Abstract
Thyroid hormones play critical roles in body growth and development as well as reproduction. They also influence the activities of a wider variety of tissues and biological functions, such as osmoregulation, metabolism, and especially metamorphosis in organisms, such as frogs. These complex activities of thyroid hormones are prone to disruption by agricultural pesticides, often leading to modulation of growth and the reproductive system in particular. These substances include Glufosinate ammonium, Glyphosates, Imazapyr, Penoxsulam, and Diquat dibromide among other herbicides. In this study, the standardized Xenopus Metamorphosis Assay protocol was used to assess the potential thyroid-modulatory properties of the Glufosinate ammonium Basta formulation, at relevant environmental concentrations (0.05 mg/L, 0.15 mg/L, and 0.25 mg/L) for 21 days. The results showed that this formulation only reduced the hind-limb length among the morphological endpoints. Histological evaluation showed that the mean thyroid gland area and the mean thyroidal follicle epithelium height were significantly increased following 0.15 and 0.25 mg/L exposures. The present study confirmed that this Basta formulation interacts with the thyroid axis and therefore potentially pose health hazard to amphibian in particular and potentially metamorphic aquatic vertebrates. Furthermore, the result is a signal of inherent potential thyroid disrupting activities that must be further investigated and characterised in some of the aquatic herbicide formulations to safeguard the aquatic biodiversity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A decline of amphibian populations on a global scale has been suggested (Hayes et al. 2010; Wagner et al. 2013), and pollution has been highlighted as one of the main contributing factors responsible for habitat degradation or physiological modulation (Hayes et al. 2010). Chemical compounds, in particular linked to agricultural practices (pesticides and fertilizers) and waste water treatment discharges, have been shown to compromise the health of aquatic vertebrates (Jobling and Tyler 2003; Nugegoda and Kibria 2017), including amphibians (Kloas et al. 1999; Hayes et al. 2010), but also terrestrial wildlife (Rosenfelt et al. 2017) and human (Bergman et al. 2012), reliant of clean, unpolluted water. Wastewater treatment plants have been shown to be inefficient in removing all compounds (micro pollutants) before discharge (Archer et al. 2017). Many of these manmade chemicals that end up in natural water bodies may act subtly through disrupting endocrine pathways or function (endocrine disruptors [EDCs]) (Bergmen et al. 2012) and modulate development and reproductive success (Hayes et al. 2010).
Although the initial focus of acute or chronic disruption of the endocrine system was on reproductive endocrinology and function, wider disruption, for example modulating the thyroid system has been implicated (Brucker-Davis 1998; Decherf et al. 2010; Nugegoda and Kibria 2017; van Wyk 2013). Thyroid hormones in vertebrates play important regulatory roles linked to a wide range of physiological systems including metabolism, osmoregulation, development, growth, and many others (Nugegoda and Kibria 2017; Opitz et al. 2006a; Tan and Zoeller 2007; Zoeller 2007), but specifically in amphibians, the thyroid axis is known to control the pre- and prometamorphic phases through final metamorphosis (Denver 2013; Kloas et al. 1999; Shi 1999; Tietge et al. 2005; van Wyk 2013). Therefore, it is not surprising that amphibian metamorphosis is regarded as a sensitive model for the detection of thyroid disruption at the whole-animal level. The standardized Xenopus Metamorphosis Assay (XEMA) considers differential development during the pre- and pro-metamorphosis phases of metamorphosis following exposure to selected chemicals or environmental water (OECD 2007, 2009; Grim et al. 2009). For aquatic wildlife species, thyroid disruption, therefore, could compromise health, fitness, and ultimately survival (Marlatt et al. 2013; Nugegoda and Kibria 2017; Opitz et al. 2006b; van Wyk 2013).
Herbicide formulations (comprises 43% of world’s pesticide sales) (EPA 2011), which are chemicals specifically designed to eradicate weeds and aquatic invasive plants, have been widely suggested to act as endocrine disrupting chemicals (Coady et al. 2005, 2013). Apart from a few studied herbicides, including 2.4D (Coady et al. 2013), Simetryn, Mefenacet, and Thiobencarb (Saka et al. 2013), Acetachlor (Crump et al. 2002), Atrazine (Kloas et al. 2009; Hayes et al. 2010), and glyphosates (Jones et al. 2011; Wagner et al. 2017, 2013), most of the current research have focused more on insecticides. Yet, many other herbicides await further study. The fact that these herbicides, particularly the aquatic herbicides, are deliberately applied into aquatic environment, the complete characterization of their expected impacts is compulsory.
A wide range of herbicides are used by the South African agriculture sector. In addition, herbicides also are used in the South African governmental plant eradication programme (in partnership with local communities as a job creation initiative) “Working for Water (WfW),” to clear natural waterways from exotic and invasive plant species (Hill and Coetzee 2017). Herbicides are mainly used on land but could be applied in areas close to receiving waters or in some cases directly in overgrown waterbodies (Hill and Coetzee 2017). The potential thyroid disrupting effects of several of these commonly used herbicide formulations, particularly those containing glufosinate ammonium as active ingredients, are still largely unknown.
Glufosinate ammonium (N-phosphonomethyl glycine), a phosphinic acid, mostly marketed as an ammonium salt, is a broad-spectrum and systemic herbicide (Ebert et al. 1990; Hack et al. 1994). Due to glufosinate ammonium’s structural analogy with glutamate, it acts as an irreversible inhibitor of glutamine synthetase activity in tissues and often leads to increases in glutamate and ammonia synthesis (Hack et al. 1994). Commercial formulations of glufosinate ammonium include Basta, Rely, Finale, and Challenge. Glufosinates have a high propensity to soil particles but also are highly soluble in water, with a half-life that varies from 3 to 43 days, but which may double under certain conditions (Jewell et al. 1998) and approximately 300 days in water respectively (Sparling 2016). The US-EPA categorized Glufosinate ammonium as persistent in plants (lack of degradation) and mobile in soil (ease of transport).
The purpose of the present study was to evaluate the thyroid disruption potential of the Basta herbicides formulation with Glufosinate ammonium as the active ingredient, using the Xenopus metamorphosis assay (Opitz et al. 2005; 2006a, b; Tietge et al. 2005, 2013; OECD 2008).
Materials and Methods
Test Chemical
Basta formulation (Bayer Crop Science AG Ltd, Germany), containing glufosinate ammonium (200 g/L) as an active ingredient, was purchased from a local agricultural outlet, Kaap Agric (Agrimark) in Stellenbosch, South Africa. The Basta formulation contains 18.5% active glufosinate ammonium and 30% of sodium polyoxyethylene alkylether sulphate (AES) surfactant (Koyama et al. 1997).
Xenopus laevis Breeding and Tadpole’s Culture
Mature males and females were selected from an in-house breeding stock of X. laevis, and separately maintained in a 15-L glass tank that was filled with reverse osmosis water (buffered with 2.5 g sea salt and 0.8 g NaHCO3/10 L) (Kloas et al. 1999). The adult frogs were fed fish pellets (Aqua-Nutro, South Africa), three times per week. The holding tanks were cleaned and refilled with fresh buffered reverse osmosis water after each feeding. Gutter down-piping sections were placed in the holding tanks to create hideouts for the frogs. The breeding induction was performed following the ASTM (1998) protocol. In brief, the male and female frogs were primed with 100 IU of human chorionic gonadotropin (hCG) (Merck Ltd Germany), which was injected into their dorsal lymph sac 4 days before the initiations of mating activities, followed by another injection of 100 IU and 300 IU hCG, to the males and females respectively, just before mating. The pair of male and female were placed together into a 15-L breeding tank, which was lined with plastic netting, to separate the eggs from the adults during oviposition. The whole breeding tank was placed in a well-ventilated dark place, away from interference and disturbance. Amplexus followed and eggs were deposited within the next 12 h post the second hCG injection. Adults were removed and returned to separate holding tanks after the egg-laying stopped. The eggs were aerated and hatched tadpoles were transferred into different 15-L holding tanks at a density of 50 tadpoles per 10-L water to reduce an overcrowding effect on growth. Feeding of the tadpoles commenced at free swimming stage (NF-stage 47–48). Tadpoles were fed twice daily until NF stage 51 with Sera Micron (Sera Heinsberg, Germany) (30 mg/animal/day). Staging (NF-stage) of the tadpoles were checked daily using developmental atlas by Nieuwkoop and Faber (1994). All the housing, breeding, and exposure procedures were approved by the Animal Research Ethical Committee of the Stellenbosch University (Approval no-SU-ACUM 12–15).
Test Procedure
Exposure Setup
At NF-stage 51, twenty tadpoles were selected from the rearing tanks and transferred into 15-L exposure tanks, with two replicates per exposure concentration, but four replicates for the control. The exposures were performed under controlled conditions with the pH between 7.5 and 8.5, water temperature to 23 ± 1 °C, dissolved oxygen concentration of > 6.5 mg/L, and a photoperiod of 12:12 h of light and dark (L12D12) regime, as recommended in the validated OECD (2008) protocol. Food ration was increased to 50 mg/animal/day to account for the increase in growth of the tadpoles. The whole exposure was repeated twice to increase the exposure precision.
Exposure Concentrations
Based on the initial 96-h-LC 50 value of 0.59 mg/L derived at NF stage 48 of X. laevis development (Babalola and Wyk 2018), three environmentally relevant exposure concentrations (0.05, 0.15, and 0.25 mg/L) (Faber et al. 1998) of the Basta formulation were derived to be representative below this lethal toxicity threshold. Mortality in exposure tanks were monitored every 8 h, and the exposure medium was completely replaced every third day. Only mortality incidence less than 10% in the control group was accepted for the experiment (OECD 2008).
Nominal Concentration Test
To confirm the exposure concentrations, samples of the exposure tank were taken 1 h after the herbicide was introduced. The samples were analysed at Envirotech Laboratory, Lagos Nigeria, using the gas chromatography, which had been effectively used to quantify Glufosinate ammonium (Kataoka et al., 1996; Qian et al., 2011; Yun et al., 2014). The detected concentrations showed low variations relative to the predicted nominal concentrations (Appendix Table 2). The limit of detection was 0.05 μg/L.
Autopsy Procedure
At the end of the 21-day exposure, the tadpoles were euthanized in 0.1% benzocaine (Sigma). The tadpoles were then blotted dry, weighed (to the nearest 0.01 g), and measured (snout–vent length (SVL) to the nearest 0.1 mm). The tadpoles were subsequently fixed in Davidson’s solution (Shi et al. 2012; OECD 2007) for 72 h and then preserved in 4% neutral buffered formalin. Developmental stages (NF) were determined for each of the tadpoles using Nieuwkoop and Faber (1994). Five tadpoles (at the same medial developmental stage of the control exposure) per concentration were randomly selected for histological assessment of the thyroid gland.
Morphometric Measurement and Histological Procedures
The front limb length (FLL) and hind limb length (HLL) were measured (to nearest the 0.1 mm) with a Leica EZ4D stereo microscope (Leica Microscope Ltd, DE). Using a metric trace ruler which has the capacity to measure both straight and curved lines, the lengths of the hind limbs were measured using the digital photographs of the tadpoles with Laz Es Software (Leica, DE). The preserved tadpoles were decapitated just posterior to the eyes, and the heads containing the thyroid glands were subjected to routine paraffin wax (Histosec, Sweden) embedding, (56 °C melting point) and histological processing (Bancroft and Stevens 1977).
Histological Procedures
The embedded heads were sectioned at 7–8 µm using a Reichert-Jung microtome (Cambridge Instruments, Germany). The sections were mounted on albumin coated glass slides. The unstained slides were used to confirm sectioning of the thyroid glands, thereafter sections were made serially. Selected sections were oven-dried overnight at 40 °C and subsequently dewaxed (xylene), hydrated in a decreasing alcohol series, and stained using hematoxylin and eosin (Bancroft and Stevens 1977). The stained sections were dehydrated in an increasing series of alcohols before being cleared in xylene and glass cover slips mounted, using a resin-based mounting medium (DPX, Sigma).
Histological Measurement of the Thyroid
The right-side thyroid glands were selected for measurements. A Leica DMLB microscope equipped with digital camera (Leica Microscope Ltd, DE) was used to photograph representative pictures of the thyroid. Image analysis was conducted using S-Viewer Analysis software module (S-View Technology, China). Thyroids that were sectioned centrally and diagonally were selected for measurement and assessment of thyroid follicles and epithelial cells. The heights of six randomly selected follicular cells were measured in ten different follicles, which produced 60 cell-height measurements per individual. The group mean for follicle cell height was derived from the calculated individual means. Using the same image, the follicular cross sectional and thyroid cross-sectional areas were calculated. Ten follicles from each of the sections were measured, producing ten estimatess for each tadpole. The data per exposure group were derived from the sum of all the tadpoles of each group. The average sum total of the cross-sectional areas produced the gland area for each exposure group.
Data Analyses
Variation in median NF-stage among exposure groups was assessed using the non-parametric Kruskal–Wallis test, followed by the Dunn’s multiple comparison test (DMCt) to determine significant pairwise differences in developmental stage. Normality and homogeneity variance of the wet body mass, whole body length, and snout-vent-length data were assessed using the Shapiro–Wilk’s and Levene’s tests. To correct for the growth impacts on front and hind limbs, their data were normalized to SVL and was further assessed using residuals “normal” probability plots. Parametric data were analysed using one-way ANOVA, whereas the Kruskal–Wallis ANOVA test (K-W ANOVA) was applied for nonparametric data. Tukey HSD test with Spjotfoll/Stoline correction was used for parametric data or the Dunn’s test for nonparametric data, pairwise variances between treatments and the control in morphological parameters including whole body length, whole body mass, SVL, and the front- and hind-limbs (normalized). The effect of treatment (specific herbicide concentration) on developmental stage and the interaction on front and hind limbs was tested using mixed model ANOVAs. In all analysis, significant differences were taken at P < 0.05. All of the statistical analyses were done using Statistica V12 (Statsoft Inc., USA).
Results
Mortality
There was no incidence of mortality in all the exposure and control tanks throughout the 21-day exposure period.
Morphometric Analyses
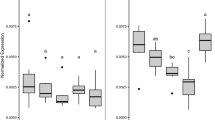
The exposure impact of tadpoles led to a trend of increased mean whole-body length (WBL) and snout–vent-length (SVL) relative to the control exposure group (Fig. 1a, b). However, a Kruskal–Wallis ANOVA could not confirm that significant variation existed in size variables, mean WBL (Fig. 1a), and mean SVL (Fig. 1b; P > 0.05) at all exposure concentrations compared with the control group. In addition, the whole-body mass (WBM) was significantly unaffected in the various treatment groups (Fig. 1c; P > 0.05) compared with the control. Similarly, the mean front limb lengths (FLL; normalized) in treated tadpoles (Fig. 1d) did not vary significantly among experimental groups at treated concentrations relative to the control (P > 0.05). However, tadpoles exposed to a concentration of 0.05 mg/L showed a significant decrease in mean hind limb lengths (HLL; normalized) compared with the control (Fig. 1e) (Tukey HSD test; P < 0.05).
Variation in Developmental Stages
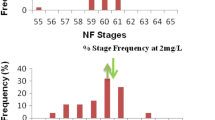
After the 21-day exposure, the emerged developmental stages of the treated tadpoles ranged from NF-stage 58 to NF-stage 63. The median of the frequency distribution in the control and lower Basta concentration (0.05 mg/L) group was NF-stage 58 (Fig. 2). The medians were higher than the control (NF-stage 60) at the higher Basta concentrations (0.15 mg/L and 0.25 mg/L) being NF-stage 60. However, the shifted median NF-stages (NF 58 vs NF 60) were not significantly different (Figs. 2 and 3; P > 0.05).
The frequency distribution (n = 30) of developmental stages of X. laevis tadpoles after 21-day treatment showing the control exposure relative concentration series of Basta formulation 0.05 mg/L, 0.15 mg/L, and 0.25 mg/L. The upward arrow indicates the median NF-stage of the control relative to downward arrow that showed the median at the various concentrations
Histopathological Endpoints
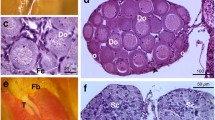
The mean thyroid colloidal (luminal) area did not vary significantly among the treated tadpoles relative to the control (P > 0.05). The mean follicle epithelium height, however, increased significantly in all three Basta treatments compared with the control (P < 0.05; Fig. 4; Table 1). Moreover, mean thyroid gland area was significantly higher in the two highest concentrations (0.15 mg/L and 0.25 mg/L) than the control (P < 0.05).
Discussion
The reality of global amphibian population decline is no longer in doubt, but what is still in doubt and very challenging is the underlying environmental factors that are responsible for this anomaly. This is because as much as the decline is concerned, the links to environmental pollutants that are potentially contributing to such declines are not well characterized. Currently, disruption of the thyroid and reproductive pathways through chemical/agrochemicals contamination has been listed amongst the potential contributing factors (Miyata and Ose 2012). Regulation by thyroid hormones is complex, and it is affecting various physiological processes (including growth, development, and reproductive), making the hypothalamus-pituitary-thyroid (HPT) axis a very important target, especially when endocrine-disrupting substances are in consideration. In particular, the potential impacts of environmental substances to modulate early developmental phases in vertebrate organisms may well have significant consequences, because organogenesis and development of functional physiological systems are regulated at this phase (Bergman et al. 2013).
In this present study, the tadpoles in the control group were in good health, and their growths were at the expected rate, with the median NF-stage at 58. Their growth pattern was consistent with the OECD prevalidation test guidelines, which recommended that control tadpoles should reach a minimum median developmental stage of NF-stage 57 at the test termination (OECD 2007). The tadpoles in the exposure groups showed varied developmental rates and were distributed between NF-stage 58–65. The concentrations used in this present study (were within predicted environmental concentrations) did not pose any lethal toxicity risk, as none of the exposed tadpoles died during the exposure. The overall MBM and SVL (± SD) of the control tadpoles, after 21-day exposure compared favourably with OECD’s phase 1 prevalidation studies, suggesting a mean body mass target of 0.944 g and a mean snout-vent length of 19.5 mm, respectively.
The fact that no significant variation occurred in size-related endpoints corroborate similar findings by Degitz et al. (2005) and Coady et al. (2010), that chemical alteration of thyroid axis do not necessarily affect the growth of tadpoles as the growth is not directly under thyroid hormone control. Tata (1998) also noted that thyroidectomised tadpoles continue to grow (under the influence of growth hormone and other growth-promoting factors), meaning that thyroid disruption does not stop the growth of the tadpoles.
Even though there was no significant difference in developmental rate in the treated tadpoles compared with the control group, histological evidence of individuals exposed to concentration of 0.15 and 0.25 mg/L of this formulation showed that significant increase in follicle epithelium and thyroid gland area occurred. Although no known thyroid activity studies using XEMA have been done for the Basta formulation, histopathological effects on thyroid glands histology in several studies have been demonstrated in the absence of developmental effects (OECD 2008), and the thyroid gland histology has been noted as the most sensitive parameter for the detection of the thyroidal effects (Brande-Lavridsen et al. 2010). The lack of developmental effects could be due to initial physiological thyroid compensatory mechanisms, which need further investigation. The observed enlarged thyroid gland following exposure to this formulation therefore suggests potential modulation in the thyroid hormone related pathways in exposed tadpoles. The enlarged gland area and increased follicle epithelium is also consistent with the results of several other related studies where tadpoles were exposed to propylthioural (PTU) (a known thyroid inhibitor), producing an enlarged thyroid gland (goitre effect) (Opitz et al. 2005; Miyata and Ose 2012). This therefore suggests that the Basta formulation may be upregulating T4 production or directly lower or inhibit circulating thyroid hormones, resulting in the overstimulation of the HPT axis (Degitz et al. 2005). The Basta formulation also may modulate the transformation of T4 to T3, hence, the normal stage development but increased T4 production.
The thyroid-disrupting potential of the Basta formulation at concentrations far below its expected and/or measured environmental concentration of 1.0 mg/L (Faber et al. 1998) must be regarded as an ecological concern in the aquatic environment. However, more research is still required to understand the interaction of the Basta formulation with the thyroid axis. Considering the observed thyroidal activities, at environmentally relevant concentrations, the full ecological consequence of this formulation on the processes of metamorphosis of many metamorphic organisms, particularly the fish and amphibian still need further research.
Conclusions
The present study applied the Xenopus metamorphosis assay to assess the sublethal/chronic toxicity of Basta formulation, a commercially available herbicide, mostly used to manage invasive alien plants along and in waterways, particularly in South Africa. Using morphometric, and histopathological endpoints, the outcome of the exposure showed that the herbicide formulations at varying concentrations did not inhibit the overall developmental programme, although aspects of the histopathology of the thyroid, including the thyroid gland, were significantly altered. These results confirm that despite the relatively low exposure concentrations, the Basta formulation is thyroid-active and could potentially cause serious physiological disruption to organisms and ecological risks in aquatic systems where the herbicide is applied. Therefore, the continued usage of this formulation needs to be reevaluated for the sake of metamorphic organisms and other important wildlife.
In South Africa, because of large volumes (and diversity) of herbicides being applied, continued attention must be directed to assessing the exposure impacts of these formulations on wildlife. In the light of thyroid gland impacts observed in this study, field observation and analysis might not be enough; chronic exposures at relevant environmental relevant dilutions will be important.
References
Archer E, Petrie B, Kasprzyk-Horden B, Wolfaardt GM (2017) The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters. Chemosphere 174:437–446
American Society for Testing and Materials (ASTM) (1998) Standard guide for conducting the frog embryo teratogenesis assay–Xenopus. E1439–98. In: Annual book of ASTM standards, vol 11.06. American Society for Testing and Materials, Philadelphia, pp 825–36
Babalola OO, van Wyk JH (2018) Comparative early life stage toxicity of African clawed frog, X. laevis following exposure to selected herbicide formulations applied to eradicate alien plants in South Africa. Arch Environ Contam Toxicol. https://doi.org/10.1007/S00244-017-0463-0
Bancroft JD, Stevens A (1977) Theory and practice of histological techniques. Churchill Livingstone, Edinburg
Bergman A, Heindel J, Jobling S, Kidd K, Zoeller RT (2012) State of the science of endocrine-disrupting chemicals 2012. WHO & UNEP, Geveva
Bergman A, Andersson A, Becher G, van den Berg M, Blumberg B, Bjerregaard P, Bornehag C, Bornman R, Brandt I, Brian JV, Casey SC, Fowler PA, Frouin H, Giudice LC, Iguchi T, Hass U, Jobling S, Juu A, Kidd KA, Kortenkamp A, Lind M, Martin OV, Muir D, Ochieng R, Olea N, Norrgren L, Ropstad RE, Ross PS, Rudén C, Scheringer M, Skakkebaek NE, Söder O, Sonnenschein C, Soto A, Swan S, Toppari J, Tyler CR, Vandenberg LR, Vinggaard AM, Wiberg K, Zoeller RT (2013) Science and policy on endocrine disrupters must not be mixed: a reply to a “common sense” intervention by toxicology journal editors. Environ Health 12:69
Brande-Lavridsen N, Christensen-Dalsgaard J, Korsgaard B (2010) Effects of ethinylestradiol and the fungicide prochloraz on metamorphosis and thyroid gland morphology in L. temporaria. Open Zool J 3:7–16
Brucker-Davis F (1998) Effects of environmental synthetic chemicals on thyroid function. Thyroid 8:827–849
Coady KK, Murphy MB, Villeneuve DL, Hecker M, Carr JA, Solomon KR, Smith EE, Van Der Kraak G, Kendall RJ, Giesy JP (2005) Effects of atrazine on metamorphosis, growth, laryngeal and gonadal development, sex steroid hormones, and aromatase activity in juvenile X. laevis. Ecotoxicol Environ Saf 62(2):160–173
Coady KK, Marino T, Thomas J, Currie R, Hancock G, Crofoot J, Mcnalley L, Mcfadden L, Geter D, Klecka G (2010) Evaluation of the amphibian metamorphosis assay: exposure to the goitrogen methimazole and the endogenous thyroid hormone-thyroxine. Environ Toxicol Chem 29(4):869–880
Coady K, Marino T, Thomas J, Sosinski L, Neal B, Hammond L (2013) An evaluation of 2,4-dichlorophenoxyacetic acid in the amphibian metamorphosis assay and the fish short-term reproduction assay. Ecotoxicol Environ Saf 90:143–150
Crump D, Werry K, Veldhoen N, Van Aggelen G, Helbing CC (2002) Exposure to the herbicide acetochlor alters thyroid hormone-dependent gene expression & metamorphosis in X. laevis. Environ Health Perspect 110:1199–1205
Decherf S, Seugnet I, Fini JB, Clerget-Froidevaux MS, Demeneix BA (2010) Disruption of thyroid hormone-dependent hypothalamic set-points by environmental contaminants. Mol Cell Endocrinol 323:172–182
Degitz SJ, Holcombe GW, Flynn KM, Kosian PA, Korte JJ, Tietge JE (2005) Progress towards development of an amphibian-based thyroid screening assay using Xenopus laevis. Organismal and thyroidal responses to the model compounds 6-propylthiouracil, methimazole, and thyroxine. Toxicol Sci 87(2):353–364
Denver RJ (2013) Neuroendocrinology of amphibian metamorphosis. Curr Top Dev Biol 103:195–227
Ebert E, Leist KH, Mayer D (1990) Summary of safety evaluation toxicity studies of glufosinate ammonium. Food Chem Toxicol 28:339–349
EPA (2011) pesticides industry sales and usage 2006 and 2007 market estimates. U.S. Environmental Protection Agency. Biological and Economic Analysis Division Office of Pesticide Programs Office of Chemical Safety and Pollution Prevention U.S. Environmental Protection Agency Washington, DC 20460. www.epa.gov/documents/pesticides.
Faber MJ, Thompson DG, Stephenson GR, Boermans HJ (1998) Impact of glufosinate-ammonium and bialaphos on the phytoplankton community of a small eutrophic northern lake. Environ Toxicol 17(7):1282–1290
Grim KC, Wolfe M, Braunbeck T, Iguchi T, Ohta Y, Tool O, Touart L, Wolf DC, Tietge J (2009) Thyroid histopathology assessments for the amphibian metamorphosis assay to detect thyroid-active substances. Toxicol Pathol 37:415–424
Hack R, Ebert E, Ehling G, Leist KH (1994) Glufosinate ammonium—some aspects of its mode of action in mammals. Food Chem Toxicol 32(5):461–470
Hayes TB, Khoury V, Narayan A, Nazir M, Park A, Brown T, Adame L, Chan E, Bucholz D, Stueve T, Gallipeau S (2010) Atrazine induces complete feminization and chemical castration in male African clawed frogs (Xenopus laevis). PNAS 107:4612–4617
Hill MP, Coetzee J (2017) The biological control of aquatic weeds in South Africa: current status and future challenges. Bothalia 47(2):190–198. https://doi.org/10.4102/abc.v47i2.2152
Jewell WT, Hess RA, Miller MG (1998) Testicular toxicity of molinate in the rat: metabolic activation via sulfoxidation. Toxicol Appl Pharmacol 149:159–166
Jobling S, Tyler CR (2003) Endocrine disruption in wild freshwater fish. Pure Appl Chem 75:2219–2223
Jones DK, Hammond J, Relyea RA (2011) Competitive stress can make the herbicide Roundup more deadly to larval amphibians. Environ Toxicol Chem 30:446–454
Kataoka H, Ryu S, Sakiyama N, Makita M (1996) Simple and rapid determination of the herbicides glyphosate and glufosinate in river water, soil and carrot samples by gas chromatography with flame photometric detection. J Chromatogr A 726(1–2):253–258
Kloas W, Lutz I, Einspanier R (1999) Amphibians as a model to study endocrine disruptors: II. Estrogenic acitivity of environmental chemicals in vitro and in vivo. Sci Total Environ 225:59–68
Kloas W, Urbatzka R, Opitz R, Würtz S, Behrends T, Hermelink B, Hofmann F, Jagnytsch O, Kroupova H, Lorenz C, Neumann N, Pietsch C, Trubiroha A, Van Ballegooy C, Wiedemann C, Lutz I (2009) Endocrine disruption in aquatic vertebrates. Trends Comp Endocrinol Neurobiol 1163:187–200
Koyama K, Koyama K, Goto K (1997) Cardiovascular effects of herbicide containing glufosinate and a surfactant: in vitro & in vivo analyses in rat. Toxicol Appl Pharmacol 145:409–414
Marlatt VL, Veldhoen N, Lo BP, Bakker D, Rehaume V, Vallée K, Haberl M, Shang D, van Aggelen GC, Skirrow RC, Elphick JR, Helbing CC (2013) Triclosan exposure alters postembryonic development in a Pacific tree frog (Pseudacris regilla) Amphibian Metamorphosis Assay (TREEMA). Aquatic Toxicol 126:85–94
Miyata K, Ose K (2012) Thyroid hormone-disrupting effects and the amphibian metamorphosis assay. J Toxicol Pathol 25:1–9
Nieuwkoop PD, Faber J (1994) Normal table of Xenopus laevis (Daudin). North-Holland Publishing Co., Amsterdam
Nugegoda D, Kibria G (2017) Effects of environmental chemicals on fish thyroid function: Implications for fisheries and aquaculture in Australia. Gen Comp Endocrinol 244:40–53
Opitz R, Braunbeck T, Bo¨gi C, Pickford DB, Nentwig G, (2005) Description and initial evaluation of a Xenopus metamorphosis assay for detection of thyroid disrupting activities of environmental compounds. Environ Toxicol Chem 24(3):653–664
Opitz R, Hartmann S, Blank T, Braunbeck T, Lutz I, Kloas W (2006a) Evaluation of histological and molecular endpoints for enhanced detection of thyroid system disruption in Xenopus laevis tadpoles. Toxicol Sci 90:337–348
Opitz R, Lutz I, Nguyen NH, Scanlan TS, Kloas W (2006b) Analysis of thyroid hormone receptor beta A mRNA expression in Xenopus laevis tadpoles as a means to detect agonism and antagonism of thyroid hormone action. Toxicol Appl Pharmacol 212:1–13
Organization for Economic Cooperation and Development (2009) Guideline for the testing of Chemicals: the amphibian metamorphosis assay. OECD Series on Testing and Assessment, Paris, p 2009
Organisation for Economic Cooperation and Development (2007)Validation of the amphibian metamorphosis assay as a screen for thyroid-active chemicals: integrated summary report. AMA integrated report.https://www.oecd.org/officialdocuments. Accessed Mar 2019.
Organisation for Economic Co-operation and Development (OECD) (2008) Series on testing and assessment. No. 91. Report of the validation of the amphibian metamorphosis assay (PHASE 3) ENV/JM/MON 18. www.oecd.org/officialdocuments. Accessed Jan 2018.
Qian K, He S, Tang T, Shi T, Li J, Cao Y (2011) A rapid liquid chromatography method for determination of glufosinate residue in maize after derivatisation. Food Chem 127(2):722–726
Rosenfeld CS, Denslow ND, Orlando EF, Gutierrez-Villagomez JM, Trudeau VL (2017) Neuroendocrine disruption of organizational and activational hormone programming in poikilothermic vertebrates. J Toxicol Environ Health B Crit Rev 20:276–304
Saka M, Tada N, Kamata Y (2013) Application of an amphibian metamorphosis assay to the testing of the chronic toxicity of three rice paddy herbicides: Simetryn, mefenacet and thiobencarb. Ecotoxicol Environ Saf 92:135–143
Shi YB (1999) Amphibian metamorphosis: from morphology to molecular biology. Wiley, New York
Shi H, Zhu P, Guo S (2012) Effects of tributyltin on metamorphosis and gonadal differentiation of Xenopus laevis at environmentally relevant concentrations. Toxicol Industrial Health 30:1–7
Sparling DW (2016) Ecotoxicology essentials: environmental contaminants and their biological effects on animals and plants. Academic Press, New York
Tan SW, Zoeller RT (2007) Integrating basic research on thyroid hormone action into screening & testing programs for thyroid disruptors. Crit Rev Toxicol 37:5–10
Tata JR (1998) Amphibian metamorphosis as a model for studying the developmental actions of thyroid hormone. Cell Res 8:259–272
Tietge JE, Holcombe GW, Flynn KM, Kosian PA, Korte JJ, Anderson LE, Wolf DC, Degitz SJ (2005) Metamorphic inhibition of Xenopus laevis by sodium perchlorate: effects on development and thyroid histology. Environ Toxicol Chem 24:926–933
Tietge JE, Degitz SJ, Haselman JT, Butterworth BC, Korte JJ, Lindberg-Livingston KPA, AJ, Burgess EM, Blackshear PE, Hornung MW, (2013) Inhibition of the thyroid hormone pathway in X. laevis by 2-mercaptobenzothiazole. Aquatic Toxicol 126:128–136
van Wyk JH (2013) Thyroid-disrupting activity in the South African aquatic environment. South Africa, Water Research Commission, Pretoria, p 138
Wagner N, Wolfram R, Hanka T, Beatrix T, Stefan L (2013) Questions concerning the potential impact of glyphosate-based herbicides on amphibians. Environ Toxicol Chem 32(8):1688–1700
Wagner N, Muller H, Viertel B (2017) Effects of a commonly used glyphosate-based herbicide formulation on early developmental stages of two anuran species. Environ Sci Pollut Res Int 24:1495–1508
Yun Z, Kai W, Junxue W, Hongyan Z (2014) Field dissipation and storage stability of glusosinate ammonium and its metabolites in soil. Int J Anal Chem 2014:1–8
Zoeller RT (2007) Environmental chemicals impacting the thyroid: targets and consequences. Thyroid 17:811–817
Funding
This study was supported by the Water Research Commission, South Africa, Research grant (Grant number K5/1952), as well as the Working for Water Department, Ministry of Water Affairs, South Africa, for the supply of all the herbicides used for this study. We declare that both the Water Research Commission and Working for Water Department, both in South Africa, did not in any way contribute to the design of the experiment, data analysis, as well as report writing and our choice of publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest in financial, relationship, or otherwise.
Ethics Approval
All the use of animals, their housing, breeding and exposure were approved by the Animal Research Ethical Committee of the Stellenbosch University (Approval no-SU-ACUM 12–15).
Appendix
Appendix
See Table 2 .
Rights and permissions
About this article
Cite this article
Babalola, O.O., Truter, J.C., Archer, E. et al. Exposure Impacts of Environmentally Relevant Concentrations of a Glufosinate Ammonium Herbicide Formulation on Larval Development and Thyroid Histology of Xenopus laevis. Arch Environ Contam Toxicol 80, 717–725 (2021). https://doi.org/10.1007/s00244-020-00758-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-020-00758-3