Abstract
Selenium (Se) is an essential nutrient which in excess causes toxicity. The disposal of incompletely combusted coal, which often is rich in Se, into aquatic settling basins is increasing the risk of Se exposure worldwide. However, very few studies have looked at the physiological effects of Se exposure on long-lived, top trophic vertebrates, such as the American alligator (Alligator mississippiensis). During a 7-week period, alligators were fed one of three dietary treatments: mice injected with deionized water or mice injected with water containing 1000 or 2000 ppm selenomethionine (SeMet). One week after the last feeding alligators were bled within 3 min of capture for plasma corticosterone (CORT). A few days later, all alligators were euthanized and whole blood and tail tissue were harvested to measure oxidative damage, an antioxidant-associated transcription factor, and antioxidant enzymes [glutathione peroxidase-1 (GPX1), superoxide dismutase-1 (SOD1), and SOD2] by Western blotting. There was a dose-dependent increase in baseline CORT levels in alligators administered SeMet. Except for blood SOD2 levels, SeMet treatment had no effect (p > 0.05 for all) on oxidative status: oxidative damage, GPX1, SOD1, and muscle SOD2 levels were similar among treatments. Our results illustrate that high levels of Se may act as a stressor to crocodilians. Future studies should investigate further the physiological effects of Se accumulation in long-lived, top-trophic vertebrates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Selenium (Se) is a naturally occurring, essential trace element. Se-containing proteins, known as selenoproteins, play an important role in thyroid hormone production and the prevention of oxidative stress (Kohrle et al. 2005; Janz et al. 2010). However, high levels of Se can lead to toxicity (Hopkins et al. 1997, 1999a; Finger et al. 2017b; Haskins et al. 2017b, c). Mechanistically, Se toxicity is thought to be mediated through its substitution for sulfur in amino acids (and consequent disruption of protein structure) and/or its induction of oxidative stress (Janz et al. 2010; Spallholz and Hoffman 2002). Selenium toxicity can manifest in reproductive impairment (Roe et al. 2004), mortality (Finger et al. 2017b), alterations in metabolic rate (Hopkins et al. 1999b; Rowe et al. 2001), and/or changes in circulating levels of stress hormones (Hopkins et al. 1997; Patterson et al. 2017).
There is an increasing risk of Se exposure worldwide due to anthropogenic activities, such as smelting, the combustion of fossil fuels for energy production, and mining (Lemly 2004; Lemly and Skorupa 2012). Coal combustion wastes (CCWs) are a byproduct of the incomplete combustion of coal and often enriched with Se (Rowe et al. 2002). Almost 20% of CCWs are deposited into aquatic settling basins (Lemly and Skorupa 2012). These basins serve as habitats for a number of organisms (Hopkins et al. 1997, 1999a, b; Rowe et al. 2002; Roe et al. 2004; Haskins et al. 2017a), heightening their risk of Se exposure.
While many studies have investigated how Se affects animals in vivo, most of these have been performed on animals that are of lower trophic status or shorter-lived (Hopkins et al. 1999a, b, 2004; Rowe et al. 2001; Haskins et al. 2017b). Because the American alligator (Alligator mississippiensis) is a long-lived, apex predator found throughout aquatic habitats in the southeastern United States, it has been suggested to serve as an indicator of environmental quality (Finger and Gogal 2013). Alligators are known to inhabit Se-contaminated areas, including ash settling basins (see Roe et al. 2004; personal observation). Alligators are capable of accumulating high levels of Se when fed prey contaminated with Se (Tuberville et al. 2016; Finger et al. 2017b). Nonetheless, few studies have investigated the effects of Se exposure and accumulation on alligator physiology (Roe et al. 2004; Finger et al. 2016).
This study was designed to determine how the consumption of prey spiked with selenomethionine (SeMet), the most bioavailable form of Se, affects oxidative status and the stress response of alligators to provide better insight into how Se accumulation affects their physiology. Baseline (i.e., unstressed levels, initially after capture) corticosterone (CORT), the main crocodilian stress hormone (Finger et al. 2015a), levels were measured in plasma of alligators exposed to one of three SeMet dietary treatments. In addition, parameters of oxidative status were measured in whole blood and tail muscle, including levels of (1) 4-hydroxynonenal (4-HNE), a lipid peroxidation byproduct; (2) levels of the antioxidant enzymes superoxide dismutase-1 (SOD1), SOD2, and glutathione peroxidase-1 (GPX1); and (3) levels of nuclear factor (erythroid-derived 2)-like 2 (Nrf2), an antioxidant-associated transcription factor that regulates the expression of multiple antioxidants (Ma 2013). Because previous studies in other species have shown that Se exposure can cause oxidative stress and affect the stress response (Hopkins et al. 1997; Patterson et al. 2017), we hypothesized that SeMet exposed alligators would exhibit higher baseline CORT levels and elevated parameters of oxidative status.
Methods
Animal Husbandry
The details of alligator husbandry employed herein have been previously described by Finger et al. (2017b). Briefly, 24 sexually immature alligators (average ± standard error, head length: 13.12 ± 0.16 cm; total length: 104.35 ± 1.73 cm), originally obtained from Rockefeller Wildlife Refuge in Grand Chenier, LA, were randomly allocated to one of three pens (n = 8) inside a climate-controlled facility (22.7 °C) with a translucent fiberglass panel roof on the Savannah River site (SRS) near Jackson, SC (Finger et al. 2015b; Hamilton et al. 2016a). Unheated (ambient temperature) water was continuously filtered throughout each pen and alligators were maintained on natural circadian rhythms through light filtration. Alligators were allowed to habituate to pens for > 5 months and fed Mazuri Crocodilian Diet pellets three times weekly until satiation before commencing treatment with SeMet. During the SeMet dosing experiment, alligators were fed pellets twice per week.
Selenomethionine Dietary Treatment
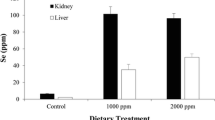
In-depth treatment details employed for dosing alligators were previously described by Finger et al. (2017b). Briefly, alligators were fed thawed, dead, small, “fuzzy” mice spiked with 1000 or 2000 ppm seleno-d,l-methionine (µg SeMet/g mouse dry weight; Sigma S3875 St. Louis, MO) or DI water (control treatment) via oral gavage once per week for 7 weeks (October–December 2014). Each pen comprised a different treatment group (Pen 1, 1000 ppm SeMet; Pen 2, 2000 ppm; Pen 3, control treatment). Alligators that were fed SeMet accumulated significantly more Se (all values in dry mass) in their kidneys (1000 ppm SeMet: 101.60 ± 8.63 ppm Se; 2000 ppm SeMet: 96.38 ± 5.81 ppm Se) and livers (1000 ppm SeMet: 35.20 ± 6.35 ppm Se; 2000 ppm SeMet: 49.97 ± 4.00 ppm Se) than control (kidney Se: 6.51 ± 0.22 ppm Se; liver Se: 2.22 ± 0.14 ppm Se) alligators (Finger et al. 2017b).
Blood Sampling and CORT Quantification
Alligators were captured and blood was sampled from the occipital sinus (with a 25-gauge, 2.54-cm nonheparinized needle and 3-mL syringe) within 3 min of capture 1 week after the last SeMet treatment at 1000 h Eastern Standard Time (December 1, 2014) to determine baseline (i.e., initial levels after capture) CORT levels (Hamilton et al. 2016a). Before entering the building housing the alligators, a timer was started to monitor the cumulative time required to capture an individual and sample it (CumTime, range: 92–708 s; Finger et al. 2015a) and the cumulative time required to bleed an individual after its capture (HandlingTime, range: 36–132 s). Within 1 h of collection, blood samples were centrifuged 3 min at 1640×g and plasma was stored at − 60 °C (Hamilton et al. 2016b).
Plasma CORT was extracted in a mixture of ethyl-acetate:hexane as described previously (Hamilton 2016; Lance and Elsey 1999; Lance et al. 2004). After extraction, all samples were analyzed with an enzyme immunoassay (EIA; ADI-900-097 Enzo, Farmingdale, NY) according to the manufacturer’s protocol. American alligator plasma samples have been previously optimized and validated for use with the specific EIA used in this study (Hamilton 2016). Intra-assay variation was 7.15%. The limit of detection (LOD; 2.40 pg/mL) for the kit was determined as described previously (Wada et al. 2007) by the following equation: \({\text{LOD}} = {\text{mean}}\, {\text{blank}}\, {\text{optical}}\, {\text{density}} + \left( {2 \times {\text{standard}}\, {\text{deviation}}\, {\text{of}}\, {\text{blank}}} \right)\). Any results that fell below the LOD for the kit were assigned this concentration.
Euthanasia and Western Blotting
Three-to-four days after this blood sampling (4–5 December 2014), all alligators were euthanized as described previously (Finger et al. 2017b). At euthanasia, whole trunk blood and tail muscle samples were obtained and stored at − 60 °C until eventual analysis of protein levels by Western blotting at the School of Kinesiology at Auburn University. Because unexpected mortalities prevented obtaining fresh tissue samples from all alligators (Finger et al. 2017b), we only investigated the oxidative status of individuals that were euthanized within 5 min of capture. Thus, Western blots were only used to analyze tissue samples from 12 alligators (i.e., 4 alligators/treatment).
Levels of 4-HNE (ab46545; Abcam, Cambridge, MA), Nrf2 (GTX103322; GeneTex, Irvine, CA), SOD1 (GTX100554; GeneTex), SOD2 (GTX116093; GeneTex), and GPX1 (GTX116040; GeneTex) in muscle and whole blood (Hill et al. 2013) were determined by Western blot as described previously (Hyatt et al. 2016; Finger et al. 2017b). Each membrane was stained with Ponceau as both a loading and transfer control. Proteins were visualized with a chemiluminescent system (GE Healthcare Life Sciences, Pittsburgh, PA), and captured images were analyzed with a ChemiDocIt Imaging System (UVP, LLC, Upland, CA).
Statistical Analysis
All statistical analyses were performed in JMP Pro 14. Linear regression was used in all analyses. Dietary treatment was used as a factor to investigate the effects of SeMet administration on plasma CORT and oxidative status. CumTime and/or HandlingTime were included as covariates (when significant) to account for disturbance/capture-associated effects on oxidative status or CORT. Post hoc multiple comparisons were made using t tests.
Unstandardized effect sizes, including regression coefficients (RC) ± standard errors (SE) and simple effect sizes (i.e., group mean differences) ± SE are presented below to indicate effect size (Finger et al. 2017b). When means are presented, these are indicated explicitly. Western blot results are in arbitrary units (AU). Significance was set at α = 0.05.
Results
Plasma CORT
Plasma CORT was significantly affected by dietary treatment (F2,17 = 9.12; p = 0.0020; Fig. 1). Both 1000 ppm (p = 0.0356; simple effect size: 5.39 ± 2.36 ng/mL) and 2000 ppm (p = 0.0005; simple effect size: 10.99 ± 2.58 ng/mL) SeMet alligators had significantly higher plasma CORT than control alligators. CORT levels also were significantly higher in alligators fed 2000 ppm SeMet than those fed 1000 ppm SeMet alligators (p = 0.0392; simple effect size: 5.59 ± 2.50 ng/mL).
Both HandlingTime (p = 0.0082, RC: 0.11 ± 0.04 ng/mL) and CumTime (p = 0.0012, RC: 0.03 ± 0.01 ng/mL) significantly affected plasma CORT when they were added individually to the Dietary Treatment model. However, when both were included together (along with Dietary Treatment) in the same model, they both had only suggestive effects on plasma CORT (HandlingTime: p = 0.0663, RC: 0.08 ± 0.04; CumTime: p = 0.103, RC: 0.02 ± 0.01). Because HandlingTime had a greater effect on CORT levels (in combined and individual models), it was included in the final Dietary Treatment model.
Simple regression analysis (independent of Dietary Treatment) revealed that neither CumTime (p = 0.85) nor HandlingTime (p = 0.1587) affected plasma CORT. Therefore, we examined their respective effects on each treatment independently. Plasma CORT levels of control alligators and alligators fed 1000 ppm SeMet were not affected by CumTime (Control, p = 0.469; 1000 ppm SeMet, p = 0.2512) or HandlingTime (Control, p = 0.1304; 1000 ppm SeMet, p = 0.624). In alligators fed 2000 ppm SeMet, both HandlingTime (p = 0.0238, RC: 0.24 ± 0.06 ng/mL) and CumTime (p = 0.0013, RC: 0.11 ± 0.01 ng/mL) significantly affected plasma CORT.
Oxidative Status
Dietary Treatment had no effect on any muscle parameter investigated (SOD1, p = 0.6436; SOD2, p = 0.2755; 4-HNE, p = 0.6638; Nrf2, p = 0.3745; GPX1, p = 0.3501; Table 1). Neither HandlingTime nor CumTime had any effects on muscle parameters (all p > 0.05).
SOD2 levels were the only blood parameter significantly affected by Dietary Treatment (F2,9 = 7.15, p = 0.0138; Table 1). SOD2 levels were higher in alligators fed 1000 (p = 0.0499; simple effect size: 0.54 ± 0.24 AU) and 2000 ppm SeMet (p = 0.0045; simple effect size: 0.88 ± 0.24 AU) than control alligators. No difference in blood SOD2 levels was observed between those fed 1000 or 2000 ppm SeMet (p = 0.1701). Blood Nrf2 (p = 0.8786), GPX1 (p = 0.7055), 4-HNE (p = 0.1036), and SOD1 (p = 0.2658) levels were unaffected by Dietary Treatment (Table 1). No effects of CumTime and HandlingTime were observed on blood parameters.
Discussion
This is the first study to investigate how dietary ingestion of Se affects stress hormone levels (i.e., CORT) and oxidative status in the American alligator, a long-lived, top-trophic carnivore. Alligators were fed SeMet-contaminated prey over a 7-week period. At the end of this period, blood and tissue samples were obtained to measure plasma CORT and oxidative status. The current study demonstrates that although SeMet treatment resulted in increased CORT levels as expected, SeMet treatment inconsistently affected oxidative status.
Plasma CORT
As important mediators of the stress response, plasma glucocorticoids rapidly increase above their baseline level in response to a stressor (Sapolsky et al. 2000). After an acute or transient stressor, negative feedback mechanisms are usually activated that eventually return plasma glucocorticoids back to baseline levels. However, a chronic or persistent stressor, such as toxicant exposure, can result in prolonged elevation of glucocorticoids (Gunderson et al. 2003; Patterson et al. 2017). As such, baseline levels of plasma glucocorticoids often are measured to assess the effects of a chronic stressor on an animal (Guillette et al. 1997; Lance and Elsey 1999; Finger et al. 2015a).
Alligators treated with 1000 and 2000 ppm SeMet had significantly higher baseline (i.e., unstressed, initial levels) plasma CORT levels than control alligators (Fig. 1), supporting our original hypothesis. Mean baseline plasma CORT levels of control alligators (0.62 ng/mL; range: 0.28–1.34 ng/mL) were low, equivalent to levels previously reported in both juvenile and adult alligators (Elsey et al. 1990a, b; Guillette et al. 1997). In contrast, alligators treated with SeMet had levels of baseline plasma CORT that were as high or higher than previously reported stress-induced levels (Lance and Elsey 1999; Gunderson et al. 2003). The mean plasma CORT level (9.66 ± 4.55 ng/mL) of alligators fed 2000 ppm SeMet was comparable to levels found in juvenile alligators housed at high stocking densities for 3.5 months (Elsey et al. 1990a), subadult alligators held in a bag for 2 h to simulate an acute stressor (Lance et al. 2004), and juvenile alligators subjected to restraint for 4 h (Lance and Elsey 1999). Alligators fed 1000 ppm SeMet (3.00 ± 1.27 ng/mL) had mean plasma CORT levels that were higher than those found in captive adult alligators housed at a high stocking density (Elsey et al. 1990b).
Similar to our results, Se exposure is associated with higher plasma glucocorticoid levels in some amphibians and fish (Hopkins et al. 1997, 1999a; Miller et al. 2007, 2009; Thomas and Janz 2011; Wiseman et al. 2011; Patterson et al. 2017). For example, white sturgeon (Acipenser transmontanus) exposed to SeMet for 72 days had higher baseline cortisol levels (the main fish glucocorticoid) (Patterson et al. 2017). Likewise, in southern toads inhabiting a Se-contaminated ash basin, higher Se levels were associated with higher baseline CORT levels (Hopkins et al. 1997). Because higher baseline plasma CORT levels are indicative of chronic stress, our results demonstrate that chronic Se exposure is a stressor to alligators.
Previously, we observed that SeMet treatment decreased alligator body condition (Finger et al. 2017b). Specifically, alligators fed 1000 and 2000 ppm SeMet decreased in body condition over the 7-week administration period, whereas the body condition of control alligators did not change. Combined, our results presented here suggest that SeMet may exert a negative effect on alligator body condition through increasing CORT levels.
Oxidative Status
Selenium is thought to induce oxidative stress through increasing reactive oxygen species (ROS) and decreasing levels of the antioxidant glutathione (Spallholz and Hoffman 2002). However, because Se displays nutritional hormesis, its effects on oxidative stress are dose dependent. In fact, Se deficiency can lead to oxidative stress, whereas Se supplementation may prevent oxidative stress through increasing levels or the activity of antioxidant enzymes (Elsey and Lance 1983; Cheng et al. 1999; Monteiro et al. 2009).
Alligators treated with SeMet had higher blood SOD2 levels than control alligators (Table 1). Higher blood SOD2 levels suggest that SeMet treatment altered alligator oxidative status and provides support for our original hypothesis. Se exposure has been shown to increase superoxide anion, a potent ROS, generation (Spallholz and Hoffman 2002). SODs detoxify superoxide by reducing it to hydrogen peroxide (H2O2; Fukai and Ushio-Fukai 2011). Interestingly, SOD1 and muscle SOD2 levels were unaffected by SeMet treatment. These null effects on SOD1 and muscle SOD2 may be a consequence of differences in tissue expression or due to the intracellular location of SODs; SOD1 is mainly found in the cytoplasm, whereas SOD2 is mainly localized in the mitochondrial matrix (Fukai and Ushio-Fukai 2011).
In contrast to our original hypothesis, levels of 4-HNE (a lipid peroxidation byproduct) were similar among control and SeMet-treated alligators (Table 1) even though alligators accumulated Se to some of the highest levels ever reported in a reptile (Finger et al. 2017b). This suggests that SeMet treatment does not increase oxidative damage in alligators. However, augmented superoxide generation (and/or glutathione depletion) induced by SeMet may have led to increased expression of certain antioxidant enzymes, such as blood SOD2, as a compensatory mechanism. In turn, this may have prevented or mitigated oxidative damage caused by SeMet exposure, masking any effects of SeMet treatment on oxidative damage (Jing et al. 2015). Regardless of the ultimate cause, similar to our results, there was no effect of chronic SeMet treatment on lipid peroxidation levels in white sturgeon (Acipenser transmontanus) and rainbow trout (Oncorhynchus mykiss) larvae (Vidal et al. 2005; Zee et al. 2016). A number of studies in birds also have failed to find a relationship between Se accumulation and lipid peroxidation (Hoffman and Heinz 1998; Ware et al. 2012; Brady et al. 2013).
Similar to 4-HNE, SeMet treatment had no effect on Nrf2 and GPX1 levels. GPX1 is a selenoprotein that reduces H2O2 and certain hydroperoxides using glutathione (Brigelius-Flohe and Maiorino 2013). Nrf2 is involved in regulating the expression of a number of antioxidant enzymes, including GPX1, in response to increased ROS and oxidative damage (Ma 2013). Because lipid peroxidation levels were not affected by SeMet treatment (i.e., damage), it is unsurprising that no changes in Nrf2 and GPX1 levels were observed. Likewise, there was no change in GPX1 levels of white sturgeon chronically treated with SeMet (Zee et al. 2016).
There are a few possible reasons why we observed no consistent effect of SeMet treatment on oxidative status. First, the oxidative effects of Se appear to be dependent on the Se species being investigated. In this study, alligators were administered SeMet, which in vitro has been shown to have no effect on superoxide generation or glutathione depletion (Stewart et al. 1999; Spallholz et al. 2001; however, see Misra et al. 2012). In vivo studies in fish have found similar findings (Vidal et al. 2005; Zee et al. 2016). Second, unexpected mortalities prevented obtaining fresh tissue samples from all alligators. Only individuals that were euthanized quickly after capture were investigated. Mortalities may also have contributed to an unintentional selection of the most Se-tolerant alligators for sampling (see Finger et al. 2017a for a discussion of this in relation to methylmercury). Lastly, because kidney and liver tissues were used to determine Se levels, we were unable to measure oxidative status of these organs. Selenium tends to preferentially bioaccumulate in the liver and kidney (Burger et al. 2000; Hopkins et al. 2002). Therefore, it is possible that using only blood or muscle to investigate oxidative status may have prevented an accurate reflection of the effects of Se on oxidative status in alligators even though chronic SeMet treatment can lead to significant Se accumulation in both blood and muscle and lead to histological alterations (Haskins et al. 2017b, c). Future studies should investigate whether nondestructive tissues (i.e., blood) can be used to adequately inform oxidative status in relation to Se exposure.
CumTime, HandlingTime, and Plasma CORT
Both CumTime and HandlingTime increased baseline plasma CORT levels. These results suggest that our presence within the facility (i.e., the disturbance and/or the capture and sampling of conspecifics) and the act of capture raised CORT levels, respectively, despite all samples being obtained within 3 min of capture. Nevertheless, when we examined each treatment independently, we found that CumTime and HandlingTime only affected CORT levels of alligators fed 2000 ppm SeMet. This highlights the possibility that chronic Se exposure enhances sensitization to stressors (such as disturbance or capture). If this was the case, however, we would have expected CumTime and HandlingTime to have also increased plasma CORT in alligators fed 1000 ppm SeMet, but this was not observed. Because one alligator in the 2000 ppm SeMet treatment had extremely high plasma CORT levels (26.63 ng/mL), which is equivalent to levels of wild juvenile alligators held in a bag for 2 h after initial capture (Guillette et al. 1997), this individual likely biased the effects of CumTime and HandlingTime on plasma CORT. In fact, when this individual was removed, there was no effect of CumTime or HandlingTime on plasma CORT levels overall or within the 2000 ppm SeMet treatment (data not shown).
Even though control alligators were sampled after all SeMet-treated alligators (507–793 s post-pen entrance), CumTime had no effect on their plasma CORT levels. This suggests that control alligators were not affected by prolonged human presence and/or the auditory (and likely visual) stimuli associated with the capture of other individuals in the SeMet treatments in nearby pens. Similar to these results, Elsey et al. (1990a) found that sampling order did not affect juvenile alligator CORT levels. In a previous study conducted on hatchling farmed saltwater crocodiles (Crocodylus porosus), CumTime significantly increased plasma CORT (Finger et al. 2015a). However, the disparity of results observed in saltwater crocodiles was most likely a consequence of agricultural management practices aimed at minimizing crocodile-human interactions to increase welfare and reduce stress. Our results suggest that crocodilians (or at least American alligators) are capable of acclimating to human presence and/or disturbance in captive settings. Consequently, these results may have significant implications in the realm of crocodilian farming and captive husbandry.
Conclusions
In conjunction with our previous study (Finger et al. 2017b), the current study is one of the first to investigate how Se accumulation affects crocodilians. Moreover, this is the first study to investigate how Se affects stress hormones and oxidative stress in a crocodilian. Our results demonstrate that chronic Se administration causes stress in alligators: alligators fed SeMet had higher levels of baseline (i.e., at initial capture) CORT. This highlights a potential concern for alligators, and other aquatic wildlife, inhabiting Se-contaminated environments.
In contrast to CORT, SeMet treatment had no consistent effect on alligator oxidative status, which is similar to a number of other studies in other taxa (Hoffman and Heinz 1998; Vidal et al. 2005; Miller et al. 2007; Ware et al. 2012; Brady et al. 2013; Zee et al. 2016). The inconsistent effects of SeMet treatment on alligator oxidative status suggests that the mortality observed in our previous study (Finger et al. 2017b) occurred independent of oxidative stress (Zee et al. 2016). However, because ours is the first study to investigate the effects of Se exposure on crocodilian oxidative status, future studies are required to investigate this further. Future studies should investigate the effects of Se on alligator physiology, such as how Se influences immune function, health, and reproduction and provides a more thorough endocrine profile following Se exposure.
References
Brady C, Petrie SA, Schummer ML, Badzinski SS, Belzile N, Chen YW (2013) Effects of dietary selenium on the health and survival of captive wintering lesser scaup. Environ Pollut 175:8–15. https://doi.org/10.1016/j.envpol.2012.12.005
Brigelius-Flohe R, Maiorino M (2013) Glutathione peroxidases. Biochim Biophys Acta 1830:3289–3303. https://doi.org/10.1016/j.bbagen.2012.11.020
Burger J, Gochfeld M, Rooney AA, Orlando EF, Woodward AR, Guillette LJ (2000) Metals and metalloids in tissues of American alligators in three Florida lakes. Arch Environ Contam Toxicol 38:501–508. https://doi.org/10.1007/s002449910066
Cheng WH, Fu YX, Porres JM, Ross DA, Lei XG (1999) Selenium-dependent cellular glutathione peroxidase protects mice against a pro-oxidant-induced oxidation of NADPH, NADH, lipids, and protein. FASEB J 13:1467–1475
Elsey RM, Lance VA (1983) Effect of diet on blood selenium and glutathione peroxidase activity in the alligator. Comp Biochem Physiol 76B:831–837
Elsey RM, Joanen T, McNease L, Lance VA (1990a) Growth rate and plasma corticosterone levels in juvenile alligators maintained at different stocking densities. J Exp Zool 255:30–36
Elsey RM, Joanen T, McNease L, Lance VA (1990b) Stress and plasma corticosterone levels in the American alligator-relationships with stocking density and nesting success. Comp Biochem Physiol 95:55–63
Finger JW Jr, Gogal RM Jr (2013) Endocrine-disrupting chemical exposure and the American alligator: a review of the potential role of environmental estrogens on the immune system of a top trophic carnivore. Arch Environ Contam Toxicol 65:704–714. https://doi.org/10.1007/s00244-013-9953-x
Finger JW Jr, Thomson PC, Adams AL, Benedict S, Moran C, Isberg SR (2015a) Reference levels for corticosterone and immune function in farmed saltwater crocodiles (Crocodylus porosus) hatchlings using current Code of Practice guidelines. Gen Comp Endocrinol 212:63–72. https://doi.org/10.1016/j.ygcen.2015.01.023
Finger JW Jr, Williams RJ, Hamilton MT, Elsey RM, Oppenheimer VA, Holladay SD, Gogal RM Jr (2015b) Influence of collection time on hematologic and immune markers in the American alligator (Alligator mississippiensis). J Immunoassay Immunochem 36:496–509. https://doi.org/10.1080/15321819.2014.1001030
Finger JW Jr, Hamilton MT, Metts BS, Glenn TC, Tuberville TD (2016) Chronic ingestion of coal fly-ash contaminated prey and its effects on health and immune parameters in juvenile American alligators (Alligator mississippiensis). Arch Environ Contam Toxicol 71:347–358. https://doi.org/10.1007/s00244-016-0301-9
Finger JW Jr, Botero J, Zhang Y, Still SE, Hoffman AJ, Kavazis AN, Cristol DA, Wada H (2017a) No effect of lifelong methylmercury exposure on oxidative status in zebra finches (Taeniopygia guttata): a demonstration of methylmercury-induced selection? Bull Environ Contam Toxicol 99:668–672. https://doi.org/10.1007/s00128-017-2202-7
Finger JW Jr, Hamilton MT, Glenn TC, Tuberville TD (2017b) Dietary selenomethionine administration in the American alligator (Alligator mississippiensis): hepatic and renal Se accumulation and its effects on growth and body condition. Arch Environ Contam Toxicol 72:439–448. https://doi.org/10.1007/s00244-017-0370-4
Fukai T, Ushio-Fukai M (2011) Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal 15:1583–1606. https://doi.org/10.1089/ars.2011.3999
Guillette LJ, Crain DA, Rooney AA, Woodward AR (1997) Effect of acute stress on plasma concentrations of sex and stress hormones in juvenile alligators living in control and contaminated lakes. J Herpetol 31:347–353
Gunderson MP, Kools SAE, Milnes MR, Guillette LJ (2003) Effect of acute stress on plasma β-corticosterone, estradiol-17β and testosterone concentrations in juvenile American alligators collected from three sites within the Kissimmee-Everglades drainage basin in Florida (USA). Comp Biochem Physiol C: Toxicol Pharmacol 135:365–374. https://doi.org/10.1016/s1532-0456(03)00138-8
Hamilton MT (2016) Characterizing stress and immune parameters in the American alligator (Alligator mississippiensis). University of Georgia
Hamilton MT, Finger JW Jr, Winzeler ME, Tuberville TD (2016a) Evaluating the effect of sample type on American alligator (Alligator mississippiensis) analyte values in a point-of-care blood analyser. Conserv Physiol 4:cov065. https://doi.org/10.1093/conphys/cov065
Hamilton MT, Kupar CA, Kelley MD, Finger JW Jr, Tuberville TD (2016b) Blood and plasma biochemistry reference intervals for wild juvenile American alligators (Alligator mississippiensis). J Wildl Dis 52:631–635. https://doi.org/10.7589/2015-10-275
Haskins DL, Hamilton MT, Jones AL, Finger JW Jr, Bringolf RB, Tuberville TD (2017a) Accumulation of coal combustion residues and their immunological effects in the yellow-bellied slider (Trachemys scripta scripta). Environ Pollut 224:810–819
Haskins DL, Hamilton MT, Stacy NI, Finger JW Jr, Tuberville TD (2017b) Effects of selenium exposure on the hematology, innate immunity, and metabolic rate of yellow-bellied sliders (Trachemys scripta scripta). Ecotoxicology 26:1134–1146
Haskins DL, Howerth EW, Tuberville TD (2017c) Experimentally induced selenosis in yellow-bellied slider turtles (Trachemys scripta scripta). Vet Pathol 55:473–477
Hill GE, Fu X, Balenger S, McGraw KJ, Giraudeau M, Hood WR (2013) Changes in concentrations of circulating heat-shock proteins in House Finches in response to different environmental stressors. J Field Ornithol 84:416–424. https://doi.org/10.1111/jofo.12040
Hoffman DJ, Heinz GH (1998) Effects of mercury and selenium on glutathione metabolism and oxidative stress in mallard ducks. Environ Toxicol Chem 17:161–166
Hopkins WA, Mendonca MT, Congdon JD (1997) Increased circulating levels of testosterone and corticosterone in southern toads, Bufo terrestris, exposed to coal combustion waste. Gen Comp Endocrinol 108:237–246
Hopkins WA, Mendonca MT, Congdon JD (1999a) Responsiveness of the hypothalamo-pituitary-interrenal axis in an amphibian (Bufo terrestris) exposed to coal combustion wastes. Comp Biochem Physiol 122:191–196
Hopkins WA, Rowe CL, Congdon JD (1999b) Elevated trace element concentrations and standard metabolic rate in banded water snakes (Nerodia fasciata) exposed to coal combustion wastes. Environ Toxicol Chem 18:1258–1263
Hopkins WA, Roe JH, Snodgrass J, Staub BP, Jackson BP, Congdon JD (2002) Effects of chronic dietary exposure to trace elements on banded water snakes (Nerodia fasciata). Environ Toxicol Chem 21:906–913
Hopkins WA, Staub BP, Baionno JA, Jackson BP, Roe JH, Ford NB (2004) Trophic and maternal transfer of selenium in brown house snakes (Lamprophis fuliginosus). Ecotoxicol Environ Saf 58:285–293. https://doi.org/10.1016/s0147-6513(03)00076-9
Hyatt HW, Kephart WC, Holland AM, Mumford P, Mobley CB, Lowery RP, Roberts MD, Wilson JM, Kavazis AN (2016) A ketogenic diet in rodents elicits improved mitochondrial adaptations in response to resistance exercise training compared to an isocaloric Western diet. Front Physiol 7:533. https://doi.org/10.3389/fphys.2016.00533
Janz DM et al (2010) Selenium toxicity to aquatic organisms. In: Chapman PM et al (eds) Ecological assessment of selenium in the aquatic environment. Society of Environmental Toxicology and Chemistry, Pensacola, FL, pp 139–230. https://doi.org/10.1201/ebk1439826775-c6
Jing CL, Dong XF, Wang ZM, Liu S, Tong JM (2015) Comparative study of DL-selenomethionine vs sodium selenite and seleno-yeast on antioxidant activity and selenium status in laying hens. Poult Sci 94:965–975. https://doi.org/10.3382/ps/pev045
Kohrle J, Jakob F, Contempre B, Dumont JE (2005) Selenium, the thyroid, and the endocrine system. Endocr Rev 26:944–984. https://doi.org/10.1210/er.2001-0034
Lance VA, Elsey RM (1999) Plasma catecholamines and plasma corticosterone following restraint stress in juvenile alligators. J Exp Zool 283:559–565
Lance VA, Elsey RM, Butterstein G, Trosclair PL (2004) Rapid suppression of testosterone secretion after capture in male American alligators (Alligator mississippiensis). Gen Comp Endocrinol 135:217–222. https://doi.org/10.1016/j.ygcen.2003.09.013
Lemly AD (2004) Aquatic selenium pollution is a global environmental safety issue. Ecotox Environ Safe 59:44–56
Lemly AD, Skorupa JP (2012) Wildlife and the coal waste policy debate: proposed rules for coal waste disposal ignore lessons from 45 years of wildlife poisoning. Environ Sci Technol 46:8595–8600. https://doi.org/10.1021/es301467q
Ma Q (2013) Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53:401–426. https://doi.org/10.1146/annurev-pharmtox-011112-140320
Miller LL, Wang F, Palace VP, Hontela A (2007) Effects of acute and subchronic exposures to waterborne selenite on the physiological stress response and oxidative stress indicators in juvenile rainbow trout. Aquat Toxicol 83:263–271. https://doi.org/10.1016/j.aquatox.2007.05.001
Miller LL, Rasmussen JB, Palace VP, Hontela A (2009) The physiological stress response and oxidative stress biomarkers in rainbow trout and brook trout from selenium-impacted streams in a coal mining region. J Appl Toxicol 29:681–688. https://doi.org/10.1002/jat.1458
Misra S, Hamilton C, Niyogi S (2012) Induction of oxidative stress by selenomethionine in isolated hepatocytes of rainbow trout (Oncorhynchus mykiss). Toxicol In Vitro 26:621–629. https://doi.org/10.1016/j.tiv.2012.02.001
Monteiro DA, Rantin FT, Kalinin AL (2009) The effects of selenium on oxidative stress biomarkers in the freshwater characid fish matrinxã, Brycon cephalus (Günther, 1869) exposed to organophosphate insecticide Folisuper 600 BR® (methyl parathion). Comp Biochem Physiol 149:40–49. https://doi.org/10.1016/j.cbpc.2008.06.012
Patterson S, Zee J, Wiseman S, Hecker M (2017) Effects of chronic exposure to dietary selenomethionine on the physiological stress response in juvenile white sturgeon (Acipenser transmontanus). Aquat Toxicol 186:77–86. https://doi.org/10.1016/j.aquatox.2017.02.003
Roe JH, Hopkins WA, Baionno JA, Staub BP, Rowe CL, Jackson BP (2004) Maternal transfer of selenium in Alligator mississippiensis nesting downstream from a coal-burning power plant. Environ Toxicol Chem 23:1969–1972
Rowe CL, Hopkins WA, Zehnder C, Congdon JD (2001) Metabolic costs incurred by crayfish (Procambarus acutus) in a trace element: polluted habitat-further evidence of similar responses among diverse taxonomic groups. Comp Biochem Physiol 129:275–283
Rowe CL, Hopkins WA, Congdon JD (2002) Ecotoxicological implications of aquatic disposal of coal combustion residues in the United States: a review. Environ Monit Assess 80:207–276
Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89. https://doi.org/10.1210/edrv.21.1.0389
Spallholz JE, Hoffman DJ (2002) Selenium toxicity: cause and effects in aquatic birds. Aquat Toxicol 57:27–37
Spallholz JE, Shriver BJ, Reid TW (2001) Dimethyldiselenide and methylseleninic acid generate superoxide in an in vitro chemiluminescence assay in the presence of glutathione: implications for the anticarcinogenic activity of L-selenomethionine and L-Se-methylselenocysteine. Nutr Cancer 40:34–41. https://doi.org/10.1207/S15327914NC401_8
Stewart MJ, Spallholz JE, Neldner KH, Pence BC (1999) Selenium compounds have disparate abilities to impose oxidative stress and induce apoptosis. Free Radic Biol Med 26:42–48
Thomas JK, Janz DM (2011) Dietary selenomethionine exposure in adult zebrafish alters swimming performance, energetics and the physiological stress response. Aquat Toxicol 102:79–86. https://doi.org/10.1016/j.aquatox.2010.12.020
Tuberville TD, Scott DE, Metts BS, Finger JW Jr, Hamilton MT (2016) Hepatic and renal trace element concentrations in American alligators (Alligator mississippiensis) following chronic dietary exposure to coal fly ash contaminated prey. Environ Pollut 214:680–689. https://doi.org/10.1016/j.envpol.2016.04.003
Vidal D, Bay SM, Schlenk D (2005) Effects of dietary selenomethionine on larval rainbow trout (Oncorhynchus mykiss). Arch Environ Contam Toxicol 49:71–75. https://doi.org/10.1007/s00244-004-0160-7
Wada H, Hahn TP, Breuner CW (2007) Development of stress reactivity in white-crowned sparrow nestlings: total corticosterone response increases with age, while free corticosterone response remains low. Gen Comp Endocrinol 150:405–413. https://doi.org/10.1016/j.ygcen.2006.10.002
Ware LL, Petrie SA, Bailey RC, Badzinski SS, Schummer ML, Chen YW, Belzile N (2012) Effects of elevated selenium on body condition, oxidative stress, and oragn health in greater scaup wintering in Lake Ontario. Wildl Soc Bull 36:506–511. https://doi.org/10.1002/wsb.158
Wiseman S, Thomas JK, McPhee L, Hursky O, Raine JC, Pietrock M, Giesy JP, Hecker M, Janz DM (2011) Attenuation of the cortisol response to stress in female rainbow trout chronically exposed to dietary selenomethionine. Aquat Toxicol 105:643–651. https://doi.org/10.1016/j.aquatox.2011.09.002
Zee J, Patterson S, Wiseman S, Hecker M (2016) Is hepatic oxidative stress a main driver of dietary selenium toxicity in white sturgeon (Acipenser transmontanus)? Ecotoxicol Environ Saf 133:334–340. https://doi.org/10.1016/j.ecoenv.2016.07.004
Acknowledgements
The authors thank Dr. Ruth Elsey and her staff at Rockefeller Wildlife Refuge for providing alligators for this study. Sharon L. Finger helped in the transport of alligators from Louisiana to South Carolina. Dan Quinn, Nick Bossebroek, David Haskins, Bess Harris, Sam Dean, and Megan E. Winzeler were instrumental in assisting with the feeding, blood sampling, and dissections of alligators. We thank two anonymous reviewers whose comments helped to improve an earlier version of this manuscript. Support was provided in part by Award Number DE-FC09-07SR22506 from Department of Energy to the University of Georgia Research Foundation, by the Crocodile Specialists Group, and Savannah River Nuclear Solutions – Area Completions Project. All experimental procedures were approved by the University of Georgia’s Institutional Animal Care and Use Committee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Finger, J.W., Hamilton, M.T., Kelley, M.D. et al. Dietary Selenomethionine Administration and Its Effects on the American Alligator (Alligator mississippiensis): Oxidative Status and Corticosterone Levels. Arch Environ Contam Toxicol 75, 37–44 (2018). https://doi.org/10.1007/s00244-018-0530-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-018-0530-1