Abstract
Globally, amphibians are experiencing widespread abnormalities and population declines. One potential contributor to these challenges is the use of pesticides, particularly aquatic herbicides applied to aquatic habitats inhabited by amphibians. Critical issues of concern are the potential toxicity and teratogenicity of these herbicides towards amphibians. Using the FETAX protocol, three globally used formulations, including diquat dibromide (Midstream), glufosinate ammonium (Basta), and imazapyr (Arsenal), were assessed for embryotoxicity, teratogenicity, and growth inhibition. Developing Xenopus laevis embryos were exposed for 96 h at concentrations of 0.5–3.0 mg/L, 1.6–3.0 mg/L, and 20–45 mg/L for Midstream, Basta, and Arsenal respectively. The 96-h LC50 estimates were 0.83 mg/L acid equivalent (a.e.), 36 mg/L a.e., and 2.2 mg/L a.e., whereas the EC50 estimates were 0.24 mg/L a.e., 28.13 mg/L a.e., and 2.01 mg/L a.e. for the Midstream, Arsenal, and Basta formulations, respectively. These two estimates produced Teratogenic Index of 3.5, 1.3, and 1.1 for Midstream, Arsenal, and Basta, respectively, indicating a high risk of malformation induction by Midstream and moderate risk for Arsenal. Regarding growth inhibition, lowest observable effect concentrations of 0.5 mg/L, 25 mg/L, and 2.0 mg/L were computed for Midstream, Arsenal, and Basta, respectively, producing the minimum concentration inhibiting growth (MCIG) ratios of 0.62, 0.69, and 0.89 for the three formulations. These MICG values are higher than the standard 0.30 growth inhibitors benchmark, suggesting that the formulations are not growth inhibitors at the evaluated concentrations. This study provides evidence of the embryotoxic and teratogenic status of Midstream and the embryotoxicity of Basta. There is a need to further characterise the physiological and ecological impacts of these formulations to ensure responsible use and the safety of amphibians and other wildlife.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Pesticides, including herbicides, insecticides, and fungicides, have been of immense benefits to mankind (European Environmental Agency (EEA) 2011; WHO 2013). These anthropogenic chemicals can increase farm yield, enhance pest control, environmental management, and improve health. But despite their positive contributions, the presence of these chemicals in the environment has continuously deteriorated the quality of water and soil resources therein (WFW 2010).
Currently, freshwater habitats are experiencing rapid biodiversity modifications, largely due to chemicals associated with agricultural practices (Downing et al. 2008). In addition, agrochemicals have become a major source of water pollution and consequent risk to the health of humans and wildlife (WFW 2010). Some estimates of freshwater biodiversity loss suggest that the current rate of extinction is the most profound in the past 100,000 years (Eldredge 1998). Amphibians’ vulnerability to pesticides has been linked to their specific ecological requirements, which usually links them to permanent or temporary shallow waterbodies that are essential to their life cycle. However, these waterbodies are frequently contaminated with pesticides. The concern regarding the global incidence of amphibian morphological abnormalities and population decline highlights the urgent necessity to characterise the potential impact of pesticides on these organisms (Gungordu 2013; Lajmanovich et al. 2013).
Pesticide ecotoxicology studies and acute toxicity LC50 estimates for aquatic organisms are mostly with reference to fish species, whereas information regarding toxicity to amphibians is limited (Mann et al. 2009; Wagner et al. 2013). Because it has been shown that amphibians may be just as sensitive to environmental contaminants as fish due to their permeable skin, speculation that exposure to pesticides could be linked to global amphibian decline exists (Brühl et al. 2013), and more amphibian related studies are needed (Blaustein et al. 1994; Wagner et al. 2013).
South Africa is the largest pesticides user in Africa, with about 180 pesticide active ingredients commercially available, and registered as approximately 400 trade names (Meinhardt 2008; Ansara-Ross et al. 2012). Several of these herbicides, including diquat dibromide, imazapyr, and glufosinate, are widely used in aquatic weeds management globally. However, the teratogenic potential and impact of the herbicide formulations applied directly to the aquatic environment, including diquat dibromide, glufosinate ammonium, imazapyr, and certain glyphosate formulations, on vertebrate growth and development are still not well characterized.
Diquat dibromide (9, 10-dihydro-8a, 10a-diazonia phenanthrene ion) is a post-emergent, nonselective contact herbicide, and crop desiccant that also is used in aquatic weeds control (Emmett 2002; WHO 2004). This herbicide is widely used in the United States, Canada, Europe, Australia, and Japan (Emmett 2002; WHO 2004). The Arsenal formulation for example contains nonylphenol ethoxylate as surfactant, which has been shown to exhibit estrogenicity (Othman et al. 2009). There have been some conflicting evidence regarding the impacts of diquat dibromide on amphibians and wildlife in general. Anderson and Prahlad (1976) showed that Diquat formulation inhibited body growth and pigmentation and resulted in distorted body shape at low concentrations 0.75–2 µg/L. In addition, Bimber and Mitchell (1978) reported increased rates of exogastrulation and mortality in Rana pipiens exposed to 0.1 mg/L diquat. In another study, Selypes et al. (1980) exposed nullipara mice to 11 mg/kg of Reglone formulation of diquat on the ninth day of gravidity and showed that the death of foetus is concentration-dependent and that the average embryonic weight decreased as the number of embryos retarded in weight increased. Selypes et al. (1980) further reported that diquat caused retardation in the embryos of females repeatedly treated with low doses, with changes occuring in the skull, vertebrae, sternum, and limbs. Conversely, Dial and Dial (1987) reported that eggs of R. pipiens to be resistant at 2.0–10 mg/L diquat, with both early and late gastrula producing no abnormalities.
Imazapyr herbicide belongs to the imidazolinone chemical family (Liu et al. 1992). These compounds are usually degraded through photolysis in water, with a half-life ranging between 2.5 and 5.3 days (WSDA 2009). According to Grisolia et al. (2004), Arsenal, one of the imazapyr formulations, consists of 25 g/L of imazapyr, 186 g/L of ammonium hydroxide, 18 g/L of nonylphenol ethoxylate (with 9 ethoxylated units), and water. There is scarcity of literature on the teratogenicity and developmental toxicity of this herbicide. However, various reviews, including USEPA, 2006 Registration Eligibility Decision (RED), and Washington State Department of Agriculture (WSDA 2003), rated the active ingredient of this herbicide as having no developmental toxicity and teratogenicity.
Glufosinate ammonium [(GA) (ammonium-D,L- homoalanin-4-yl methyl) phosphate] is a broad-spectrum herbicide. One of the prominent glufosinate ammonium formulations is Basta, which contains an anionic sodium polyoxyethylene alkyether sulphate (AES) that constitute 30% alkylether-sulphate as surfactant, solvent (propylene glycol ether), defoamer, and a blue dyestuff (Koyama et al. 1997). Even though the herbicide and its analogues are now registered and used in more than 40 countries (Qian et al. 2008), not much is known concerning its potential teratogenicity and thyroid disrupting activity. Watanabe and Iwase (1996) examined developmental and dysmorphogenic effects of GA on mouse embryos in culture and observed that 8-day-old embryos cultured for 48 h showed a significant overall growth retardation and increased embryolethality (37.5% at 10 mg/L). Accordingly, all embryos in the treated group exhibited specific morphological defects, including blisters in the lateral head (100%), hypoplasia of the prosencephalon (57.1%), and visceral arches (42.9%). Using the micromass cell culture method, GA also inhibited the differentiation of midbrain cells in Day 12 embryos, with 50% inhibition occurring at 0.55 µg/L. The ratios of the half maximal inhibitory concentration (IC50) for cell proliferation to differentiation in limb bud cells were 0.76 and 1.52 in embryos (Days 11 and 12). Watanabe and Iwase (1996) concluded that GA was embryotoxic in vitro.
The Frog Embryo Teratogenesis Assay-Xenopus (FETAX) is a standardised 4-day flexible bioassay for assessment of potential developmental and teratogenic effects (Mann and Bidwell 2000; Yu et al. 2013). It is widely used in aquatic toxicity testing and is well-suited to environmental testing (Bantle et al. 1999). FETAX protocol assesses mortality, malformation, and growth inhibition during the embryonic developmental phase and has been applied to assess numerous environmental chemicals, including nonylphenol ethoxylate (Mann and Bidwell 2000), heptanol (Bernardini et al. 1994), soil extract (Fort et al. 1995), acidic mine water (Dawson et al. 1985; Oberholster et al. 2014), atrazine herbicides (Morgan et al. 1996), glyphosate herbicides (Babalola and van Wyk 2019), organophosphate (Boga et al. 2009), and organochlorine insecticides (Schuytema et al. 1994), nicotine (Dawson et al. 1988).
In South Africa for example, the exposure impacts of several pesticides applied directly to aquatic ecosystems have not been well studied (Ansara-Ross et al. 2012), particularly the developmental toxicity of formulations, such as Midstream, Basta, and Arsenal, remain unknown. The aim of the present study was to assess the potential developmental toxicity (including teratogenicity, growth inhibition, and malformation) of diquat dibromide (Midstream), glufosinate ammonium (Basta), and imazapyr (Arsenal) herbicide formulations using X. laevis as model organism.
Materials and Methods
Test Chemicals
The herbicide formulations include: Midstream (373 g/L, diquat dibromide, Syngenta Ltd, South Africa), Basta (200 g/L, glufosinate ammonium, Bayer Crop Science AG Ltd, Germany), and Arsenal (250 g/L, Imazapyr, Base Chemical Ltd).
Exposure Concentrations
Following the initial pilot studies (Babalola and van Wyk 2017) and some acute toxicity results from the literature, between four and six concentrations were selected for each of the herbicide formulations (Table 1).
Nominal Concentration Test
Analytical Assessment of Experimental Concentrations
To confirm the exposure concentrations, at about 2 h after the introduction of the herbicide formulation, water samples were randomly taken from 70% of the exposure tanks, including the controls. The water samples were stored in 150-mL glass bottles for each sample, frozen in an ice pack before being transported to the laboratory for analysis. The analysis was performed on the water sample around 8 h after collection. The analysis was done at Envirotech Laboratory, Lagos Nigeria, using gas chromatography (Anisuzzaman et al. 2000; Budde 2003; Shen and Lee 2003; Liu et al. 2004; de Almeida and Yoramine 2007). The detected concentrations (at detection limit of 0.05 µg/L) showed low variations relative to the predicted nominal concentrations (Supplementary Material Table 1).
Care of Xenopus laevis and Breeding of Tadpoles
For the breeding, three sexually mature male and female X. laevis were selected from the established breeding colony of the laboratory and separately maintained in two 15-L glass tanks containing carbon filtered water. The frogs were fed with fish pellets (Aqua-Nutro, RSA) every 3 days. Following the American Society for testing and materials (ASTM) 2014 protocol, breeding induction was performed. In short, males were initially primed with 100 IU human chorionic gonadotropin (hCG) (Merck Ltd, Germany), which was injected into their dorsal lymph sac, 4 days before mating. The males and females were injected with another 100 IU and 300 IU hCG respectively to initiate mating three days after the first injection. Each breeding pair was housed in a separate 15-L exposure tanks lined with plastic netting (to separate the eggs from the adults) and positioned in a well-ventilated dark climate room.
FETAX Bioassay
Following the basic guidelines described by American Society for Testing and Materials (ASTM 1998), the FETAX bioassay exposure was performed. In brief, the fertilised eggs harvested from one of the breeding pairs were de-jellied by swirling in 2% l-cysteine (Sigma, Germany) (prepared in FETAX solution and adjusted to pH 8.1 with NaOH) for 3 min (Dawson and Bantle 1987). After de-jellying, normal cleaving embryos were first individually selected using the microscope. Second-level sorting was performed about an hour later to guarantee the quality of embryos used for the experiment (Fort and Mathis 2018). The embryos were staged using the NF developmental atlas of Nieuwkoop and Faber (1956), with NF stages 8-11 (mid-blastula to early gastrula embryos) selected for the exposure. All breeding and exposure procedures followed the ethical protocol approved by the Animal Ethical Committee of Stellenbosch University (SU-ACUM12-00014).
Exposure Set-Up
Twenty haphazardly selected NF-stage 8-11 embryos were introduced into each exposure vessel (500 mL). Each concentration was represented by two replicates (totaling 40 embryos), while four replicates were used for the positive and negative controls (80 embryos each). The whole experiment was haphazardly arranged in a controlled-climate room under the following conditions: water temperature 24 ± 1 °C, pH of 6.5–7.4, dissolved oxygen of > 6.5 mg/L, and 12 h light:dark photoperiod (L12D12) (OECD 2008). All of the herbicide stocks were freshly prepared in distilled water daily, to avoid chemical degradation/breakdown by environmental factors. For the study, a semi-static exposure approach was adopted, where the exposure medium was changed every 24 h. The chemical 6-ammonicotinade (6-AN) at 99% purity at concentrations of 5.5 mg/L and 2500 mg/L was used as positive control, as part of FETAX requirement for the 96-h toxicity test without metabolic activation (ASTM 2014). The experiments were repeated twice.
Mortality Assessment
Mortality observations were recorded every 8 h throughout the exposure period. The dead embryos were counted and removed to reduce contamination. At 96 h, the exposure was terminated, and the cumulative mortality data were used to define the 96-h LC5, LC50, and LC95 for each of the herbicides, where the average of the replicates per concentration was used. All of the exposed embryos attained stage 46, including the control groups. The surviving tadpoles were euthanized using MS 222 (Tricaine methane sulfonate) (200 mg/L buffered with sodium bicarbonate at 0.42–1.05 g/L) (OECD 2007).
Growth Inhibition Assessment
The lowest observable effect concentration (LOEC) was calculated by statistically comparing the mean 96-h head-to-tail length larval at each concentration relative to the control. The minimum concentrations inhibiting growth (MCIG) was derived by dividing the LOEC with 96-h LC50 (Fort and Mathis 2018).
Malformations and Teratogenic Index
Developmental malformations, including facial and axial malformations, in the treated embryos/larva were accessed using the Atlas of Abnormalities (Bantle et al. 1999). In addition, characteristic methods described by Fort and Paul (2002) was adopted in the assessment of malformation. The percentage incidences of abnormalities were used to determine the 96-h malformation (EC50) index. The LC50 and EC50 values were subsequently used to derive the teratogenic index (TI) using the equation TI = LC/EC) (NICEATM 2000; ASTM 2014; Fort and Mathis 2018).
Data Analysis
The mortality and malformation data were used to compute the LC50 and EC50 values using the probit analysis program (USEPA 1998). Variance in body length data between the treated embryos/larva and the control were used to derive the minimum concentration inhibiting growth (MCIG). Normality and heterogeneity of variance of the embryo length data was assessed using normal probability plots and Levene’s test, respectively. For parametric data, variance among treatments were assessed using one-way ANOVA, and pairwise differences using Tukey’s HSD test with the Spjotvoll-Stoline correction for unequal sample sizes. Nonparametric data were analysed using Kruskal–Wallis ANOVA in combination with Dunn’s pairwise comparisons test. α = 0.05 was deemed as significant. Statistica v 13.5 (Tibco Inc., USA) was used for all ANOVA and post hoc tests.
Results
Embryolethality/Mortality
In this study, the Midstream formulation was found to be the most embryolethal, followed by the Basta formulation, whereas the Arsenal formulation was the least toxic among the three formulations. The 96-h LC50 for Midstream, Basta and Arsenal were 0.83, 2.24, and 36 mg/L a.e., respectively (Table 2).
Growth Effects
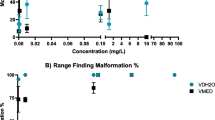
Exposure to the Midstream formulation resulted in a significant decrease in total length across the concentrations tested (0.5–3 mg/L) relative to the control (Fig. 1a). In the Arsenal formulation exposure, there was a concentration dependent decrease in length (Fig. 1b). The mean total lengths of tadpoles were only significantly reduced relative to the control at concentrations of 30–45 mg/L (Fig. 1b). Exposure to the Basta formulation resulted in a similar concentration dependent decrease in length in the treated tadpoles (Fig. 1c). The length reduction was only significant between 2 and 3 mg/L relative to the control (Fig. 1c). The growth inhibition potential (MCIG/LC50) derived for the herbicides were 0.60, 0.69, and 0.89 for the Midstream, Arsenal, and Basta formulations, respectively (Table 2).
Total length (mean ± SE ± SD) of Xenopus laevis tadpoles exposed to the Midstream (Diquat dibromide) (a), Arsenal (Imazapyr) (b), and Basta formulations (Glufosinate ammonium) (c) for 96-h relative to their negative control. Asterisks indicate significant differences relative to the negative control: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001
Malformation Index (MI) and Teratogenic Index (TI)
The 96-h EC50 values for malformation induction were 0.241 mg/L, 28.13 mg/L, and 2.01 mg/L for Midstream, Arsenal, and Basta formulations, respectively. TI produced was the highest for Midstream: 3.5, followed by 1.3, and 1.1 for the Arsenal and Basta formulations, respectively (Table 2).
Observed Malformations
Midstream Formulation
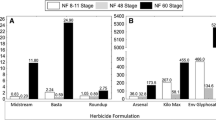
Following the characteristic malformation approach, the observed malformations following the Midstream treatments included generalised edema, cardiac and abdominal edema, blistering, improper gut formation/coiling abnormalities, wavy tails, and tail flexures that occurred in concentration dependent manner (Fig. 2). The most common malformation associated with Midstream exposure was generalised edema. But the most unique malformation was the occurrence of two-headed and multiple tails tadpole (Fig. 2h). The percentage incidences of malformations were as follows: edema (generalised, cardiac, and abdominal) (43%), gut abnormalities (11.4%), blistering (14%), axial malformation (wavy and curved tail) (10.04%), eye abnormalities (2.9%), and head (1.4%).
Arsenal Formulation
The observed characteristic malformations in the larva exposed to the Arsenal formulation include edema (generalised, cardiac, and abdominal), improper gut formation/coiling, and blistering (Fig. 2). The percentage incidences of malformations were as follows: edema (generalised, cardiac, and abdominal) (58.1%) (Fig. 2e), gut abnormalities (47.6%) (Fig. 2d), blistering (8.1%), head (2.3%), and eye (1.2%).
Basta Formulation
The observed characteristic malformations included edema (abdominal, generalised, and cardiac edema), gut abnormalities, and axial malformation (including wavy tails), which occurred with increasing concentration (Fig. 2). The percentage of various malformations occurred in the following order: edema (generalised, abdominal, and cardiac) (Fig. 2e) (50.4%), axial malformation (wavy and curved tail) (Fig. 2c) (38.8%), gut abnormalities(Fig. 2d) (15.4%), as well as eye and head abnormalities with (3.8%).
Discussion
There is increasing global concern regarding the health and ecological implications of pesticides in the environment (WHO 2013). Apart from numerous human health issues, including developmental, thyroid, and reproductive disruption (Lajmanovich et al. 2013; WHO 2013), the exposure impacts of pesticides to other nontarget organisms continues to incentivise research. This present study examined the toxicity, teratogenic, and malformation potential of three globally used herbicide formulations, including Midstream, Arsenal, and Basta, which are used extensively in commercial farming as well as to control alien plants in South Africa’s water catchment areas (Working for Waters Programme) (Bold 2007; Mensah et al. 2013).
This study confirms that Midstream formulation has a high toxicity with an LC50 of 0.83 mg/L. The lethal concentration of this formulation is close to the expected environmental concentration (EEC), i.e., 0.73 mg/L, at the recommended application rate of 0.1–2.0 mg/L (Dial and Dial 1987; Peterson et al. 1994). This supports the findings of Anderson and Prahlad (1976), who reported that diquat at concentrations of 1 or 2 mg/L, was highly embryotoxic to X. laevis. Our results therefore suggest that more than 30% mortality will occur at the EEC concentration of 0.73 mg/L. Similarly, the related quaternary ammonium paraquat herbicide also has been reported to exhibit high toxicity in X. laevis, with an LC50 of 0.67 mg/L (Osano et al. 2002). The present results show that diquat dibromide is not appropriate for weed control in the aquatic environment, inhabited by amphibians and other equally sensitive organisms.
The Arsenal formulation was slightly toxic towards developing X. laevis, with an LC50 of 36 mg/L, which exceeds the expected environmental concentration of the herbicide, being 5.77 mg/L. The amphibian embryo therefore will not be at risk of acute toxicity at the normal application dosage of Arsenal. The Basta formulation was shown to be moderately toxic, with a 96-h LC50 of 2.24 mg/L. This result supports the findings of Ebert et al. (1990), who noted that glufosinate ammonium is slightly toxic following oral exposure in rats and dogs. However, with the Basta EEC of 1.0 mg/L in water, the safety of the tadpoles cannot be totally guaranteed because of the 96-h LC50 of 2.24 mg/L, as there will always be localised spots where concentrations higher than the EEC would be present, particularly immediately after application. The safety of the sensitive aquatic organisms is therefore in question.
Exposure to the Midstream formulation resulted in relatively high degree of growth inhibition, as the growth was inhibited even at the lowest exposure concentration of 0.5 mg/L (Fig. 1). The inhibition in the 0.5 mg/L treatment resulted in mean body length of 4.98 mm compared with 11.07 mm in the control. However, the minimum concentration inhibiting growth ratio (MCIG) was 0.6 and exceeded the 0.30 benchmark for a substance to be classified as growth inhibitor standard (NICEATM 2000). Nonetheless, the mere fact that even the lowest exposure concentration in this study inhibited the growth of the treated larva suggests that this formulation may be a high-risk growth inhibitor and requires further investigation. In particular, concentrations below 0.5 mg/L also could inhibit growth and lower MCIG due to the LOEC/LC50 ratio change. This current growth inhibition result supports the findings of Anderson and Prahlad (1976), who reported that diquat inhibited general body growth in X. laevis at a concentration of 1.5 mg/L. In the case of Arsenal and Basta, even though the two formulations caused significant concentration dependent growth reductions, particularly from 30–45 mg/L and 2.0–3.5 mg/L, respectively (Fig. 1), MCIG ratios were high at 0.69 and 0.89, respectively, compared with the 0.30 benchmark growth inhibitor level (NICEATM 2000). The data hence suggests that these two formulations are not growth inhibitors at the exposure concentrations tested. The occurrence of growth inhibition as observed in these two formulations therefore could be due to toxicity, which should be further investigated.
A high Teratogenic Index (TI) was observed for the Midstream formulation (Diquat). A TI value above 1.5 indicates teratogenic potential (ASTM 2014; Fort and Mathis 2018). The high TI observed for Midstream supports the findings of Osano et al. (2002), who reported a TI of 3.72 for paraquat, a related quaternary ammonium herbicide. High teratogenicity could therefore be a trait of members of the quaternary ammonium group. Severe generalised edema was one of the most common abnormalities observed in tadpoles exposed to Midstream. These edema abnormalities according to Osano et al. (2002) may be due to altered osmoregulation associated with disruption of cell membrane lipid layers. It is notable that the rare double headed and multiple tailed embryo-larva was observed in the Midstream exposed cohort. For Arsenal formulation (Imazapyr), the teratogenic index was 1.3, which is slightly below the 1.5 standard indicating teratogenic potential. According to Leconte and Mouche (2013), a TI > 1.2 should be regarded as positive dysmorphogenic, instead of 1.5 recommended by the ASTM. The TI of Arsenal therefore exceeds the 1.2 reference value and can be considered as teratogenic based on a study by Leconte and Mouche (2013). The leading abnormalities observed in individuals exposed to Arsenal were gut malformations that include the slightly improper gut coiling as well as complex improper gut formation. A high frequency of severe generalised edema was furthermore observed, as was the case with Midstream exposed tadpoles. The Basta formulation did not reach the teratogenic reference values of Leconte and Mouche (2013) or the ASTM, with a low TI of 1.1 suggesting the herbicide is not teratogenic towards X. laevis.
Conclusions
The results of this study indicate high toxicity and teratogenicity exhibited by Midstream (diquat dibromide) towards X. laevis embryo-larva, whereas Basta (glufosinate ammonium) was moderately toxic. The two formulations therefore have the potential to disrupt population dynamics of the embryo-larval stage of amphibians in a way that could lead to serious population decline if extensively applied. The current results furthermore indicate that the Midstream formulation has the potential to cause widespread malformations, given its high teratogenic index of 3.5. Further research regarding the ecological impacts of diquat dibromide and glufosinate ammonium-containing formulations are therefore imperative, not only related to amphibians, but other sensitive wildlife as well. Importantly, to protect all sensitive wildlife species, the continued use of diquat dibromide and glufosinate ammonium-containing formulations, particularly in aquatic ecosystems, should be revisited. In addition, the activity of these herbicides in terms of endocrine-disrupting potential in wildlife should be evaluated.
References
American Society for Testing and Materials (2014) Standard guide for conducting frog embryo teratogenesis assay-Xenopus, ASTM E1439-98: annual book of ASTM standards, vol 11.05. ASTM, Philadelphia, PA, pp 826–36
American Society for Testing and Materials (ASTM) (1998) Standard guide for conducting the frog embryo teratogenesis assay-Xenopus (FETAX). E1439-98
Anderson RJ, Prahlad KV (1976) The deleterious effects of fungicides and herbicides on Xenopus laevis embryos. Arch Environ Contam Toxicol 4(3):312–323
Anisuzzaman KM, Amin M, Ogg N, Hoq F, Kanithi MR, Jenkins RE (2000) Synthesis of dimethyl derivatives of imidazolinone herbicides: their use in efficient gas chromatographic methods for the determination of these herbicides. J Agric Food Chem 48(12):5893–5902. https://doi.org/10.1021/jf000428h
Ansara-Ross TM, Wepener V, van den Brink PJ, Ross MJ (2012) Pesticides in South African fresh waters. African J Aquatic Sci 37(1):1–16
Babalola OO, van Wyk JH (2017) Comparative early life stage toxicity of African clawed frog, X. laevis following exposure to selected herbicide formulations applied to eradicate alien plants in South Africa. Arch Environ Contam Toxicol. https://doi.org/10.1007/S00244-017-0463-0
Babalola OO, van Wyk JH (2019) Mortality, teratogenicity and growth inhibition of three glyphosate formulations using frog embryo teratogenesis assay-Xenopus. J Appl Toxicol 2019:1–10. https://doi.org/10.1002/jat.3811
Bantle J, Dumont J, Finch R, Linder G (1999) Atlas of abnormalities: a guide for the performance of FETAX. Oklahoma State Publications Department, Stillwater, OK
Bernardini G, Vismara C, Boracchi P, Camatini M (1994) Lethality, teratogenicity and growth inhibition of heptanol in Xenopus assayed by a modified frog embryo teratogenesis assay: Xenopus (FETAX) procedure. Sci Environ 151:1–8
Bimber DL, Mitchell RA (1978) Effects of diquat on amphibian embryo development. Ohio J Sci 78(1):50–51
Blaustein AR, Wake DB, Sousa WP (1994) Amphibian declines: judging stability, persistence, and susceptibility of populations to local and global extinctions. Conserv Biol 8:60–71
Boga A, Binokay S, Sertdemir Y (2009) The toxicity and teratogenicity of gibberellic acid (GA3) based on the frog embryo teratogenesis assay-Xenopus (FETAX). Turk J Biol 33:181–188
Bold T (2007) Management treatments summary guide: aquatics. Working for Waters National Office. www.dwaf.gov.za/wfw/control. Accessed 16 Jan 2017
Brühl CA, Schmidt T, Pieper S, Alscher A (2013) Terrestrial pesticide exposure of amphibians: An underestimated cause of global decline? Sci Rep 3:1135
Budde WL (2003) Analytical mass spectrometry of herbicides. Mass Spectrom Rev 23(1):1–24. https://doi.org/10.1002/mas.10070
Dawson DA, Bantle JA (1987) Development of a reconstituted water medium and initial validation of FETAX. J Appl Toxicol 7:237–244
Dawson DA, Fort DJ, Smith GJ, Newell DL, Bantle JA (1988) Evaluation of developmental toxicity of nicotine and cotinine with FETAX. Teratogen Carcinogen Mutagen 8:329–338
Dawson DA, Mcmcormick CA, Bantle JA (1985) Detection of teratogenic substances in acidic mine water samples using the Frog Embryo Teratogenesis Assay-Xenopus (FETAX). J Appl Toxicol 5:234–244
de Almeida RM, Yoramine M (2007) Gas chromatography-mass spectrometric method for the determination of the herbicide paraquat and diquat in plasma and urine samples. J Chromatogr B 853:260–264
Dial NA, Dial CAB (1987) Lethal effects of diquat and paraquat on developing frog embryos and 15-day-old R. pipien. Bull Environ Contam Toxicol 38:1006–1011
Downing JA, Cole JJ, Middelburg JJ, Striegl RG, Duarte CM, Kortelainen P, Prairie YT, Laube KA (2008) Sediment organic carbon burial in agriculturally eutrophic impoundments over the last century. Global Biogeochem Cycles 22:GB1018. https://doi.org/10.1029/2006gb002854
Ebert E, Leist KH, Mayer D (1990) Summary of safety evaluation toxicity studies of glufosinate ammonium. Food Chem Toxicol 28:339–349
Eldredge N (1998) Life in the balance: humanity & the biodiversity crisis. Princeton Univ. Press, Princeton, NJ
Emmett K (2002) Final risk assessment for diquat bromide. The Water Quality Program of the Washington State Department of Ecology. 02-10-046
European Environment Agency (EEA) (2011) Safe water and health water services in a changing environment. EEA technical report no. 7. ISSN 1725-2237
Fort DJ, Mathis M (2018) Frog embryo teratogenesis assay—xenopus (FETAX): use in alternative preclinical safety assessment. Cold Spring Harb Protoc. https://doi.org/10.1101/pdb.prot098319
Fort DJ, Paul RR (2002) Enhancing the Predictive Validity of Frog Embryo Teratogenesis Assay—Xenopus (FETAX). J Appl Toxicol 22:185–191. https://doi.org/10.1002/jat.848
Fort DJ, Stower EL, Norton D (1995) Ecological hazard assessment of aqueous soil extracts using FETAX. J Appl Toxicol 15:183–191
Grisolia CK, Bilich MR, Formigli LM (2004) A comparative toxicologic and genotoxic study of the herbicide arsenal, its active ingredient imazapyr, and the surfactant nonylphenol ethoxylate. Ecotoxicol Environ Saf 59:123–126
Gungordu A (2013) Comparative toxicity of methidathion and glyphosate on early life stages of three amphibian species: Pelophylax ridibundus, Pseudepidalea viridis, and Xenopus laevis. Aquat Toxicol 140–141:220–228. https://doi.org/10.1016/j.aquatox.2013.06.012
Koyama K, Koyama K, Goto K (1997) Cardiovascular effects of herbicide containing glufosinate and a surfactant: in vitro and in vivo analysis in rat. Toxicol Appl Pharmacol 145:409–414
Lajmanovich RC, Junges CM, Attademo AM, Peltzer PM, Cabagna-Zenklusen MC, Basso A (2013) Individual and mixture toxicity of commercial formulations containing glyphosate, metsulfuron-methyl, bispyribac-sodium, and picloram on Rhinella arenarum tadpoles. Water Air Soil Pollut 224:1404
Leconte I, Mouche I (2013) Frog embryo teratogenesis assay on Xenopus and predictivity compared with in vivo mammalian studies. Methods Mol Biol 947:403–421
Liu W, Pusino A, Gessa C (1992) High-performance liquid chromatographic determination of the herbicide imazapyr residues in water and soil. Sci Total Environ 123(124):39–43
Liu R, Zhou JL, Wilding A (2004) Microwave-assisted extraction followed by gas chromatography–mass spectrometry for the determination of endocrine disrupting chemicals in river sediments. J Chromatogr A 1038(1–2):19–26. https://doi.org/10.1016/j.chroma.2004.03.030
Mann RM, Bidwell JR (2000) Application of the FETAX protocol to assess developmental toxicity of nonylphenol ethoxylate to Xenopus laevis and two Australian frogs. Aquatic Toxicol 51:19–29
Mann RM, Hyne RV, Choung CB, Wilson SP (2009) Amphibians and agricultural chemicals: review of risks in a complex environment. Environ Pollut 157:2903–2927
Meinhardt HR (2008) Evaluation of predictive models for pesticide behaviour in South African soils. PhD thesis, University of the North-West, South Africa
Mensah PK, Palmer CG, Muller WJ (2013) Derivation of South African water quality guidelines for Roundups using species sensitivity distribution. Ecotoxicol Environ Saf 96:24–31
Morgan MK, Scheuerman PR, Bishop CS, Pyles RA (1996) Teratogenic potential of atrazine & 2, 4-D using FETAX. J Toxicol Environ Health 48:151–168
National Toxicology Program Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM) (2000) Frog Embryo Teratogenesis Assay-Xenopus-background review document. Retrieved December 12, 2018 from www.niceatm.org
Nieuwkoop PD, Faber J (1956) Normal table of X. laevis. North Holland, Amsterdam
Oberholster PA, Botha KA, Babalola OO, Ndlela L, Staebe K, van Wyk JH (2014) The adverse effects of anthropogenic pollution on xenopus laevis with special reference to Acid Mine Drainage (AMD) in a freshwater Wetland. In M. Lonbardi (Ed.), Amphibian, Anatomy, Ecological Significance and Conservation Strategies. Animal Science, Issues & Professions. Novinka Science Publishers Inc, New York
Organisation for Economic Cooperation and Development (OECD) (2007) Validation of the amphibian metamorphosis assay as a screen for thyroid-active chemicals: integrated AMA summary report. Retrieved November 6, 2018 from www.oecd.org/document
Organisation for Economic Co-operation and Development (OECD) (2008) Series on testing and assessment. No. 91. Report of the validation of the amphibian metamorphosis assay (PHASE 3) ENV/JM/MONO(2008)18. Retrieved November 9, 2018 fromwww.oecd.org/document
Osano O, Oladimeji A, Kraak MS, Admiraal W (2002) Teratogenic effects of amitraz, 2, 4-dimethylaniline, and paraquat on developing (Xenopus) embryos. Arch Environ Contam Toxicol 43:42–49
Othman MZ, Ding L, Jiao Y (2009) Effect of anionic and non-ionic surfactants on activated sludge oxygen uptake rate and nitrification. World Academy of Science, Engineering and Technology 58
Peterson HG, Boutin G, Martin PA, Freemark KE, Ruecker NJ, Moody MJ (1994) Aquatic phyto-toxicity of 23 pesticides applied at expected environmental concentrations. Aquatic Toxicol 28:275–292
Qian H, Chen W, Sheng GD, Xu X, Liu W, Fu Z (2008) Effects of glufosinate on antioxidant enzymes, subcellular structure, and gene expression in unicellular green alga Chlorella vulgaris. Aquatic Toxicol 88:301–307
Schuytema GS, Nebeker AV, Griffis WL (1994) Toxicity of Guithion and Guthion 2S to Xenopus laevis embryos. Arch Environ Contam Toxicol 27:250–255
Selypes A, Nagymojtenyi L, Berensi G (1980) Mutagenic and embryotoxic effects of paraquat and diquat. Bull Environ Contam Toxicol 25:513–517
Shen G, Lee HK (2003) Determination of triazines in soil by microwave-assisted extraction followed by solid-phase microextraction and gas chromatography–mass spectrometry. J Chromatogr A 985(1–2):167–174. https://doi.org/10.1016/S0021-9673(02)01222-0
USEPA (1998) US Environmental Protection Agency - Office of Pollution Prevention and Toxics. Chemical hazard data. Availability study. What do we really know about the safety of high production volume chemicals? Washington DC. Retrieved March 15, 2018 from http://www.epa.gov/HPV/pubs/general/hazchem.htm
Wagner N, Wolfram R, Hanka T, Beatrix T, Stefan L (2013) Questions concerning the potential impact of glyphosate-based herbicides on amphibians. Environ Toxicol Chem 32:1688–1700. https://doi.org/10.1002/etc.2268
Washington State Department of Agriculture (WSDA) (2003) Ecological risk assessment of proposed use of imazapyr to control invasive cordgrass in estuarine habitat of Washington State. Project no. 3000901. Retrieved October 4, 2019 from www.ecy.wa.gov
Washington State Department of Agriculture (WSDA) (2009) Human health and ecological effects imazapyr risk assessment, Washington State. Retrieved October 4, 2019 from www.ecy.wa.gov
Watanabe T, Iwase T (1996) Developmental & dysmorphogenic effects of glufosinate ammonium on mouse embryo in culture. Teratogen Carcinogen Mutagen 16:287–299
World Health Organisation (WHO) (2004) Diquat in drinking water. background document for development of who guidelines for drinking-water quality. World Health Organisation, WHO/SDE/WSH/03.04/91
World Health Organisation (WHO) (2013) State of the Science of Endocrine Disrupting Chemicals (2012). United Nations Environmental Programme and World Health Organisation. ISBN 978-92-807-3274-0 (UNEP) and 978 92 4 150503 1 (WHO)
WWF- CHEM TRUST (2010) Protecting future generations by reducing exposure to endocrine disruptors. CHEM Trust and WWF-EPO proposals for the regulation of chemicals with endocrine disrupting properties under REACH (EC 1907/2006) and under the Plant Protection Products Regulation (EC No 1107/2009)
Yu S, Wages MR, Cai Q, Maul JD, Cobb GP (2013) Lethal and sublethal effects of three insecticides on two developmental stages of Xenopus laevis and comparison with other amphibians. Environ Toxicol Chem 32:2056–2064
Acknowledgements
The authors thank Dr. Olatunde Oladapo, formerly of Zoology and Environmental Biology Department, Lagos State University in Nigeria, for all his support. They wish him happy and good health in retirement.
Funding
This study was supported by the Water Research Commission, South Africa, Research grant (Grant Number K5/1952), as well as the Working for Water Department, Ministry of Water Affairs, South Africa, for the supply of all the herbicides used for this study. The authors declare that both the Water Research Commission and Working for Water Department, both in South Africa, did not in any way contribute to the design of the experiment, data analysis, as well as report writing and choice of publication.
Author information
Authors and Affiliations
Contributions
Babalola performed the laboratory work and manuscript writing. Truter performed all of the statistical and software analysis, and van Wyk performed the general supervision and conceptualization.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest in financial, relationship, or otherwise.
Ethical Approval
The authors declare that all experiments used in this study comply with the current laws in South Africa (Animal Ethics Permit No. SU-ACUM 12-00014).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Babalola, O.O., Truter, J.C. & Van Wyk, J.H. Lethal and Teratogenic Impacts of Imazapyr, Diquat Dibromide, and Glufosinate Ammonium Herbicide Formulations Using Frog Embryo Teratogenesis Assay-Xenopus (FETAX). Arch Environ Contam Toxicol 80, 708–716 (2021). https://doi.org/10.1007/s00244-020-00756-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-020-00756-5