJ, Campbell K (2010) Change in natural history of Duchenne muscular dystrophy with long-term corticosteroid treatment: implications for management. J Child Neurol 25(9):1116–1129
Passamano L, Taglia A, Palladino A, Viggiano E, D’Aambrosio P, Scutifero M et al (2012) Improvement of survival in Duchenne muscular dystrophy: retrospective analysis of 835 patients. Acta Myol 3(2):121–125
Ishikawa Y, Toshihiko M, Ishikawa Y, Aoyagi T, Ogata H, Hamada S et al (2011) Duchenne muscular dystrophy: survival by cardio-respiratory interventions. Neuromuscul Disord 21(1):47–51
Saito T, Kawai M, Kimura E, Ogata K, Takahashi T, Kobayasi M et al (2017) Study of Duchenne muscular dystrophy long term survivors aged 40 years and older living in specialized institutions in Japan. Neuromuscul Disord 27:107–114
Bach JR, Martinez D (2011) Duchenne muscular dystrophy: continuous noninvasive ventilator support prolongs survival. Respir Care 56(6):744–750
Kieny P, Chollet S, Delalande P, Le Fort M, Magot A, Pereon Y, Verbe BP (2013) Evolution of life expectancy of patients with Duchenne muscular dystrophy at AFM Yolaine de Kepper centre between 1981 and 2011. Ann Phys Rehabil Med 56(6):443–454
Rall S, Grimm T (2012) Survival in Duchenne muscular dystrophy. Acta Myol 31(2):117–120
Cheeran D, Khan S, Khera R, Bhatt A, Garg S, Grodin JL et al (2017) Predictors of death in adults with Duchenne muscular dystrophy-associated cardiomyopathy. J Am Heart Assoc 6(10):e006340
Biggar WD, Harris VA, Eliasoph L, Alman B (2006) Long-term benefits of Deflazacort treatment for boys with Duchenne muscular dystrophy in their second decade. Neuromuscul Disord 16(4):249–255
Houde S, Filiatrault M, Fournier A et al (2008) Deflazacort use in Duchenne muscular dystrophy; an 8-year follow-up. Pediatr Neurol 38(3):200–206
Markham LW, Kinnett K, Wong BL, Woodrow BD, Cripe LH (2008) Corticosteroid treatment retards development of ventricular dysfunction in Duchenne muscular dystrophy. Neuromuscul Disord 18(5):365–370
Schram G, Fournier A, Leduc H, Dahdah N, Therien J, Vanasse M et al (2013) All-cause mortality and cardiovascular outcomes with prophylactic steroid therapy in Duchenne muscular dystrophy. J Am Coll Cardiol 61:948–954
Barber BJ, Andrews JG, Lu Z, West NA, Meaney J, Price ET et al (2013) Oral corticosteroid and onset of cardiomyopathy in Duchenne muscular dystrophy. J Pediatr 163:1080–1084
Kim S, Zhu Y, Romitti PA, Fox DJ, Sheehan DW, Valdez R, Matthews D, Barber BJ (2017) Associations between timing of corticosteroid treatment initiation and clinical outcomes in Duchenne muscular dystrophy. Neuromuscul Disord 27(8):730–737
Birnkrant DJ, Bushby K, Bann CM, Apkon SD, Blackwell A, Brumbaugh D (2018) Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol 17(3):251–267
Birnkrant DJ, Bushby K, Bann CM, Alman BA, Apkon SD, Blackwell A (2018) Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol 17(3):251–267
Santaguida PL, Don-Wauchope AC, Oremus M, McKelvie R, Ali U, Hill SA et al (2014) BNP and NT-proBNP as prognostic markers in person with acute decompensated heart failure: a systematic review. Heart Fail Rev 19:453–470
Villa CR, Czosek RJ, Ahmed H, Khoury PR, Anderson JB, Knilans TK et al (2015) Ambulatory monitoring and arrhythmic outcomes in pediatric and adolescent patients with Duchenne muscular dystrophy. J Am Heart Assoc 5(1):e002620
Funding
Database creation and management was funded by the Division of Pediatric Cardiology at Golisano Children’s Hospital, University of Rochester Medical Center, Rochester, New York. David R. Weber received salary support from NIH Grant K23DK114477.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest are reported for any of the authors on this manuscript.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
A waiver of informed consent was obtained for this study given the retrospective nature.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wittlieb-Weber, C.A., Knecht, K.R., Villa, C.R. et al. Risk Factors for Cardiac and Non-cardiac Causes of Death in Males with Duchenne Muscular Dystrophy. Pediatr Cardiol 41, 764–771 (2020). https://doi.org/10.1007/s00246-020-02309-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-020-02309-y