Abstract
Anthropogenic impact over the Pasvik River (Arctic Norway) is mainly caused by emissions from runoff from smelter and mine wastes, as well as by domestic sewage from the Russian, Norwegian, and Finnish settlements situated on its catchment area. In this study, sediment samples from sites within the Pasvik River area with different histories of metal input were analyzed for metal contamination and occurrence of metal-resistant bacteria in late spring and summer of 2014. The major differences in microbial and chemical parameters were mostly dependent on local inputs than seasonality. Higher concentrations of metals were generally detected in July rather than May, with inner stations that became particularly enriched in Cr, Ni, Cu, and Zn, but without significant differences. Bacterial resistance to metals, which resulted from viable counts on amended agar plates, was in the order Ni2+>Pb2+>Co2+>Zn2+>Cu2+>Cd2+>Hg2+, with higher values that were generally determined at inner stations. Among a total of 286 bacterial isolates (mainly achieved from Ni- and Pb-amended plates), the 7.2% showed multiresistance at increasing metal concentration (up to 10,000 ppm). Selected multiresistant isolates belonged to the genera Stenotrophomonas, Arthrobacter, and Serratia. Results highlighted that bacteria, rapidly responding to changing conditions, could be considered as true indicators of the harmful effect caused by contaminants on human health and environment and suggested their potential application in bioremediation processes of metal-polluted cold sites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Environmental metal pollution is an emerging phenomenon, caused by the increased use of metals and chemicals in industrial processes and the consequent production of contaminated effluents (Ahluwalia and Goyal 2007; Raja and Selvam 2006). Some metals (e.g. zinc, copper, nickel, chrome, iron, magnesium, potassium, and sodium) are considered and defined as essential micronutrients for microbial growth (Nies 1999). Also, they participate in biological processes inside the cells, such as redox reactions, coenzymes, and osmotic regulation (Bruins et al. 2000). Differently, other metals, such as cadmium, mercury, and lead, are toxic, nonessential, and do not play biological roles. Indeed, they are harmful for physiological functions (Bruins et al. 2000; Olaniran et al. 2013). Generally, the toxic effect is exercised by the interaction between the ionic forms of metals and other ligands, such as sulfhydryl groups of enzymes, thus inhibiting their enzymatic activity involved in a number biological and physiological functions (Olaniran et al. 2013).
It is nonetheless true that microorganisms if exposed for a long time to high concentrations of contaminants (e.g. metals and antibiotics) can develop resistance by evolving different strategies to counteract the stress induced by toxic compounds (Habi and Daba 2009; Laganà et al. 2018). A positive relationship between the number of colonies on metal-amended plates and metals found generally exists, and plate counts thus become a useful approach to ascertain the impact deriving from anthropogenic stress in a natural environment. Microbial adaptive strategies make metal-resistant bacteria potential bioindicators of pollution events and represent a useful tool to improve the knowledge of sublethal toxicity. Furthermore, bacterial metal tolerance was proposed as a possible indicator of potential toxicity to all other forms of life in a number of environments (AMAP 2011; Das et al. 2009; Ellis et al. 2001; Papale et al. 2018; Sumampouw and Risjani 2014; Zakaria et al. 2004).
Currently, several studies on metal resistance of bacteria isolated from wastewater, sewage, and polluted soils are available, but scarce is the literature about resistant bacteria isolated from cold environments, such as the Arctic and Antarctica (González-Aravena et al. 2016; Lo Giudice et al. 2013; Neethu et al. 2015; Tomova et al. 2014). The Pasvik River is the largest river system in the northern Fennoscandia and represents a border area between Finland, Russia, and Norway. The human activity developed in recent years has filled the river with numerous pollutants, including metals, which remain minimally as ions dissolved in the water and are partially absorbed by particulate matter, and then by sediments after settling, resulting in harm to waterbirds, fish, and benthic fauna. The human impact over the Pasvik area seems to be linked to several causes, also at a local scale, such as the direct discharges of Russian, Norwegian, and Finnish wastewater, and emissions by the Company Pechenganikel, a foundry located in the Russian town of Nikel that was classified as one of the world’s top polluted places. Moreover, iron mines in the Kirkenes area were abundantly exploited during the world wars and until 1966, when the last mine was closed. A new mining program was initiated in 2007, when the Sydvaranger Gruve AS started production of high-grade iron ore concentrate. Furthermore, the environmental metal concentration may seasonally vary. This is particularly true in spring and summer, during which the effect of increasing temperatures and the decomposition of organic matter stimulates the release of metals from sediments (Ali and Abdel-Satar 2005).
In this context, this study was designed to answer two main questions: (1) Are metal contamination levels and bacterial metal resistance driven by local inputs and/or seasonality in sediments from the Pasvik area? (2) Does a relationship exist between the development of bacterial resistance to metals and metal contamination level in the area? To reach the main goals, sediment samples from sites with different histories of metal input within the Pasvik area were chemically and microbiologically analysed. Inter- and intra-site variability of pollution and its impact on bacterial community also was examined during two seasons: late Spring (May 2014) and summer (July 2014). Furthermore, bacterial colonies growing on high concentrations of metals were isolated, tested for multiresistance, and phylogenetically identified.
Materials and Methods
Sampling Area
The Pasvik flows from Lake Inari (Finland) into the Arctic Ocean. It is 166.6-km long, with an area of 20,890 km2, of which 69.8% belongs to Finland, 25.2% to Russia, and 5% to Norway. Many smaller river systems also flow into the river and end in Kirkenes, a port city within an inlet of Varangerfjord, which is then enriched with pollutants (Dauvalter and Rognerud 2001). The Pasvik River is regulated by seven hydropower plants and has no remaining natural rapids or waterfalls. Fluctuations in the water level is low, less than 80 cm, with periods of absence of ice between May/June and October/November. The average temperature is 12 °C, and maximum temperatures are registered during the summer (17–18 °C) (Amundsen et al. 1997).
Collection and Preliminary Treatment of Samples
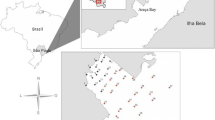
In the framework of the SpongePOP project, sediment samples (3 replicates) were collected during two sampling campaigns, namely SpongePop 1.0 (SP1; 17 May-1 June 2014) and SpongePop 2.0 (SP2; 15-31 July 2014), from nine stations covering different sites along the Pasvik River and the adjacent fjords. Two additional stations (i.e. 10 and 11) were sampled only in July. The location of sampling stations is depicted in Fig. 1. Based on their location, stations were subdivided in three groups: outer (station 10), middle (stations 4, 11, 6, 7, and 3), and inner (stations 8, 2, 1, 5, and 9) stations. Thereinafter, each station will be indicated by the sampling campaign (i.e. SP1 or SP2) followed by the station number (e.g. SP2-8 refers to the station 8 sampled during the SpongePop 2.0 campaign). Sediment samples for chemical analyses were collected using a pre-cleaned stainless-steel bailer and stored at −20 °C in pre-cleaned glass jars until analysis. Samples for microbiological analyses were aseptically collected by using pre-sterilized polycarbonate containers.
All samples were preliminary processed after sampling (approximately 2 h) in the laboratory of the NIBIO Svanhovd Research Station (Svanvik, Pasvik Valley), as described in the following sections. Physicochemical parameters of water and sediment were recorded at each sampling point (Table 1). Sediment were mainly composed by sand (fraction between approximately 0.062 and 2 mm) and mud (fraction < 62 µm). The analyses described below were performed on sediment fraction smaller than 62 µm.
Metal Concentration in Sediment Samples
Reagents and Material
All solutions were prepared using LC–MS Ultra Chromasolv water (Sigma Aldrich) in a class 100 laboratory dotted with a workbench with vertical airflow. Nitric acid (67–69% Suprapur®, Romil) and H2O2 (30% Suprapur®, Romil) were applied. All the material was soaked in HNO3 10% for at least 16 h and then rinsed with double deionised water.
Microwave-Assisted Digestion
Each sample aliquot (approximately 0.2 g) was weighed using an analytical balance (AE 200 Mettler) in a Teflon container and then placed in an HPR 1000/6 rotor. Before their use, the inner liners were soaked in 10% HNO3 overnight. The following microwave cleaning procedure was performed: 5 mL of 67–69% HNO3 was added to each liner, the digestion vessels were sealed, and the temperature was raised to 180 °C within 10 min and held at 180 °C for 10 min. After cooling, the contents of the vessels were discarded, and the liners were thoroughly rinsed with double-distilled water. Ten milliliters of 67–69% nitric acid and 3 mL of 30% hydrogen peroxide were added to the samples, submitted to microwave-assisted mineralization in a high-performance microwave digestion unit, MLS 1200 mega (Milestone, Brøndby, Denmark) with an MLS Mega 240 terminal and EM 45 A exhaust module.
The following optimized microwave program was implemented: 250 W: 5 min; 400 W: 6 min; 0 W: 5 min; 500 W: 2 min; 300 W: 3 min; ventilation: 8 min. The program used was based on the manufacturer’s recommendations for use (Milestone Cookbook of Microwave Application Notes for MDR technology, January 1995). The rotor was then cooled down for a period of 1 h. Mineralized solutions were quantitatively transferred into 50-mL volumetric flasks and diluted to volume with ultrapure water. Quartz containers were rinsed at least three times with ultrapure water to dilute any possible rest of colloids attached in the vessels’ walls. Triplicate digested reagent blank solutions for each digestion run were analyzed for determination of the method detection limit (MDL).
Analysis
A quadrupole ICP-MS Agilent model 7700 (Agilent Technologies, Tokyo, Japan) equipped with a collision cell system for elimination of isobaric interferences was used for water and sediment sample analysis (Table S1). Samples were introduced into the plasma by an autosampler Agilent model ASX-520 (Agilent Technologies, Tokyo, Japan). The instrument was fitted with a MicroMist nebulizer (Agilent nebulizer standard for 7700) with a Scott-type double-pass glass spray chamber cooled down to 2 °C.
The external calibration solutions must include known concentrations of each target analyte. They were prepared from standard certified elemental solutions (ICUS-1239; Sb, As, Ba, Be, Cd, Cr, Co, Cu, Fe, Pb, Mg, Mn, Mo, Ni, Se, Tl, Sn, Zn, V; 100 µg/mL) and LC–MS Ultra Chromasolv water containing 2% HNO3 to get a range of concentrations: 1.0, 2.0, 5.0, 10.0, 20.0, 50.0, 100.0, 200.0, and 500.0 μg/L. A blank solution consisting in LC–MS Ultra Chromasolv water containing 2% HNO3 completed the calibration curve (counts versus μg/L) for each analyte.
The nonspectral matrix effects associated to the ICP-MS measurements were resolved by the addition of internal standards. The standard solution was prepared by diluting a single elemental stock solution with LC–MS Ultra Chromasolv water containing 2% HNO3 up to get 10 μg/L of iridium (193Ir).
Method Validation
Each individual sample was measured with three main acquisition runs during the experiment, providing mean values and standard deviation. The average recovery of the internal standard (iridium) was 100 ± 5%, indicating the absence of a significative matrix effect in the adopted procedure. Because some metal concentrations are near the detection limits of ICP-MS, a validation of the analytical technique was mandatory. To evaluate the accuracy of the instrument itself, marine sediment sample certified for metals MURST-ISS-A1 (Antarctic Marine Sediment, Programma Nazionale Ricerche in Antartide, Istituto Superiore Sanità) was analyzed (Table S2). The limit of detection (LOD) for this study, based on the mean of the blank samples from all runs plus three times its standard deviation, were obtained within the range of 0.002 μg/g (Cd) to 0.1 μg/g (Mn) for sediment samples. Sample blanks and standard solutions were run with each batch of samples as quality control. Digestion and ICP-MS analysis were repeated three times for six samples, to estimate the reproducibility of the entire procedure. The maximum relative standard deviation obtained was 11%.
Estimation of Bacterial Viable Counts
Bacterial cells were first detached from sediment particles through a treatment in a detachment solution (0.1% sodium pyrophosphate in phosphate buffer saline, PBS), followed by sonication for 10 cycles in ice. Serial dilutions were prepared (1:10 and 1:100, using 0.9% sodium chloride solution), and 100 µL of each dilution was spread-plated in duplicate on Plate Count Agar (PCA, Difco; composition per liter: tryptone, 5.0 g; yeast extract, 2.5 g; glucose, 1.0 g; bacteriological agar, 12.0 g) and PCA amended with metals under aerobic conditions. PCA plates were amended with seven different metal salts, i.e. ZnCl2 (Zn), CuCl2· 2H2O (Cu), HgCl2 (Hg), CdCl2·2H2O (Cd), NiCl2· 6H2O (Ni), CoCl2 · 6H2O (Co) and Pb(CH3COO)2 (Pb). Metal salt solutions were prepared in filter-sterilized 1X PBS and stored at 4 °C until use (Selvin et al. 2009). Individual metal salts were added at two different concentrations: 50 and 100 ppm. Inoculated plates were incubated in the dark at 4 °C for 1 month. Results were expressed as CFU/g of wet sediment.
Isolation of Metal-Resistant Bacteria and Screening for Multiresistance
Metal-resistant bacterial colonies were randomly isolated from metal-amended agar plates used for CFU counts, picked and subcultured almost three times under the same conditions. All isolates were tested for growth at increasing concentrations (up to 10,000 ppm) of the seven metals used in this study. The following concentrations (ppm) were used: 50, 100, 500, 1000, 5,000, 7500, and 10,000 ppm. Metal salt solutions were prepared and added to the medium as described above for viable counts. Isolates were streaked on metal-amended media and plates were incubated at 4 °C for 1 month under aerobic conditions.
Phylogenetic Affiliation of Bacterial Isolates
16S rRNA Gene Amplification
Phylogenetic affiliation of metal-resistant bacterial isolates was determined by PCR amplification, sequencing, and analysis of 16S rRNA gene. DNA was extracted through thermal shocking of individual colonies, and the 16S rRNA gene was amplified using Bacteria-specific primers 27F (5′-AGAGTTTGATC(AC)TGGCTCAG–3′) and 1492R (5′-TACGGYTACCTTGTTACGAC-3′) (Mastercycler GeneAmp PCR-System 9700, Applied Biosystem, USA). The reaction mixtures and the PCR program were performed as reported by Michaud et al. (2004).
Sequencing and Analysis of 16S rRNA Genes
After the purification of PCR templates (QIAquick PCR purification kit, Qiagen), sequencing was performed at the Macrogen Laboratory (The Netherland). The NCBI GenBank and the EMBL databases were used to compare and determine next relative isolates (Altschul et al. 1997). Sequence alignment was performed by using the program Clustal W (Thompson et al. 1994. Each alignment was checked manually and corrected.
Nucleotide Sequence Accession Numbers
The nucleotide sequences of the metal-resistant bacteria have been deposited in the GenBank database under the accession numbers MH232035-MH232046.
Statistical Analysis
Correlation coefficients between microbial counts and metal concentrations were calculated to establish possible significant relationships. One-way ANOVA test and post hoc analysis (Tukey test) were used to perform statistical analysis, by comparing the metal concentrations and microbial abundance values between stations of SP1 and SP2 campaigns, thus measuring significant differences between seasons. Results were considered significant when p < 0.05.
All data were opportunely transformed to compute the statistical analysis. Two-way ANOVA was performed to establish if influence of season or geographical position of stations on metal concentrations occurred. Additionally, the same analysis was performed to verify the influence of station and metal concentration on the bacterial counts obtained (MiniTab software, version 16.0). Nonmetric multidimensional analysis (nMDS) analysis was performed to observe the grouping of the stations in dependence of metals concentrations and in dependence of microbial abundance. Principal Component Analysis (PCA) and Cluster Analysis were used to provide the visual grouping of the microbiological and chemical data both in May and July campaign (Primer 6 Plymouth Marine Laboratory, Roborough, United Kingdom). The metal concentration values determined in the present study were further compared with those recorded during a previous sampling campaign (within the SedMicro project) performed in the same area in July 2013.
Results
Metal Concentration in Sediment Samples
Results on metal concentration in sediment samples are reported in Table 2. Higher levels of metals were generally recorded in May (SP1) than in July (SP2), except for the middle station 7. The inner stations 1 and 5 resulted particularly polluted by metals both in May and July and mainly were enriched in Zn, Cu, Ni, and Cr. Remarkable amounts of Zn and Ba were detected in samples from the inner station SP2-2, as well as for sediments from the middle station 7, which also showed high values for Zn, Ba, and Cr, mostly in July. The middle station SP1-3 showed the occurrence of metals that were absent in SP2-3, despite this latter presented two higher peaks for Cr and Ni. Conversely, a constant trend was observed in samples derived from the middle station 6, which resulted more pollution in May than July, with considerable concentrations of Cr, Zn, Pb, Ni, and Cu. Sediments from the middle station 4 showed a decrease in Zn, Cu, and Ni concentrations from May to July. Sediment samples from the inner station 9 showed a high amount of Cr, Ni, Ba, and Zn in May, with values that decreased in July. A similar trend was observed for the inner station 8. Manganese occurred in all stations at high concentrations, even if a dilution effect was observed in July.
Sediment from the outer station SP2-10, sampled in July, were particularly enriched in Zn, Ba, V, Cr, Cu, Ni, Co, and Pb. The middle station SP2-11, sample only in July too, was characterized by high amounts of Zn and Ba.
No significative differences were shown by one-way ANOVA between stations in relation to metal concentration (p > 0.05), nor between campaigns. Otherwise, significantly higher concentrations were detected for Fe and Mg in all stations and both campaigns, as shown by the statistical analysis (p < 0.05). The metal concentrations were divided for area (inner, middle, and outer areas) and studied to detect potential significant differences among areas by one-way ANOVA and post hoc analysis. All metal amounts determined at inner, middle, and outer stations resulted comparable.
All data of metal concentrations were square root transformed and a cluster analysis was performed. The non-metric multi-dimensional scaling (nMDS) analysis that was computed on data of metal concentrations during the campaigns SP1 and SP2, by imposing as factor the station location (outer, middle, inner), is shown in Fig. 2. The clustering of stations seemed not to be influenced by the sampling period, and the separation of the stations did not reflect the station localization. Three main clusters were highlighted for SP1 (Fig. 2a), whereas two clusters were observed for SP2 (Fig. 2b). All groups were constituted by stations of different localization (inner, middle, and outer areas). Furthermore, some stations grouping together in July separated in different clusters in May. Differently, stations 1 and 7 were clusterized in the same group during both campaigns. A significant difference was highlighted by the ANOSIM test computed by imposing main clusters obtained from previous analysis as factors (Global R = 0.9, p = 0.004 for SP1; Global R = 1, p = 0.006 for SP2), and the SIMPER analysis attributes this difference mainly to Mg and Fe concentrations both in May and July.
The two-way ANOVA analysis revealed that there were not significant influence of season or geographical position of stations on metal concentrations at all stations. The one-way ANOVA between metal concentration values determined in this study with those obtained in 2013 within the SedMicro project revealed that no significant differences occurred between metal concentration at each station after a 1-year interval.
Estimation of Metal-Resistant Bacterial Abundances in Sediment Samples
Viable counts in absence of metals were two orders of magnitude higher than in metal-amended agar plates, being in the range 0.1–10.0 and 0.8–1515.0 × 105 CFU/g in May (SP1) and July (SP2), respectively (Table 3). Overall, bacterial growth was generally observed at both 50 and 100 ppm of metals. Viable counts on metal-amended plates were in the range 0.0–41.0 and 0.0–35.0 × 103 CFU/g during SP1 and SP2, respectively (both values were obtained on Ni-amended plates). Overall, the metal resistance of the bacterial communities was in the order Ni2+>Pb2+>Co2+>Zn2+>Cu2+>Cd2+>Hg2+ for both SP1 and SP2 campaigns.
Bacterial growth was rarely observed in the presence of Hg and Cd, whereas resistance to all other tested metals was generally recorded at all. Bacterial growth in samples from SP2-1, SP2-10, and SP2-11 was negligible. Viable counts in presence of different metals were higher in SP1-4, SP1-5, SP1-6, SP2-2, SP2-5, and SP2-8 (between 19.0 and 41.0 × 103 CFU/g). Higher bacterial abundances were generally recorded on Ni2+- and Co2+-amended plates for both SP1 and SP2.
Data on viable counts on 50-ppm, metal-amended plates from each campaign were used to perform a one-way ANOVA, from which resulted that during SP1 bacterial abundances were significantly higher at stations SP1-3, SP1-4, and SP1-5 (p < 0.05). Differently, viable counts obtained on 100-ppm, metal-amended plates resulted significantly lower at stations SP1-2 and SP1-4. The same analysis performed on data from SP2 campaign revealed significantly higher values at station SP2-5 on both 50-ppm and 100-ppm, metal-amended plates (p < 0.05).
Viable counts on metal-amended plates also were compared between SP1 and SP2, and no significant differences were detected nor on 50-ppm neither 100-ppm, metal-amended agar plates. In relation to metal concentrations, bacterial abundances on Ni-amended plates were significantly higher, whereas bacterial abundances on Cd2+- and Hg2+-amended plates were significantly lower for both SP1 and SP2 (p < 0.05).
Figure 3 shows the nMDS analysis performed on data from viable counts obtained on 50-ppm (Fig. 3a, b) and 100-ppm (Fig. 3c, d), metal-amended plates during both campaigns. Overall, stations sampled during SP1 formed similar clusters at both metal concentration (Fig. 3a, c), with a big cluster composed of stations SP1-1, SP1-2, SP1-3, SP1-5, SP1-8, and SP1-9. This also was true for viable counts obtained during SP2 (Fig. 3b, d), with the separation of SP2-4 and SP2-11 from the other stations.
Viable Counts versus Metal Concentrations in Sediments
No significant positive or negative correlations were generally detected between viable counts and metal concentrations measured in the same sediment samples. However, viable counts on both 50- and 100-ppm Cd2+-amended plates, although not significant, showed the highest positive correlation with Cd concentrations during SP2 (data not shown).
Two-way ANOVA analysis showed that both station and metal affected significantly the bacterial counts obtained. In particular, the type of metal appeared to have a strongest effect than station on metal-resistant bacterial community.
The Principal Component Analysis (PCA) was performed elaborating chemical data (metal concentrations) and microbiological data (microbial abundance on all metal-amended plates) at each station (Fig. 4). In the case of SP1 (Fig. 4a), the first component represented the 39.7% of the total variability and was mainly constituted by a mix of chemical and microbiological parameters, with particular regard to microbial abundance on 50-ppm amended plates. The second component represented the 22.3% of the total variability and was represented by metal concentrations. The identification of clusters was obtained by superimposing cluster analysis based on Euclidean distance: stations SP1-5 and SP1-9 formed two distinct clusters, whereas a bigger group was represented by all the other stations. The obtained clusters were used to perform the ANOSIM Pairwise test, which showed significant difference (R = 0.9; p < 0.05), and the subsequent SIMPER analysis explained the PCA plot by highlighting that the higher average dissimilarity occurred between stations SP1-5 and SP1-9 and was mainly due to microbial abundance on Ni-amended plates, with cumulative values of 89.8% and 92.3% on 50- and 100-ppm, amended plates, respectively. The average dissimilarity with the bigger cluster was due to a mix of microbiological and chemical data.
With regard to SP2, the first component represented the 35 of the total variability and was mainly represented by a mix of chemical and microbiological parameters (Fig. 4b). The second component represented the 27.5% of the total variability and was dependent on metal concentrations. The identification of clusters was obtained by superimposing the cluster analysis based on Euclidean distance: stations SP2-5, SP2-7, and SP1-10 formed three distinct clusters, whereas a bigger group was represented by the other stations. The obtained clusters were used to perform the ANOSIM Pairwise test, which showed significant difference (R = 0.9; p < 0.05). The SIMPER analysis explained that the average dissimilarity occurred between stations SP2-5 and the stations of the bigger cluster, and it was mainly due to microbial abundance metal-amended plates, whereas a mix of microbiological and chemical data explained the average dissimilarity between the bigger cluster and the stations SP2-7 and SP2-10.
Isolation of Metal-Resistant Bacteria and Screening for Multiresistance
A total of 286 strains (142 and 144 from SP1 and SP2, respectively) were isolated from plates used for viable counts and amended with 100 ppm of metals (Table 4). They mainly derived from Ni2+- and Pb2+-amended plates (76 and 70 isolates, respectively), followed by Co2+-, Cu2+, and Zn2+-amended plates (51, 44, and 32 isolates, respectively) and, at a lesser extent, from Cd2+- and Hg2+-amended media (10 and 3 isolates, respectively). With regard to the sampling station, the number of obtained isolates ranged between 2 and 55, with lower numbers (between 2 and 14 isolates) from SP2-10 and SP2-11 in July and SP1-4 and SP1-6 in May.
Multiresistance to Metals
All isolates were screened for growth at increasing concentrations of the metals used in this study. The concentration of 500 ppm was considered as a threshold in order to individuate more resistant strains. Among them, 12 isolates (Table 5) were selected as multiresistant and identified by the 16S rRNA gene sequencing. They belonged to the Gammaproteobacteria (9; genera Stenotrophomonas and Serratia) and Actinobacteria (3; all within the genus Arthrobacter).
All isolates tolerated Pb2+ and Zn2+, whereas Hg2+ and Co2+ were tolerated only by few isolates (1 and 5, respectively). Metals were generally tolerated up to 1000 ppm. However, Pb2+ was the sole metal among those tested to be seldom tolerated up to the maximum concentration used (10,000 ppm; Stenotrophomonas spp. 2.3SPb8 and 2.4SPb2). The other metals were tolerated up to 5000 ppm.
In particular, no isolate tolerated all tested metals. Two isolates showed multiresistance to six metals (i.e. Stenotrophomonas spp. 2.4SPb1 and 2.4SPb2), whereas three isolates grew in the presence of five metals (i.e. Stenotrophomonas sp.2.5SCu5, 2.7SCu8, and 2.7SCd10). All remaining isolates generally tolerated three to four metals amending the agar plates.
Discussion
The massive use of metals in industrial processes represents a serious menace for the environment and its biological components (Ahluwalia and Goyal 2007; Raja and Selvam 2006). Polar regions are more sensible to the consequent damages due to the aggravating effect of low temperature, which prevents the mobilization of metals from sediments and rocks, and the correct expulsion by the organisms. Several complexing agents could chelate metals in the environment, but their bioavailable fraction is the most dangerous for the biota, including bacteria. Bacterial population density, distribution, and metabolic activity may be strongly affected by the occurrence of metals in the bulk environment. In turn, bacteria have developed several tolerance mechanisms (e.g. formation of metal-complexes, reduction of certain metals to less-toxic species, metal efflux from cells) to combat stress caused by metals (Nies 2000; Rathnayake et al. 2009), thus being potentially exploited in the bioremediation of contaminated areas. This becomes particularly useful under extreme environments as bacteria active at in situ conditions could be applied. Metals, as well as other kinds of pollutants, accumulate in sediments due to their adsorption and storage capacities. This makes sediments useful indicators of both acute and chronic pollution events, whereas the occurrence of metal-resistant bacteria represents an important tool to detect or highlight the metal bioavailable fraction. In this study, we attempted to establish whether metal-resistant bacteria abundance and distribution were influenced, seasonally and/or locally, by the occurrence of metals in sediment samples from sites differently affected by metal inputs, depending on the surrounding anthropogenic activities, within the Pasvik River area.
On the base of the chemical survey metal distribution among stations seemed to be independent from the geographical position, with a slightly different distribution among stations between SP1 and SP2. This finding could be linked to a seasonal factor as well as meteorology and hydrological dynamisms. Seasonality was probably a determinant factor in those stations where a decrease in metal concentrations was observed between May and July, probably as a consequence of a mixing process deriving from the water flow along the river and dispersion effect on sediments. Similarly, a lower dynamism in the fjords than in the river system was probably the cause of the widespread rate of pollution at station 3, located in the Langfjorden. The higher contamination levels determined in July could be due to the wind coming from Nikel and causing the precipitation of pollutants into areas far from the emission source. Due to the prevailing wind direction, the area to the north of the complex receives the greatest deposition load. Despite this, the seasonal factor seems to be inconsistent to explain the differences occurred in sediment samples among stations in both campaigns. Indeed, each station showed a singular pattern in metal concentrations, and if some of them reported an increase of pollutants in July, others showed a value decrease or the disappearance of some metals. Moreover, the pattern of metal concentration was not constant or uniform for all metals at all stations. For example, even if station 2 showed a general decrease of metal amounts from May to July, an increase in Zn and Ba concentrations occurred. Similarly, station 3 was characterized by a general reduction of metal concentrations during the second campaign, with the exception of Ni, Cr, and Co, for which a strong increase in amounts was recorded. The high contamination level at station 11 was probably due to its location, as the water coming from the Kirkenes area finds an obstacle at this station, so becoming unable to move freely in the open sea with dissolved contaminants that tend to accumulate in its sediment.
Differences in metal contamination appeared to have an effect in shaping the bacterial resistance profile in the considered area. Metal contamination generally results in an increase in the resistance level among the inhabiting bacterial community and reduces the bacterial diversity as a consequence of bottleneck events (Del Busso et al. 2016). Therefore, a higher occurrence of metal-resistant bacteria could be the reflection of a higher metal concentration. This finding was confirmed by the statistical analysis, especially for Pb and Ni, in addition to Zn, Co, and Cu, during both seasons. Station 7, located in the Kirkenes harbour, presented a strong increase in concentration of metals used for the isolation of the metal-resistant bacterial fraction, especially of Zn and Ni. At this station, the metal contamination may be mainly considered chronic and attributable to vessel traffic, in addition to Norwegian wastewaters.
As previously reported by other authors, in this study an increase in metal concentration was generally reflected by an increase in bacterial resistance level (Malik et al. 2002; Papale et al. 2018). The only exception of this trend was represented by station 1, in which an important increase of metals from May to July corresponded to a drastic reduction of metal-resistant bacteria by suggesting a possible acute effect of metal pollution on the microbial community. Such findings confirm that bacteria could develop adaptive strategies in response to environmental stressors (Lo Giudice et al. 2013).
On the base of obtained viable counts, the higher resistance towards Ni2+ and Pb2+ than Zn2+ is in line with the toxicity trend reported by Neethu et al. (2015) for bacterial isolates from Arctic Kongsfjord. Even if Hg and Cd are being monitored as the major pollutants in the Arctic environment and a worrisome trend in their concentrations is observed in marine ecosystems (AMAP 2002; Neethu et al. 2015); they occurred at low concentrations in the Pasvik area. As a consequence, they were found to be the most toxic for bacterial communities among tested metals, as indicated by both the low bacterial abundances on agar plates amended with these metals and the very low bacterial tolerance showed by individual isolates towards them.
In this study, the occurrence and distribution of metal-resistant bacterial communities at local and seasonal scale was assayed on a rich medium, which did not contain ionic forms able to chelate metals. This allowed us ensuring bacterial isolation, but also limiting the possible metal ionic complexation, with consequent reduced metal bioavailability and overestimation of metal bacterial resistance. Similarly, both the presence of organic matter (which could complex metals) and the long incubation period (which could allow, for example, the volatilization of mercury) may have affected the metal bioavailability. For this reason, further analyses will be performed to test metal bacterial resistance in poor media and with a shorter incubation time.
Previous data on metal-tolerant, cold-adapted bacterial isolates are scant. Selected isolates were assayed to evaluate their multiresistance profile and phylogenetically identified. Among multiresistant strains, the frequency of Stenotrophomonas spp. was substantially higher than the other genera found, and they may be the dominant genus in the investigated metal-contaminated area. Stenotrophomonas spp. have been previously reported as copper resistant bacteria (Altimira et al. 2012), and a strong tolerance towards lead, nickel, and chrome was highlighted for Stenotrophomonas isolates from Antarctic soils (Tomova et al. 2014). Also, Arthrobacter representatives from Antarctic soils were reported as multitolerant bacteria (towards Pb, Cu, and Ni) (Tomova et al. 2015), suggesting their potential application in bioremediation processes of metal-polluted cold sites.
The comparison of the metal resistance profile at genus and species level revealed some distinctive features, which can derive from the development and/or acquisition of different resistance mechanisms, as previously observed (Vaz-Moreira et al. 2011). Interestingly, growth profile and metal-amended agar plates often highly differed among isolates in the same species (e.g. Stenotrophomonas isolates), thus suggesting that resistance could be more likely strain- rather than species-specific (Lo Giudice et al. 2013; Mangano et al. 2014).
Multitolerant isolates were generally more resistant to those metals occurring at higher concentrations in the study area, e.g. Zn and Ni. The exception was Pb that was well tolerated, despite its lower concentration than the other metal. In addition, they mainly derived from stations characterized by the highest amounts of Zn. The order of resistance showed by multitolerant strains was Pb2+>Zn2+>Co2+>Ni2+>Cu2+>Cd2+>Hg2+. Pb2+ was the most tolerated metal (up to 10,000 ppm) by members within the genera Stenotrophomonas and Sporosarcina. Ni2+, Zn2+, Cu2+, and Co2+ also were well tolerated, with no toxic effects towards bacterial growth up to 5000 ppm. The high resistance towards copper, cobalt, nickel, and zinc could be reasonably correlated to their natural essential character for the cell survival, as well as because they serve as micronutrients involved in many metabolic processes, i.e. osmotic regulation, molecular interaction, and biological reactions (Bruins et al. 2000). For example, zinc is an important metabolic cofactor (Nies 2000), whereas nickel, tolerated up to 5000 ppm, is considered essential for microorganisms when in trace but can cause oxidative stress at high concentrations (Alboghobeish et al. 2014). Contrastingly, metals as cadmium, mercury, and lead are considered very dangerous and toxic towards microorganisms, because their ionic forms tend to form intracellular complexes (Bruins et al. 2000; Nies 1999) and are recognized as cause of biological damages in the Arctic (AMAP 2011). Even if Cd is considered as the second most important metal contaminating the Arctic (AMAP 2002), its amounts in analyzed sediments were very low, and it was tolerated rarely at high concentrations (between 3500 and 5000 ppm). The same was true for Hg, which was tolerated by few isolates, in line with previous reports (Neethu et al. 2015). Lead toxicity occurs in its chemical form Pb(II), because it causes damage to molecules of biological importance, such as nucleic acids, protein, and enzymes, and strongly affects membrane functions and cellular osmotic balance (Bruins et al. 2000). Bacteria multitolerance has been reported by several authors, which observed concomitant resistance towards zinc, copper, and lead (Ceylan and Ugur 2012; Tada and Inoue 2000). Such results suggest that the bacterial community in the Pasvik area have developed moderate resistance mechanisms towards such toxic metals whose amounts were negligible in the area.
Conclusions
Bacterial tolerance towards metals in polar environments is still poorly treated and limited to few contributions (González-Aravena et al. 2016; Lo Giudice et al. 2013; Mangano et al. 2014; Neethu et al. 2015). Indeed, whereas our findings strongly support the biotechnological importance of metal-resistant bacteria, our results also represent an important ecological afford to previous monitoring observations in a such sensitive area.
We highlighted the importance of bacteria as useful indicators of metal pollution in areas, such as the Pasvik one, that are subjected to contamination events due to domestic sewage and waste from harbour and mine activities, as just recently assessed also for metal-resistant microorganisms from nonpolar environments (Papale et al. 2018; Zakaria et al. 2004). In fact, despite eventual discrepancies between metal concentrations and resistant bacteria counts in relation to the bioavailable/not bioavailable metal form, resistance to metals appeared to be linked to the amounts of metals tested, with an increase/decrease of metal concentrations that influenced bacterial distribution. Microorganisms could develop different mechanisms of tolerance/resistance to metals, thus resulting in several possible scenarios of their application (Filali et al. 2000). Strains that showed high metal resistance or multiresistance ability have to be considered optimal candidate exploitable for bioremediation application. Indeed, as suggested by Pagès et al. (2008), high metal resistance could be correlated to the overproduction of multidrug resistance efflux pumps, hydrolytic enzymes, or other bioactive molecules. Whereas generally the most studied metals correlated to bacterial tolerance in polar areas have been zinc, copper, or cadmium and mercury, we added information about nickel and lead.
This work, which provides new data on the yet scarce knowledge on Arctic metal-resistant bacterial communities, was designed to clarify whether the bacterial resistance in sediments from the Pasvik area was driven by local inputs and/or by a seasonal factor. This study underlines the strong influence of local metal inputs into the sediment of the Pasvik area and clarifies that the seasonal influence is really weak and not determinant. A relationship between the development of bacterial metal resistance and metal contamination in the area was confirmed.
References
Ahluwalia SS, Goyal D (2007) Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour Technol 98:2243–2257
Alboghobeish H, Tahmourespour A, Doudi M (2014) The study of Nickel Resistant Bacteria (NiRB) isolated from wastewaters polluted with different industrial sources. J Environ Health Sci Eng 12:44
Ali M, Abdel-Satar A (2005) Studies of some heavy metals in water, sediment, fish and fish diets in some fish farms in El-Fayoum province. Egypt J Aquat Res 31:261–273
Altimira F, Yáñez C, Bravo G, González M, Rojas LA, Seeger M (2012) Characterization of copper-resistant bacteria and bacterial communities from copper-polluted agricultural soils of central Chile. BMC Microbiol 12:193
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
AMAP (2002) AMAP assessment: heavy metals in the Arctic. Arctic Monitoring and Assessment Programme (AMAP), Oslo
AMAP (2011) AMAP assessment 2011: mercury in the Arctic. Arctic Monitoring and Assessment Programme (AMAP), Oslo, Norway
Amundsen P-A, Staldvik FJ, Lukin AA, Kashulin NA, Popova OA, Reshetnikov YS (1997) Heavy metal contamination in freshwater fish from the border region between Norway and Russia. Sci Total Environ 201:211–214
Bruins MR, Kapil S, Oehme FW (2000) Microbial resistance to metals in the environment. Ecotoxicol Environ Saf 45:198–207
Ceylan O, Ugur A (2012) Bio-monitoring of heavy metal resistance in Pseudomonas and Pseudomonas related genus. J Biol Environ Sci 6:233–242
Das S, Elavarasi A, Somasundharan PL, Khan SA (2009) Biosorption of heavy metals by marine bacteria: potential tool for detecting marine pollution. J Environ Health 9:38–43
Dauvalter V, Rognerud S (2001) Heavy metals pollution in sediments of the Pasvik River drainage. Chemosphere 42:9–18
Ellis RJ, Neish B, Trett MW, Best JG, Weightman AJ, Morgan P, Fry JC (2001) Comparison of microbial and meiofaunal community analyses for determining impact of heavy metal contamination. J Microbiol Methodol 45:171–185
Filali BK, Taoufik J, Zeroual Y, Dzairi FZ, Talbi M, Blaghen M (2000) Waste water bacterial isolates resistant to heavy metals and antibiotics. Curr Microbiol 41:151–156
González-Aravena M, Urtubia R, Del Campo K, Lavín P, Wong CMVL, Cárdenas CA, González-Rocha G (2016) Antibiotic and metal resistance of cultivable bacteria in the Antarctic sea urchin. Antarct Sci 28:261–268
Habi S, Daba H (2009) Plasmid incidence, antibiotic and metal resistance among enterobacteriaceae isolated from Algeria stream. Pak J Appl Sci 12:1474–1482
Laganà P, Votano L, Caruso G, Azzaro M, Lo Giudice A, Delia S (2018) Bacterial isolates from the Arctic region (Pasvik River, Norway): assessment of biofilm production and antibiotic susceptibility profiles. Environ Sci Pollut Res 25:1089–1102
Lo Giudice A, Casella P, Bruni V, Michaud L (2013) Response of bacterial isolates from Antarctic shallow sediments towards heavy metals, antibiotics and polychlorinated biphenyls. Ecotoxicology 22:240–250
Malik A, Khan IF, Aleem A (2002) Plasmid incidence in bacteria from agricultural and industrial soils. World J Microbiol Biotechnol 18:827–833
Mangano S, Michaud L, Caruso C, Lo Giudice A (2014) Metal and antibiotic-resistance in psychrotrophic bacteria associated with the Antarctic sponge Hemigellius pilosus (Kirkpatrick, 1907). Polar Biol 37:227–235
Michaud L, Di Cello F, Brilli M, Fani R, Lo Giudice A, Bruni V (2004) Biodiversity of cultivable psychrotrophic marine bacteria isolated from Terra Nova Bay (Ross Sea, Antarctica). FEMS Microbiol Lett 230:63–71
Neethu CS, Mujeeb Rahiman KM, Saramma AV, Mohamed Hatha AA (2015) Heavy-metal resistance in Gram-negative bacteria isolated from Kongsfjord, Arctic. Can J Microbiol 61:429–435
Nies DH (1999) Microbial heavy metals resistances. Appl Microbiol Biotechnol 51:730–750
Nies DH (2000) Heavy metal resistant bacteria as extremophiles: molecular physiology and biotechnological use of Ralstonia sp. CH34. Extremophiles 4:77–82
Olaniran AO, Balgobind A, Pillay B (2013) Bioavailability of heavy metals in soil: impact on microbial biodegradation of organic compounds and possible improvement strategies. Int J Mol Sci 14:10197–10228
Pagès JM, James CE, Winterhalter M (2008) The porin and the permeating antibiotic: a selective diffusion barrier in Gram-negative bacteria. Nat Rev Microbiol 6:893–903
Papale M, Conte A, Del Core M, Zito E, Sprovieri M, De Leo F, Rizzo C, Urzì C, De Domenico E, Luna GM, Michaud L, Lo Giudice A (2018) Heavy-metal resistant microorganisms in sediments from submarine canyons and the adjacent continental slope in the northeastern Ligurian margin (Western Mediterranean Sea). Prog Ocean 168:155–168
Raja ECA, Selvam GS (2006) Isolation and characterization of a metalresistant Pseudomonas aeruginosa strain. World J Microbiol Biotechnol 22:577–586
Rathnayake IVN, Megharaj M, Bolan N, Naidu R (2009) Tolerance of heavy metals by Gram positive soil bacteria. World Acad Sci Eng Technol 53:1185–1189
Selvin J, Shanmughapriya S, Gandhimathi R, Kiran GS, Ravji TR, Natarajaseenivasan K, Hema TA (2009) Optimization and production of novel antimicrobial agents from sponge associated marine actinomycetes Nocardiopsis dassonvillei MAD08. Appl Microbiol Biotechnol 83:435–445
Sumampouw OJ, Risjani Y (2014) Bacteria as indicators of environmental pollution: review. Int J Ecosyst 4:251–258
Tada Y, Inoue T (2000) Use of oligotrophic bacteria for the biological monitoring of heavy metals. J Appl Microbiol 88:154–160
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tomova I, Stoilova-Disheva M, Vasileva-Tonkova E (2014) Characterization of heavy metals resistant heterotrophic bacteria from soils in the Windmill Islands region, Wilkes Land, East Antarctica. Polish Pol Res 35:593–607
Tomova I, Stoilova-Disheva M, Lazarkevich I, Vasileva-Tonkova E (2015) Antimicrobial activity and resistance to heavy metals and antibiotics of heterotrophic bacteria isolated from sediment and soil samples collected from two Antarctic islands. Front Life Sci 8:348–357
Vaz-Moreira I, Nunes OC, Manaia CM (2011) Diversity and antibiotic resistance patterns of Sphingomonadaceae isolates from drinking water. Appl Environ Microbiol 77:5697–5706
Zakaria ZA, Jaapar J, Ahmad WA (2004) Bacteria as bioindicators for metal contamination. In :Biomonitoring in tropical coastal ecosystems, Phang and Brown, 131–135
Zampieri BDB, Bartelochi PA, Schultz L, de Oliveira MA, de Oliveira AJFD (2016) Diversity and distribution of heavy metal-resistant bacteria in polluted sediments of the Araça Bay, São Sebastião (SP), and the relationship between heavy metals and organic matter concentrations. Microb Ecol 72:582–594
Acknowledgements
This research was supported by grants from the INTERACT Transnational Access EU Program within the project SpongePOP “Sponge associated culturable microbiome able to degrade persistent organic pollutant along the Pasvik River and the Bokfjorden (Norway).” The authors thank the INTERACT coordinator Hannele Savela, and Lars Ola Nillson at the NIBIO Svanhovd Research Station (Svanvik, Pasvik Valley) for his continuous logistic support, which allowed us to perform successfully all of the lab and field work planned.
Author information
Authors and Affiliations
Contributions
Angelina Lo Giudice and Carmen Rizzo wrote the paper; Simona Caputo helped to write the paper and performed microbiological analyses; Federica Moscheo performed microbiological analyses; Stefania Giannarelli, Massimo Onor and Stefano Miserocchi performed sediment analyses and revised the paper; Maurizio Azzaro, Antonella Conte, Maria Papale, Marco Graziano and Paul Eric Aspholm participated to the sampling campaigns and helped to write the paper; Luigi Michaud, Angelina Lo Giudice and Maurizio Azzaro designed the research experimentation; Emilio De Domenico revised the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Caputo, S., Papale, M., Rizzo, C. et al. Metal Resistance in Bacteria from Contaminated Arctic Sediment is Driven by Metal Local Inputs. Arch Environ Contam Toxicol 77, 291–307 (2019). https://doi.org/10.1007/s00244-019-00628-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-019-00628-7