Abstract
The native T1 value at 3.0 Tesla is a sensitive marker of diffuse myocardial damage. We evaluated the clinical usefulness of native T1 mapping in symptomatic adults with congenital heart disease (CHD), particularly in the systemic right ventricle (RV). Prospectively, 45 consecutive symptomatic adults with CHD were enrolled: 20 with systemic RV and 25 with tetralogy of Fallot underwent cardiac magnetic resonance (CMR) imaging at 3.0 Tesla. The Modified Look-Locker Inversion recovery sequence was used for T1 mapping. Cardiovascular events in the systemic RV were defined as heart failure and tachyarrhythmia. Brain natriuretic peptide (BNP) and indexed systemic ventricular end-diastolic volume were significantly higher in the systemic RV group. The native T1 value and extracellular volume (ECV) of the septal and lateral walls were higher in the systemic RV group, suggesting high impairment of the myocardium in the systemic RV group. There was a strong correlation between the native T1 value and ECV of the septum (r = 0.58, P = 0.03) and lateral wall (r = 0.56, P = 0.046) in the systemic RV group. Seven patients with systemic RV had cardiovascular events. In univariate logistic regression analysis, BNP and native T1 values of the insertion point were important for predicting cardiovascular events. The native T1 value at 3.0 Tesla may be a sensitive, contrast-free, and non-invasive adjunct marker of myocardial damage in CHD and predictive of cardiovascular events in the systemic RV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
T1 mapping techniques in cardiac magnetic resonance (CMR) imaging allow quantitative tissue characterization in the myocardium, adding new information to that provided by T1-weighted techniques such as late gadolinium enhancement (LGE) imaging. For focal fibrosis, LGE provides excellent delineation of lesions with some means of quantifying their volume [1]; however, LGE does not give a T1 estimate and may not be able to identify widely distributed or diffuse myocardial diseases. These are the obvious limitations of LGE techniques. Mapping of the longitudinal relaxation time (T1) and extracellular volume (ECV) offers a means of identifying and quantifying pathological changes in myocardial tissue, including diffuse changes that may be invisible to LGE [1,2,3]. Use of myocardial T1 mapping is now widespread in various cardiomyopathies and infiltrative diseases [3,4,5]. In other words, myocardial T1 mapping is a non-invasive modality to assess ECV for estimating diffuse myocardial fibrosis, by measuring myocardial and blood T1 relaxation time before and after contrast enhancement.
Several T1 mapping studies in congenital heart disease (CHD) gave the highest priority to the assessment of ECV; however, adults with CHD sometimes have chronic renal failure, and the contrast medium administration is contraindicated. Furthermore, flow measurements are often very complex and time-consuming; therefore, not all patients can tolerate the large amount of time required for scanning with gadolinium. As an alternative, native T1 values may be a sensitive, contrast-free, and non-invasive CMR marker of myocardial damage. Recently, some studies in non-CHD patients reported that native myocardial T1 values of 3.0 Tesla CMR imaging provide better discrimination between normal and diffusely diseased myocardium, including hypertrophied cardiomyopathy (HCM) and dilated cardiomyopathy (DCM) [3,4,5]. It is important to detect potential myocardial impairment in systemic right ventricle (RV) impairment, such as congenitally collected transposition of the great arteries (CCTGA), transposition of the great arteries (TGA), and single ventricle. The reason is that patients with systemic RV have anatomically vulnerable myocardia that are intolerant of systemic pressure for long periods of time. To optimize the timing of interventions, including surgical valve replacement and/or cardiac resynchronization therapy, visualization of ventricular tissue deterioration may be clinically useful.
In this study, we focused on native myocardial T1 values at 3.0 Tesla in symptomatic adults with CHD, particularly in the systemic RV, and we evaluated the potential value of using this method for detecting myocardial damage.
Materials and Methods
We prospectively enrolled 45 consecutive symptomatic adult CHD patients who were in New York Heart Association functional class ≥ 2: 20 consecutive adult patients with systemic RV (such as TGA and CCTGA) and 25 consecutive adult patients with tetralogy of Fallot (TOF) who had undergone intracardiac repair. Each patient underwent CMR imaging at Tokyo Women’s Medical University between March 2018 and March 2019. Cardiovascular events in the systemic RV were defined as heart failure requiring hospitalization, tachyarrhythmias requiring medication, and ablation and/or implantation of a cardiac defibrillator within the previous 2 years. A blood test was performed within 3 months, and catheterization was performed within 2 years of CMR imaging. Twenty-five age-matched, healthy adults were also enrolled as a control group. These controls underwent CMR imaging for screening of palpitations and non-specific chest pain and showed normal results on all tests including electrocardiography, brain natriuretic peptide (BNP), transthoracic echocardiography, CMR and/or coronary magnetic resonance, and/or computed tomography angiography. No controls had hypertension, diabetes mellitus, or ischemic heart disease.
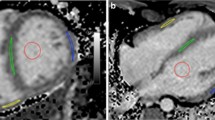
All participants underwent CMR imaging with a 3.0-Tesla whole-body imager (Ingenia 3 T; Philips Healthcare, Best, the Netherlands) equipped with dual-source, parallel radiofrequency transmission, and 32-element cardiac phased-array coils for radiofrequency reception. The Modified Look-Locker Inversion recovery sequence was used for T1 mapping. Mid-ventricular short-axis slices were scanned for myocardial T1 values before and after contrast imaging. Other scan parameters were as follows: field of view: 360 mm, matrix size: 128 × 256, SENSE factor: 2, repetition time: 2.7 ms, echo time: 1.26 ms, slice thickness: 8 mm, flip angle: 10°, TFE factors: 33, and shot mode: single-shot. Post-contrast imaging was performed approximately 12–15 min following administration of 0.1 mmol/kg gadoteric meglumine (Magnescope; Guerbet, Tokyo, Japan). Hematocrit was determined from a venous blood sample following CMR imaging. An elliptical region of interest (ROI) was drawn in the septal free walls and insertion points of the left ventricle (LV) and the RV on mid-ventricular short-axis images, avoiding regions with artifacts and ensuring that there was neither pooled blood nor epicardial fat in the ROI (Fig. 1). ECV was calculated based on pre- and post-contrast T1 values and hematocrit, as proposed by Flett et al. [6].
Native T1 mapping and late gadolinium enhancement in adults with congenitally corrected transposition of the great arteries. A ellipse region of interest (ROI) was drawn in the septal, free walls, and insertion points of the LV and the RV at mid-ventricular short-axis images, avoiding regions with artifact and ensuring that neither blood pool nor epicardial fat in the ROI
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. A priori approval was received from the institution's human research committee, and the ethical committee of our hospital approved our study. All patients provided informed consent for undergoing CMR imaging.
Statistics
Statistical analyses were performed using one-way analysis of variance. Continuous variables were assessed using Pearson’s or Spearman’s correlation coefficient. Logistic regression analysis was used to assess important parameters of cardiovascular events. Intra- and interobserver variabilities were assessed using intra- and interclass correlation coefficients, absolute values, and 95% confidence intervals.
Results
Basic Characteristics and CMR Imaging Data (Table 1)
BNP and indexed systemic ventricular end-diastolic volume (EDV) were significantly higher in the systemic RV group than in the TOF group. Peripheral capillary oxygen saturation and indexed pulmonary ventricular EDV were significantly lower in the systemic RV group than in the TOF group. There was no significant difference in age, blood pressure, systemic or pulmonary ejection fraction (EF), end-diastolic pressure, cardiac index, or mean pulmonary arterial pressure between the systemic RV and TOF groups.
T1 Mapping Data at 3.0 Tesla (Table 2) (Fig. 2)
Systemic ventricular native T1 values in the systemic RV group, pulmonary ventricular native T1 values in the TOF groups, and insertion point native T1 values were prolonged compared to those in the control group. Systemic and pulmonary ventricular ECV in the systemic RV and TOF groups were higher than those in the control group. Septal and lateral native T1 and ECV in the systemic RV group were higher than those in TOF group. There was a strong correlation between native T1 values and ECV of the septum (r = 0.58, P = 0.03) and lateral wall (r = 0.56, P = 0.046) in the systemic RV group.
Cardiovascular Events in Symptomatic Patients with Systemic RV (Table 3)
Seven patients with systemic RV had cardiovascular events: 4 patients had heart failure and 5 had atrial arrhythmias (tachycardia/atrial flutter/atrial fibrillation, overlapped) within the previous 2 years. There were no episodes of sustained ventricular tachycardia or death. In univariate logistic regression analysis, BNP and native T1 values of the insertion point were important parameters predicting cardiovascular events. Age, lower RV EF, and septal native T1 values also showed the tendency of important markers (P = 0.06, 0.08, and 0.06, respectively) but were not significant statistically.
Reproducibility
The intraclass correlation coefficients for the interventricular septum, RV insertion points, and LV free wall T1 values were 0.82, 0.79, and 0.80, respectively. The interclass correlation coefficients for the septum, RV insertion points, and LV free wall T1 values were 0.80, 0.76, and 0.75, respectively.
Discussions
This is a preliminary study of native T1 mapping and its potential clinical usefulness in adults with CHD. Our results showed that (1) the septal and lateral native T1 values and ECVs were higher in the systemic RV group than in the TOF group (2) there was a strong correlation between native T1 values and ECV in the systemic RV group, and (3) native T1 values of the insertion point, as well as BNP, were relevant predictive factors for cardiovascular events in the systemic RV group using 3.0 Tesla CMR imaging.
Native T1 Values in CHD and the Systemic RV in Particular
Generally, myocardial damage in CHD can be caused by volume and/or pressure overload, prolonged cyanosis before intracardiac repair in the childhood, and longer cross-clamp times during surgery [7,8,9,10]. Systemic ventricular myocardium deterioration is common in middle-aged adults with systemic RV [11]. Systemic RV function with significant tricuspid regurgitation becomes more easily deteriorated; however, it is difficult to assess genuine ventricular damage only by EF using echocardiography or CMR cine images because of Frank-Starling's law [12]. Therefore, additional parameters are needed to evaluate real myocardial damage proactively.
Before evaluating T1 map, we should know the following details. Normal myocardium is composed of three major compartments: intracellular, intravascular, and interstitial [2]. The intracellular compartment is the largest of the three in normal myocardium and consists predominantly of myocytes, but also includes fibroblasts, endothelial cells, and smooth muscle cells. The interstitial and intravascular compartments are often referred to as the ECV. In the majority of cardiovascular diseases such as extracellular edema, interstitial replacement, or infiltrative fibrosis, the ECV becomes expanded, primarily due to expansion of the interstitial component [2, 3]. Native T1 mapping refers to the acquisition of T1 maps without the use of contrast agents. In edema and other fibrotic conditions, expansion of the extracellular space results in an increase of native T1 values as well.
Myocardial fibrosis is not considered rare in the systemic RV [13, 14] and is associated with higher BNP [15]. Elevation of ECV was reported to be associated with adverse clinical outcomes as well [15]. An increased native T1 value and/or ECV reflect the proportionally larger extracellular matrix component subsequent to cardiomyocytes atrophy and/or death [3, 9, 16]. If a contrast-free CMR imaging marker was available, it would be more convenient. Our result showed a strong correlation between native T1 values and ECV; therefore, native T1 values may be an alternative marker of potential myocardial damage in this population as reported in the population with HCM or DCM [4, 5]. In adults with CHD with low pre-test possibility for myocardial impairment or for those in whom contrast administration is contraindicated, native T1 values may serve as an effective screening test.
Some studies also reported that native T1 values and ECV for 1.5 Tesla CMR imaging were higher in the systemic RV than in TOF [15]; therefore, our results were reasonable. A profibrotic state through genetically driven collagen metabolism precedes the overt phenotype with LV hypertrophy or fibrosis visible on LGE in cardiomyopathy [16]; therefore, native T1 values may be adjunct but can be clinically informative. Contrast-free markers of adverse cardiac remodeling may be able to predict therapy response and/or guide treatment strategies. It has been reported that TOF after PVR may improve native T1 value and/or ECV compared to that before PVR; therefore, there is a possibility that native T1 value and/or ECV can be useful parameters of reversible myocardial damage. These parameters may be informative when we decide the optimal timing of tricuspid valve replacement in the systemic RV before irreversible myocardial damage occurs.
Why was the Native T1 Value of the Insertion Points Relevant?
LGE and/or elevated native T1 values of the insertion points are often identified in patients with HCM and pulmonary arterial hypertension (PAH) [17,18,19]. These findings are considered to represent focal plexiform fibrosis associated with myocardial disarray and increased connective tissue density rather than replacement fibrosis [19,20,21,22]. On the other hand, some studies have reported the prognostic significance of them in PAH and HCM [20,21,22].
In the systemic RV, ventricles become hypertrophied and become a similar shape to HCM. This may be one of the reasons why patients with systemic RV often show elevated native T1 values of the RV insertion point. Another possibility is that there is secondary pulmonary hypertension associated with left-sided heart failure. Not only patients with genuine PAH but also those with secondary PAH show elevated native T1 values of the insertion point [23]. Few studies have focused on native T1 values of the insertion point as well as septum and/or free wall in CHD, so further studies are required.
The Possibility of 3.0 Tesla CMR Imaging in Adults with CHD
Previous CHD studies at 1.5 Tesla mainly focused on ECV [24] rather than native T1 values because there appeared to be no statistical significance in native T1 values among various CHDs. This may be partially due to the technical difference/limitation between 1.5 and 3.0 Tesla. Native T1 values at 1.5 Tesla are reported to underestimate myocardial infarction compared to those at 3.0 Tesla [25]; therefore, native T1 images at 3.0 Tesla in adults with CHD may be more sensitive, but further studies in a large cohort are needed to confirm that these findings are clinically useful.
Limitations
The limitations of this study are the very small population size and the lack of a cox-hazard analysis due to the short follow-up period; therefore, the prognostic values of native T1 mapping remain unknown. This is a preliminary study of native T1 mapping at 3.0 Tesla in adults with CHD, but we believe that this study is clinically informative. Furthermore, the native T1 values of the insertion points were found to be less reproducible than those of the interventricular septum. This may be due to higher T1 heterogeneity in this region leading to greater T1 variation when ROI placement differs.
Conclusions
The native T1 value at 3.0 Tesla may be a sensitive, contrast-free, and non-invasive adjunct marker of myocardial damage in CHD and may be predictive of cardiovascular events in the systemic RV.
Abbreviations
- CHD:
-
Congenital heart disease
- LGE:
-
Late gadolinium enhancement
- CMR:
-
Cardiac magnetic resonance
- BNP:
-
Brain natriuretic peptide
- ECV:
-
Extracellular volume
- HCM:
-
Hypertrophic cardiomyopathy
- DCM:
-
Dilated cardiomyopathy
- RV:
-
Right ventricle
- CCTGA:
-
Congenitally collected transposition of the great arteries
- TGA:
-
Transposition of the great arteries
- TOF:
-
Tetralogy of Fallot
- ROI:
-
Region of interest
- LV:
-
Left ventricle
- EDV:
-
End-diastolic volume
- EF:
-
Ejection fraction
- PAH:
-
Pulmonary arterial hypertension
References
Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM (2000) The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 343:1445–1453
Aherne E, Chow K, Carr J (2019) Cardiac T1 mapping: techniques and applications. J Magn Reson Imaging. https://doi.org/10.1002/jmri.26866
Haaf P, Garg P, Messroghli DR, Broadbent DA, Greenwood JP, Plein S (2016) Cardiac T1 Mapping and extracellular volume (ECV) in clinical practice: a comprehensive review. J Cardiovasc Magn Reson 18(1):89
Puntmann VO, Voigt T, Chen Z, Mayr M, Karim R, Rhode K, Pastor A, Carr-White G, Razavi R, Schaeffter T, Nagel E (2013) Native T1 mapping in differentiation of normal myocardium from diffuse disease in hypertrophic and dilated cardiomyopathy. JACC Cardiovasc Imaging 6(4):475–484
Nakamori S, Dohi K, Ishida M, Goto Y, Imanaka-Yoshida K, Omori T, Goto I, Kumagai N, Fujimoto N, Ichikawa Y, Kitagawa K, Yamada N, Sakuma H, Ito M (2018) native t1 mapping and extracellular volume mapping for the assessment of diffuse myocardial fibrosis in dilated cardiomyopathy. JACC Cardiovasc Imaging 11(1):48–59
Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, McGregor C, Moon JC (2010) Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation 122(2):138–144
Hanneman K, Crean AM, Wintersperger BJ, Thavendiranathan P, Nguyen ET, Kayedpour C, Wald RM (2018) The relationship between cardiovascular magnetic resonance imaging measurement of extracellular volume fraction and clinical outcomes in adults with repaired tetralogy of Fallot. Eur Heart J Cardiovasc Imaging 19:777–784
Riesenkampff E, Luining W, Seed M, Chungsomprasong P, Manlhiot C, Elders B, McCrindle BW, Yoo SJ, Grosse-Wortmann L (2016) Increased left ventricular myocardial extracellular volume is associated with longer cardiopulmonary bypass times, biventricular enlargement and reduced exercise tolerance in children after repair of Tetralogy of Fallot. J Cardiovasc Magn Reson 18:75
Chen CA, Dusenbery SM, Valente AM, Powell AJ, Geva T (2016) Myocardial ECV fraction assessed by CMR is associated with type of hemodynamic load and arrhythmia in repaired tetralogy of fallot. JACC Cardiovasc Imaging 9:1–10
Yim D, Riesenkampff E, Caro-Dominguez P, Yoo SJ, Seed M, Grosse-Wortmann L (2017) assessment of diffuse ventricular myocardial fibrosis using native t1 in children with repaired tetralogy of fallot. Circ Cardiovasc Imaging 10(3):e005695
Lee J, Ruygrok PN, Gerber IL, Finucane AK (2019) Natural history of a systemic right ventricle. Intern Med J 49(3):388–391
Sequeira V, van der Velden J (2015) Historical perspective on heart function: the Frank-Starling Law. Biophys Rev. 7(4):421–447
Broberg CS, Valente AM, Huang J, Burchill LJ, Holt J, Van Woerkom R, Powell AJ, Pantely GA, Jerosch-Herold M (2018) Myocardial fibrosis and its relation to adverse outcome in transposition of the great arteries with a systemic right ventricle. Int J Cardiol 15(271):60–65
Ladouceur M, Baron S, Nivet-Antoine V, Maruani G, Soulat G, Pereira H, Blanchard A, Boutouyrie P, Paul JL, Mousseaux E (2018) Role of myocardial collagen degradation and fibrosis in right ventricle dysfunction in transposition of the great arteries after atrial switch. Int J Cardiol 258:76–82
Broberg CS, Chugh SS, Conklin C, Sahn DJ, Jerosch-Herold M (2010) Quantification of diffuse myocardial fibrosis and its association with myocardial dysfunction in congenital heart disease. Circ Cardiovasc Imaging 3(6):727–734
Kellman P, Wilson JR, Xue H, Bandettini WP, Shanbhag SM, Druey KM, Ugander M, Arai AE (2012) Extracellular volume fraction mapping in the myocardium, part 2: initial clinical experience. J Cardiovasc Magn Reson 14:64
Ho CY, López B, Coelho-Filho OR, Lakdawala NK, Cirino AL, Jarolim P, Kwong R, González A, Colan SD, Seidman JG, Díez J, Seidman CE (2010) Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N Engl J Med 363:552–563
Saunders LC, Johns CS, Stewart NJ, Oram CJE, Capener DA, Puntmann VO, Elliot CA, Condliffe RC, Kiely DG, Graves MJ, Wild JM, Swift AJ (2018) Diagnostic and prognostic significance of cardiovascular magnetic resonance native myocardial T1 mapping in patients with pulmonary hypertension. J Cardiovasc Magn Reson 20(1):78
Bravo PE, Luo HC, Pozios I, Zimmerman SL, Corona-Villalobos CP, Sorensen L, Kamel IR, Bluemke DA, Wahl RL, Abraham MR, Abraham TP (2016) Late gadolinium enhancement confined to the right ventricular insertion points in hypertrophic cardiomyopathy: an intermediate stage phenotype? Eur Heart J Cardiovasc Imaging 17(3):293–303
van Wolferen SA, Marcus JT, Boonstra A, Marques KM, Bronzwaer JG, Spreeuwenberg MD, Postmus PE, Vonk-Noordegraaf A (2017) Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J 28(10):1250–1257
McCann GP, Gan CT, Beek AM, Niessen HW, Vonk Noordegraaf A, van Rossum AC (2007) Extent of MRI delayed enhancement of myocardial mass is related to right ventricular dysfunction in pulmonary artery hypertension. AJR Am J Roentgenol 188(2):349–355
Bradlow WM, Assomull R, Kilner PJ, Gibbs JS, Sheppard MN, Mohiaddin RH (2010) Understanding late gadolinium enhancement in pulmonary hypertension. Circ Cardiovasc Imaging 3(4):501–503
Zhu Y, Park EA, Lee W, Kim HK, Chu A, Chung JW, Park JH (2015) Extent of late gadolinium enhancement at right ventricular insertion points in patients with hypertrophic cardiomyopathy: relation with diastolic dysfunction. Eur Radiol 25(4):1190–1200
Riesenkampff E, Messroghli DR, Redington AN, Grosse-Wortmann L (2015) Myocardial T1 mapping in pediatric and congenital heart disease. Circ Cardiovasc Imaging 8(2):e0025
Kali A, Cokic I, Tang RL, Yang HJ, Sharif B, Marbán E, Li D, Berman DS, Dharmakumar R (2014) Determination of location, size, and transmurality of chronic myocardial infarction without exogenous contrast media by using cardiac magnetic resonance imaging at 3 T. Circ Cardiovasc Imaging 7(3):471–481
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Ethical Approval
Institutional Review Board approval was obtained. Study subjects or cohorts overlap: No study subjects or cohorts have been previously reported. Methodology: Prospective diagnostic or prognostic study performed at one institution.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shiina, Y., Inai, K., Taniguchi, K. et al. Potential Value of Native T1 Mapping in Symptomatic Adults with Congenital Heart Disease: A Preliminary Study of 3.0 Tesla Cardiac Magnetic Resonance Imaging. Pediatr Cardiol 41, 94–100 (2020). https://doi.org/10.1007/s00246-019-02227-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-019-02227-8