Abstract
Chemicals in the water of urban areas are representative of the occurrence of these chemicals in the city surrounding water systems and reflect recent human or industrial usage of those chemicals in the sampling areas. In this study, the levels of eight endocrine-disrupting chemicals [including bisphenol analogues, parabens, and triclosan (TCS)] were determined in urban river water and sediments in Guangzhou, South China, and their related ecological risks were evaluated. The eight target chemicals were frequently detected in our samples, with concentrations ranging from not detected (ND) to 65,600 ng/L and from ND to 492 ng/g dw in river water and sediments, respectively. Among these chemicals, the three most abundant were bisphenol A (BPA) (accounting for 35% of the total amount), methyl paraben (MeP) (23%), and TCS (14%) in river water and BPA (43%), TCS (37%), and MeP (14%) in sediments. Significant correlations were found between most target EDCs, particularly MeP and TCS, in river water and sediments (both p < 0.01), indicating their similar sources and wide usage. The ecological risk assessment methods used suggested that TCS was the chemical of primary concern, with an average hazard quotient (HQ) = 1.57 (up to 11.5) in river water and an average HQ = 0.74 (up to 3.63) in sediments. In addition, the ecological risk assessment of different sampling sites indicated a suspected high-risk level for some sites in the study area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Endocrine-disrupting chemicals (EDCs), such as bisphenol A (BPA) and its analogues, parabens and triclosan (TCS), are known as a group of substances that can interfere with human or animal endocrine systems and cause adverse health effects. In the past 20 years, due to their increasing usage as plastic additives, preservatives, and antibacterial agents in various plastics, personal care products (PCPs), and consumer products, those EDCs have become ubiquitous in our environment. EDCs in water and sediments have gained attention, because their concentrations in environmental aquatic media may prove to present an unacceptable risk to aquatic organisms, which may lead to adverse ecological consequences (Gong et al. 2009, 2011; Peng et al. 2008; Zhao et al. 2010).
Bisphenol A (BPA) and its analogues are a group of chemicals with a main structure consisting of two hydroxyphenyl. They are widely used in the manufacture of epoxy resins, polycarbonate plastics, and lacquer coatings as important industrial raw materials (Chen et al. 2002). Due to its property of release into the environment, BPA has been found in various environment media, such as foodstuffs, indoor dust, and air (Liao and Kannan 2013; Liao et al. 2012a; Xue et al. 2016). In recent years, because of its potential toxicities to human and animals, BPA has been gradually replaced by bisphenol S (BPS) and bisphenol F (BPF) (Chen et al. 2002; Tišler et al. 2016). However, several studies have indicated that BPS and BPF may be more toxic than BPA (Ike et al. 2006; Wu et al. 2017). Parabens are commonly used as preservatives in PCPs, pharmaceuticals, and food. It was reported that concentrations of methyl paraben (MeP), ethyl paraben (EtP), propyl paraben (PrP), and butyl paraben (BuP) in PCPs in China were up to 2830, 379, 1560, and 160 µg/g, respectively (Guo et al. 2014). Studies have reported that those four parabens may interfere with the endocrine system and cause breast or skin cancer in humans through the application of PCPs (Darbre and Harvey 2008). In addition, high concentrations of parabens in aquatic systems may affect the endocrine systems of aquatic organisms. For example, after exposure to a certain dose of PrP, the vitellogenin concentration in the plasma of adult male Japanese medaka fish consequently changed (Inui et al. 2003). TCS is a broad-spectrum antibacterial agent that is widely used in daily consumer products, such as soap and toothpaste. The parabens and TCS used in PCPs may partly pass into aquatic system through wastewater pipes and may cause potential health risks to humans and aquatic organisms (Singer et al. 2002). In recent years, parabens were frequently detected in river water (Peng et al. 2008), sediments, and waste water treatment plant effluents (Liao et al. 2013; Yang et al. 2017).
Previous studies have reported the occurrence of these EDCs in the main river systems in South China, and little was known about their profiles in urban rivers. However, urban rivers are considered to be important sources of contaminants for main rivers, because pollutants in urban streams are usually highly affected by industrial and human activities (Zhao et al. 2010). Direct measurement of EDCs in urban river water may predict the profiles of EDCs in the main river systems and also can reflect the recent usage of EDCs in urban populations or products. In the present study, we determined eight emerging EDCs (BPA, BPS, BPF, MeP, EtP, PrP, BuP, and TCS) in urban tributaries of the Liuxi River, which is one of the main tributaries of the Pearl River in South China. The purpose of this study was to (1) explore the spatial distribution and potential sources of target EDCs in river water and sediments, (2) to estimate the content of substances passing from urban rivers to the Liuxi River, and (3) to assess the ecological risks from the eight EDCs in this urban region.
Materials and Methods
Standards and Materials
All standard solutions, including BPA, BPF, and BPS, were purchased from Sigma-Aldrich (purity > 97%, St. Louis, MO). MeP, EtP, PrP, BuP, and TCS were from AccuStandard Inc. (purity > 99%, New Haven, CT). 13C12-BPA and 13C12-BPS, as internal standards for BPA and BPF, and BPS, respectively, were purchased from Cambridge Isotope Laboratories (Andover, MA), as well as 13C12-MeP (for MeP, EtP, and PrP), 13C12-BuP (BuP), and 13C12-TCS (TCS). High-performance liquid chromatography grade methanol, ethyl acetate, methyl tert-butyl ether, and water were purchased from Thermo Fisher Scientific (Waltham, MA).
Sample Collection and Preparation
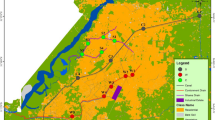
Urban river water (n = 28) and sediment (n = 20) samples were collected in December 2016 (L1–L12 and W1–W3 for river water and S1–S10 for sediment) and January 2017 (L(1)–L(12) and W(1) for river water and S(1)–S(10) for sediment) at the same sites in tributaries of the Liuxi River, which is located in the middle of Guangzhou, South China (Fig. 1). The water samples were obtained using a stainless-steel bucket (1 L of mixed water from both sides and the middle), while surface sediment samples (< 10 cm) were collected with a stainless-steel grasp sampler. All samples were sealed in amber brown bottles that had been previously rinsed with methanol and baked at 450 °C for 5 h. The sediment samples were freeze-dried and passed thought an 80-mesh sieve. Water samples were stored at 4 °C, while sediment samples were kept at − 20 °C until further analysis. The characteristic parameters of water and sediment samples are shown in Table S1 (S is defined as Supporting Information).
Water samples were treated without filtration of small particles. After mixture, samples were treated by liquid–liquid extraction, followed by a solid-phase extraction. Specifically, 20 ng of internal standards was added to 20-mL water samples before extraction. A 10-mL mixture of ethyl acetate and methyl tert-butyl ether (3:1, v/v) was used for extraction. After 20 min of ultrasonic treatment, 30 min of shaking, and 20 min of centrifugal treatment at 4000 r/min, the supernatant was transferred to another glass tube. The extraction step was repeated twice. The final combined supernatant was concentrated to near dryness by rotary evaporators. After that, an Oasis HLB cartridge (60 mg/3 cm3; Waters, Milford, MA) was preconditioned with 3 mL of methanol and 3 mL of water. After loading the sample, the cartridge was washed with 3 mL of water, and target chemicals were eluted with 3 mL of methanol. The eluate was concentrated to 0.4 mL and passed through a 0.22-μm filter before instrumental analysis.
Approximately 0.5 g of dry sediment was transferred to a glass tube and spiked with 20 ng of internal standard chemicals. After 2-h equilibration, 3 mL of methanol was added to the sediment, followed by 20 min of ultrasound, 20 s of vortex, 1 h of shaking, and 5 min of centrifugal treatment at 4000 r/min, and the supernatant was moved to another glass tube. The steps were repeated twice, except for the ultrasonic step. The combined extraction was concentrated to ~ 100 μL under a gentle nitrogen stream. An oasis HLB cartridge was used for further purification similar to the process for water samples. In addition, the total organic carbon (TOC) in the sediment was determined by an Elementar Vario elemental analyzer (Hanau, Germany).
Instrumental Analysis
All analyses were performed using an HPLC (Shimadzu LC-30AD, Japan) coupled with an auto injection sampler and a Betasil C18 column (100 mm × 2.1 mm, 5 μm; Thermo, MA). The mobile phases were water (A) and methanol (B), and the total flow rate was 300 μL/min. The injection volume was set at 5 μL. The flow gradient program is presented in Table S2. Target compounds were quantified by a tandem mass spectrometer (AB SCIEX QTRAP5500, CA, USA). The negative electrospray ionization (ESI) and multiple reaction monitoring (MRM) mode were selected. More information about the mass spectrometer is shown in Table S2.
Quality Assurance, Quality Control, and Data Analysis
The procedural blanks were used to determine the EDC laboratory background, and their concentration values were subtracted from all samples. Matrix spike was applied to identify the matrix-effects. Recoveries of internal standards and target analyses are summarized in Table S3. The recoveries of target EDCs spiked in duplicated samples were 74 ± 8% (mean ± SD), 90 ± 8, 94 ± 12, 85 ± 5, 104 ± 16, 113 ± 34, 107 ± 6, and 75 ± 20% for BPA, BPF, BPS, MeP, EtP, PrP, BuP, and TCS, respectively. The limit of quantification (LOQ) was determined as 10 times the signal-to-noise ratio and ranged from 0.30 to 12 ng/L in river water and 0.11–2.00 ng/g in sediments. A nine-point calibration curve ranged from 0.20 to 100 ng/mL. The regression coefficients (r) were > 0.99 for all calibration curves.
All data were acquired with Analyst Software Version 1.6.2 (AB SCIEX, USA), and other statistical analysis was performed using SPSS 19.0 or SigmaPlot 10.0. Concentrations of EDCs below LOQ were replaced with a value equal to zero. A Pearson correlation analysis was used to evaluate the relationship between target EDCs in river water and sediments. Statistical significant was set at p < 0.05.
Results and Discussion
Occurrence of EDCs in River Water
The mean and median concentrations of target EDCs in river water and sediments are shown in Table 1. For bisphenol analogues, BPA and BPS were detected in all samples, whereas BPF showed a lower detection rate (DR) of 89%. The concentration of total bisphenol analogues ranged from 128 to 66,200 ng/L, with a median value of 818 ng/L. Compared with other Chinese lakes (Table 2), the concentration levels of total bisphenol analogues in water samples of this study were significantly higher (16–50 times) than those in the Hunhe River (range/median 7.6–160/53 ng/L), the Liaohe River (8.7–173/63 ng/L), and Taihu Lake (5.4–8.7/16 ng/L) in 2013 (Jin and Zhu 2016) but comparable to those in Taihu Lake (range 81–3000 ng/L, mean 1100 ng/L) in 2016 (Yan et al. 2017). Among the three analogues, BPA was dominant in river water (median 572 ng/L), followed by BPS (173 ng/L) and BPF (33.5 ng/L), but this sequence is different compared with many previous studies in rivers or lakes worldwide (Table 2). For example, the median concentrations of BPA, BPF, and BPS were 13–325, 2.8–7.8, and 171–2290 ng/L, respectively, in several rivers and Tokyo Bay in Japan, and were 39–151, ND-41, and ND-555 ng/L in water bodies of Korea, respectively (Yamazaki et al. 2015). Significantly higher concentrations of BPF and lower concentrations of BPA and BPS were found in waters in Japan and Korea than those of this study (Yamazaki et al. 2015). In addition, for water bodies in India, the median concentrations of BPA and BPF (391–512 and ND-20 ng/L, respectively) were almost similar to our data but had higher levels of BPS (30–6840 ng/L) (Huang et al. 2012).
Regarding the parabens, MeP, EtP, and PrP were found in more than 93% of samples, and BuP was found in all samples. The total concentrations of the four compounds ranged from 42.9 to 22,600 (median 876) ng/L. The concentration of MeP (median 387 ng/L) was significantly higher than EtP (57.6 ng/L), PrP (192 ng/L), and BuP (4.24 ng/L). The concentration levels of parabens in our study were higher than those reported in other waters, such as the Pearl River, South China (ND-1000 ng/mL) (Peng et al. 2008), South Wales in the United Kingdom (< 0.3–400 ng/L) (Kasprzyk-Hordern et al. 2008), and southern India (ND-147 ng/L) (Ramaswamy et al. 2011) but comparable to those in North American (20–24,300 ng/L) (Lee et al. 2005).
In addition, TCS was detected in all river water with concentrations ranging from 14.7 to 4470 ng/L. The median concentration was 235 ng/L, much higher than the concentration in the main river, Liuxi River (11.9 ng/L), which is probably affected by water dilution (Zhao et al. 2010). However, the concentration of TCS in our study was similar to that found in the Tamiraparani River in India (up to 5160 ng/L), whose TCS was suggested to be sourced from industry and domestic sewage (Ramaswamy et al. 2011).
Occurrence of EDCs in Sediments
Generally, the value of the octanol–water partition coefficient (Kow) of chemicals can affect the distribution of chemicals in different environmental media. The log Kow of target EDCs in our study ranged from 1.65 (BPS) to 4.76 (TCS) (Chen et al. 2016; Dhillon et al. 2015; Golden et al. 2005), which indicates these chemicals are highly distributed in medium with a high content of organic substances.
Regarding the bisphenol analogues, BPA, BPS, and BPF were found in almost all of the sediment samples. Their total concentrations ranged from 1.17 to 421 ng/g dw. The median concentrations of BPS (2.42 ng/g dw) and BPF (7.50 ng/g dw) were at comparable levels with those of Taihu Lake, whereas BPA (77.0 ng/g dw) was several times higher than that of Taihu Lake (Yan et al. 2017) (Table 2). The total concentrations of parabens were 1.72–70.9 ng/g dw. MeP was the main compound, with concentration up to 69.9 ng/g dw, which were higher than those in sediments collected from several locations in the United States, Japan, and Korea (Liao et al. 2013). In addition, the median concentrations of EtP and BuP were lower than 1.0 ng/g dw. The concentration levels of TCS in sediments of urban rivers in this study were 5.90–492 ng/g dw, higher than the values of the main water (< LOQ-116 ng/g dw) reported by Zhao et al. (2010).
Compared with the results of other studies that analyzed EDCs in river water and sediments collected from lakes or bays worldwide, concentration levels of EDCs in our urban river samples were relative higher (Carmona et al. 2014; Jin and Zhu 2016; Liao et al. 2013; Yamazaki et al. 2015; Zhao et al. 2010) (Table 2). The higher concentrations of EDCs in this study are probably affected by meteorological conditions, seasonal variability, and the surrounding environment. The rivers in this study are branches of the Liuxi River in Guangzhou and are located in urban areas where domestic or industrial wastewater is directly discharged into the rivers without any treatment. In addition, the samples of this study were collected in a dry period with low rainfall and fluidity (December and January) (Bledzka et al. 2014; Zhao et al. 2010). Zhao et al. (2010) has reported that there was no significant variation in concentrations of some target compounds in the Liuxi River due to the influence of water dilution and massive discharge during wet weather.
Relationships of EDCs in River Water and Sediments
The composition and distribution of EDCs in river water and sediments are shown in Fig. 2. The most abundant chemical in the water samples was BPA, accounting for 35% of total amount of EDCs. The contribution of target chemicals to total amount of EDCs ranked as BPA (35%) > MeP (23%) > TCS (14%) > PrP (12%) > BPS (10%) > EtP (3%) > BPF (2%) > BuP (< 1%). For bisphenols, the abundance sequence (BPA > BPS > BPF) was similar to most water bodies in China (Jin and Zhu 2016) but different from some specific areas studied in recent years (BPS > BPA > BPF in India, and BPF > BPA > BPS in Japan or Korea) (Yamazaki et al. 2015). This indicates a substantial difference in bisphenol usage among these countries and the slow progress of replacing BPA with BPS in China (Wu et al. 2017). For parabens, the sum of MeP and PrP accounted for 35% of total target EDCs, and the two compounds were also frequently detected in PCPs (Guo and Kannan 2013; Guo et al. 2014). The residue of PCPs entering the river through the sewage system may be an important source of the parabens in water bodies.
In sediments, however, the abundances of target EDCs ranked as BPA (43%) > TCS (37%) > MeP (14%) > BPF (4%) > BPS (1%) > others (the sum of PrP, EtP and BuP). BPA was also the leading congener. Compared with its value in river water, TCS had a higher composition ratio in sediments (37, and 14% in water), which may be explained by its high Kow value and its rapid photodegradation rate of a half-life of 41 min in aqueous solutions (USEPA 2008).
To better understand the potential sources of these chemicals, Pearson Correlation Analysis was performed to check the relationships between concentrations of EDCs and total organic carbon (TOC). The results of the Pearson correlation are shown in Table 3. A significant positive correlation was observed between BPA and PrP/BuP (p < 0.05) in river water, and the relationship was much stronger between BPA and MeP (p < 0.01). In addition, significant positive correlations were found between BPF and BPS, MeP and EtP/BuP/TCS, and EtP and BuP/TCS (p < 0.01). The correlation results were slightly different for sediments compared with river water. Positive and significant (p < 0.05) correlation were found between BPS and EtP/PrP/BuP, BPF and MeP/TCS, BPA and BPS, and BuP and PrP. Significant correlations among these EDCs indicated that they may have some similar sources, particularly for MeP and TCS. Significant relationships were found between MeP and TCS in both river water and sediments (Fig. 3), suggesting they may have a common source, such as additives in PCPs (Liao and Kannan 2014b). Research also has reported that these target chemicals were simultaneously detected in teethers (Asimakopoulos et al. 2016), paper products (Liao and Kannan 2014a; Liao et al. 2012b), and household cleaning products (Wong and Durrani 2017). These products are generally used in our daily life and could be potential sources. Moreover, no significant correlations were found between TOC and any chemicals in sediments, which was not consistent with previous studies (Liao et al. 2012c, 2013). This discrepancy may be attributed to the sampling locations of this study. Because the sediment samples were collected from urban area, where domestic or industrial wastewater may be directly discharged into the rivers, it is hard to reach the dynamic equilibrium between pollutants and TOC in sediments.
Estimated Fluxes of Target EDCs into the Liuxi River
The flux of target EDCs into the main river was estimated by following the previously reported method with some modifications (Wang et al. 2007).
Q (m3) was the total flux of a river in a month (average value of L7 and L(7)), f was the flow rate (m3/day), and d was the number of days in a year (d = 365). \(Ci\) was the concentration of each target chemical in a river (ng/L). Si and S were the total amount of individual chemicals and all target EDCs discharging from a river in a year (kg), respectively. Based on the concentration of target EDCs and flow rates in this river water (Table S1), the annual total fluxes of total and individual chemicals flowing into the Liuxi River were estimated.
Our results indicated the total discharge of all target EDCs into the Liuxi River from its tributaries investigated in this study was ~ 480 kg per year. In this case, ~ 130 kg TCS, ~ 100 kg BPA/MeP/PrP, ~ 30 kg BPF, ~ 10 kg BPS/EtP, and ~ 1.0 kg BuP were discharged into the main river every year.
Ecological Risk Assessment
In this study, the hazard quotient (HQ) method was used to evaluate the potential ecological risks from target EDCs for river water and sediments (Dobbins et al. 2009). The sum of HQ (HQs) value of target EDCs was used to estimate the possible cumulative ecological risk at different sampling sites (Yan et al. 2017). An HQ value > 1 indicated a suspected high ecological risk level, and 0.1 < HQ < 1 and HQ < 0.1 indicated possible medium- and low-risk levels, respectively (Hernando et al. 2006). Generally, the value of HQ is calculated by Eq. (2).
MEC was the measured environmental concentration of a pollutant, and PNEC was the predicted no effect concentration. Commonly, the value of the median effect concentration (EC50) and the lowest observed effect concentration (LOEC), which related to the acute and chronic toxicity with assessment factors of 1000 and 100, respectively, obtained from the literature, were used to calculate the PNEC (Pintado-Herrera et al. 2017). In this study, Daphnia magna was selected as a representative species for aquatic organisms (Table S4). More information is explained by Eq. (3-1) for acute toxicity and (3-2) for chronic toxicity.
By using the above equations and hypothesis, the HQ values of target EDCs were estimated for organisms in river water and sediments (Table 4; Fig. 4). In river water, only TCS had relatively high HQ values, with average HQ = 1.57 (maximum 11.5), accounting for 80% of the total value. This is probably affected by the high concentration of TCS in river water and its relative high toxicity to aquatic organisms (48 h-EC50 = 390 µg/L for Daphnia magna) (Orvos et al. 2002). The other target EDCs, except for PrP (average HQ = 0.19), presented low risk (average HQ < 0.1) to aquatic organisms. In addition, the HQ values of individual chemicals at different sampling sites were different. For example, the maximum HQ values of BPA (0.75), BPS (1.19), and PrP (1.48) in river water were found in samples of W3, W2, and L(9), respectively. In fact, the aquatic system was complex, with a mixture of different chemicals. Therefore, the possible cumulative environmental risk in each sampling site was estimated using the sum of HQ (HQs) of all target EDCs (Yan et al. 2017). HQs values ranged from 0.27 to 12.0, with mean and median values of 1.96 and 1.10, respectively (Table 4; Fig. 5). The values of HQs were higher than 1.0 in 54% of the sampling sites. Due to the high concentration of TCS, some significantly high HQ values were found in river water, such as in samples L4 (3.91), L12 (12.0), and W3 (6.08). All these points were located near industrial areas and sewage outlets (Table S1; Fig. 1), reflecting the large effect of human and industrial production activities on aquatic ecosystems.
To evaluate the environmental risks in sediments, the concentrations of target chemicals were converted to their corresponding concentrations in pore water using the following equation (Zhao et al. 2010)
Cs,i was the concentration of the target compound in sediment, and Koc was the organic carbon partitioning coefficient. The risk assessment in sediments was replaced by their corresponding pore water with the same method used for river water. Some missing parameters of Koc were replaced with Kow (Yan et al. 2017).
As shown in Table 4, except for MeP (average HQ = 0.39) and TCS (0.74), the HQ values of other EDCs (BPA, BPF, BPS, EtP, PrP, and BuP) were low (HQ < 0.1). However, the possible cumulative ecological risks of target chemicals were relative higher (average HQs = 1.35). HQs were 1.37 and 5.66 in L9 and L10, respectively. Compared with river water, ecological risks to aquatic organisms from sediments were slightly lower.
The HQ values of EDCs also were reported in some main streams or lakes in other areas in China. The HQ values of total bisphenol analogues in Taihu Lake ranged from 6.8 × 10−3 to 0.25 for surface water and from 2.0 × 10−4 to 3.6 × 10−3 for sediments (Yan et al. 2017). The values were slightly lower than those in our study, in which the respective highest HQ value of total bisphenols was 1.95 and 0.71 in water and sediments, respectively. In a previous study of the Liuxi River by Zhao et al. (2010), the HQ value of TCS calculated by the “worst-case scenario” was 0.28 for river water and 5.11 for sediments (Zhao et al. 2010), which were still lower than our maximum HQ of TCS (11.5 for river water, and 3.36 for sediments; Table 4). It seems that the ecological risk from exposure to EDCs for organisms in this urban river was slightly higher than those reported in other studies in China.
Conclusions
In this study, we investigated the pollution status of eight endocrine-disrupting chemicals in urban water in Guangzhou, South China. The target EDCs were frequently found in both river water and sediments in this urban area. BPA, MeP, and TCS were the three most abundant chemicals, with total contributions of 72% in river water and 93% in sediments. Significant Pearson correlations between MeP and TCS in both river water and sediments indicated that the two compounds may have common sources, e.g., PCPs. Our ecological risk assessment of EDCs in river water and sediments indicated that TCS was the primary chemical of concern among the target EDCs of this aquatic system, whereas the others (BPA, BPF, BPS, MeP, PrP, EtP, and BuP) showed a relatively low-risk level. The possible cumulative ecological risk assessment indicated that the aquatic organisms of some sampling sites may have potential risk exposure to EDCs, with higher HQs in river water than in sediments. With the increasing usage of new EDCs in our daily lives and industries, further similar research with more sampling locations in urban areas is necessary. In addition, site-specific work, such as bioaccessibility tests of EDCs in sediments or total toxicity tests with extraction of water and sediments collected from these areas using native species in a controlled laboratory environment, is required in urban areas.
References
Asimakopoulos AG, Elangovan M, Kannan K (2016) Migration of parabens, bisphenols, benzophenone-type UV filters, triclosan, and triclocarban from teethers and its implications for infant exposure. Environ Sci Technol 50:13539–13547
Bledzka D, Gromadzinska J, Wasowicz W (2014) Parabens. From environmental studies to human health. Environ Int 67:27–42
Carmona E, Andreu V, Pico Y (2014) Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin: from waste to drinking water. Sci Total Environ 484:53–63
Chen M-Y, Ike M, Fujita M (2002) Acute toxicity, mutagenicity, and estrogenicity of bisphenol-A and other bisphenols. Environ Toxicol 17:80–86
Chen D, Kannan K, Tan H, Zheng Z, Feng Y-L, Wu Y, Widelka M (2016) Bisphenol analogues other than BPA: environmental occurrence, human exposure, and toxicity—a review. Environ Sci Technol 50:5438–5453
Darbre PD, Harvey PW (2008) Paraben esters: review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J Appl Toxicol 28:561–578
Dhillon GS, Kaur S, Pulicharla R, Brar SK, Cledon M, Verma M, Surampalli RY (2015) Triclosan: current status, occurrence, environmental risks and bioaccumulation potential. Int J Environ Res Public Health 12:5657–5684
Dobbins LL, Usenko S, Brain RA, Brooks BW (2009) Probabilistic ecological hazard assessment of parabens usingdaphnia magna and pimephales promelas. Environ Toxicol Chem 28:2744–2753
Golden R, Gandy J, Vollmer G (2005) A review of the endocrine activity of parabens and implications for potential risks to human health. Crit Rev Toxicol 35:435–458
Gong J, Ran Y, Chen D, Yang Y, Ma X (2009) Occurrence and environmental risk of endocrine-disrupting chemicals in surface waters of the Pearl River, South China. Environ Monit Assess 156:199–210
Gong J, Ran Y, Chen DY, Yang Y (2011) Occurrence of endocrine-disrupting chemicals in riverine sediments from the Pearl River Delta, China. Mar Pollut Bull 63:556–563
Guo Y, Kannan K (2013) A survey of phthalates and parabens in personal care products from the United States and its implications for human exposure. Environ Sci Technol 47:14442–14449
Guo Y, Wang L, Kannan K (2014) Phthalates and parabens in personal care products from China: concentrations and human exposure. Arch Environ Contam Toxicol 66:113–119
Hernando MD, Mezcua M, Fernandez-Alba AR, Barcelo D (2006) Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 69:334–342
Huang YQ et al (2012) Bisphenol A (BPA) in China: a review of sources, environmental levels, and potential human health impacts. Environ Int 42:91–99
Ike M, Chen MY, Danzl E, Sei K, Fujita M (2006) Biodegradation of a variety of bisphenols underaerobic and anaerobic conditions. Water Sci Technol 53:153–159
Inui M et al (2003) Effect of UV screens and preservatives on vitellogenin and choriogenin production in male medaka (Oryzias latipes). Toxicology 194:43–50
Jin H, Zhu L (2016) Occurrence and partitioning of bisphenol analogues in water and sediment from Liaohe River Basin and Taihu Lake, China. Water Res 103:343–351
Kasprzyk-Hordern B, Dinsdale RM, Guwy AJ (2008) The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales, UK. Water Res 42:3498–3518
Lee HB, Peart TE, Svoboda ML (2005) Determination of endocrine-disrupting phenols, acidic pharmaceuticals, and personal-care products in sewage by solid-phase extraction and gas chromatography–mass spectrometry. J Chromatogr A 1094:122–129
Liao C, Kannan K (2013) Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. J Agric Food Chem 61:4655–4662
Liao C, Kannan K (2014a) Concentrations and composition profiles of parabens in currency bills and paper products including sanitary wipes. Sci Total Environ 475:8–15
Liao C, Kannan K (2014b) A survey of alkylphenols, bisphenols, and triclosan in personal care products from China and the United States. Arch Environ Contam Toxicol 67:50–59
Liao C, Liu F, Guo Y, Moon HB, Nakata H, Wu Q, Kannan K (2012a) Occurrence of eight bisphenol analogues in indoor dust from the United States and several asian countries: implications for human exposure. Environ Sci Technol 46:9138–9145
Liao C, Liu F, Kannan K (2012b) Bisphenol s, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol a residues. Environ Sci Technol 46:6515–6522
Liao C, Liu F, Moon HB, Yamashita N, Yun S, Kannan K (2012c) Bisphenol analogues in sediments from industrialized areas in the United States, Japan, and Korea: spatial and temporal distributions. Environ Sci Technol 46:11558–11565
Liao C, Lee S, Moon HB, Yamashita N, Kannan K (2013) Parabens in sediment and sewage sludge from the United States, Japan, and Korea: spatial distribution and temporal trends. Environ Sci Technol 47:10895–10902
Liu Y et al (2017) Occurrence, distribution and sources of bisphenol analogues in a shallow Chinese freshwater lake (Taihu Lake)-implications for ecological and human health risk. Sci Total Environ 599–600:1090–1098
Orvos DR, Versteed DJ, Inauen J, Capdevielle M, Rothenstien A, Cunninghan V (2002) Aquatic toxicity of triclosan. Environ Toxicol Chem 21:1338–1349
Peng X et al (2017) Persistence, temporal and spatial profiles of ultraviolet absorbents and phenolic personal care products in riverine and estuarine sediment of the Pearl River catchment, China. J Hazard Mater 323:139–146
Peng X, Yu Y, Tang C, Tan J, Huang Q, Wang Z (2008) Occurrence of steroid estrogens, endocrine-disrupting phenols, and acid pharmaceutical residues in urban riverine water of the Pearl River Delta, South China. Sci Total Environ 397:158–166
Pintado-Herrera MG, Wang C, Lu J, Chang YP, Chen W, Li X, Lara-Martin PA (2017) Distribution, mass inventories, and ecological risk assessment of legacy and emerging contaminants in sediments from the Pearl River Estuary in China. J Hazard Mater 323:128–138
Ramaswamy BR, Shanmugam G, Velu G, Rengarajan B, Larsson DGJ (2011) GC-MS analysis and ecotoxicological risk assessment of triclosan, carbamazepine and parabens in Indian rivers. J Hazard Mater 186:1586–1593
Singer H, Muller S, Tixier C, Pillonel L (2002) Triclosan-Occurrence and fate of a widely used biocide in the aquatic environment—field measurements in wastewater treatment plants, surface waters, and lake sediments. Environ Sci Technol 36:4998–5004
Tišler T, Krel A, Gerželj U, Erjavec B, Dolenc MS (2016) Hazard identification and risk characterization of bisphenols A, F and AF to aquatic organisms. Environ Pollut 212:472–479
USEPA (2008) Reregistration Eligibility Decision for Triclosan, List B Case No. 2340, Office of Prevention, Pesticides And Toxic Substances https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/red_PC-054901_18-Sep-08.pdf. Accessed 18 Sept 2008
Wang J-Z, Guan Y-F, Ni H-G, Luo X-L, Zeng EY (2007) Polycyclic aromatic hydrocarbons in riverine runoff of the Pearl RiverDelta (China): concentrations, fluxes, and fate. Environ Sci Technol 41:5614–5619
Wong KH, Durrani TS (2017) Exposures to endocrine disrupting chemicals in consumer products—a guide for pediatricians. Curr Probl Pediatric Adolesc Health Care 47:107–118
Wu LH et al (2017) Occurrence of bisphenol S in the environment and implications for human exposure: a short review. Sci Total Environ 615:87–98
Xue J, Wan Y, Kannan K (2016) Occurrence of bisphenols, bisphenol A diglycidyl ethers (BADGEs), and novolac glycidyl ethers (NOGEs) in indoor air from Albany, New York, USA, and its implications for inhalation exposure. Chemosphere 151:1–8
Yamazaki E et al (2015) Bisphenol A and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India. Ecotoxicol Environ Saf 122:565–572
Yan Z et al (2017) Bisphenol analogues in surface water and sediment from the shallow Chinese freshwater lakes: occurrence, distribution, source apportionment, and ecological and human health risk. Chemosphere 184:318–328
Yang Y, Lu L, Zhang J, Yang Y, Wu Y, Shao B (2014) Simultaneous determination of seven bisphenols in environmental water and solid samples by liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr A 1328:26–34
Yang Y, Ok YS, Kim K-H, Kwon EE, Tsang YF (2017) Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: a review. Sci Total Environ 596–597:303–320
Zhao JL, Ying GG, Liu YS, Chen F, Yang JF, Wang L (2010) Occurrence and risks of triclosan and triclocarban in the Pearl River system, South China: from source to the receiving environment. J Hazard Mater 179:215–222
Acknowledgements
The present study was financially supported by the Guangdong Innovative and Entrepreneurial Research Team Program, China (No. 2016ZT06N258); the National Natural Science Funds for Distinguished Young Scholar of Guangdong Province, China (No. 2016A030306015); and the National Natural Science Foundation of China (No. 21577050).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, C., Wu, LH., Liu, GQ. et al. Occurrence and Ecological Risk Assessment of Eight Endocrine-Disrupting Chemicals in Urban River Water and Sediments of South China. Arch Environ Contam Toxicol 75, 224–235 (2018). https://doi.org/10.1007/s00244-018-0527-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-018-0527-9