Abstract
Urolithiasis is closely linked to lifestyle factors. However, the causal relationship and underlying mechanisms remain unclear. This study aims to investigate the relationship between lifestyle factors and the onset of urolithiasis and explore potential blood metabolite mediators and their role in mediating this relationship. In this study, we selected single nucleotide polymorphisms (SNPs) as instrumental variables if they exhibited significant associations with our exposures in genome-wide association studies (GWAS) (p < 5.0 × 10-8). Summary data for urolithiasis came from the FinnGen database, including 8597 cases and 333,128 controls. We employed multiple MR analysis methods to assess causal links between genetically predicted lifestyle factors and urolithiasis, as well as the mediating role of blood metabolites. A series of sensitivity and pleiotropy analyses were also conducted. Our results show that cigarettes smoked per day (odds ratio [OR] = 1.159, 95% confidence interval [CI] = 1.004-1.338, p = 0.044) and alcohol intake frequency (OR = 1.286, 95% CI = 1.056-1.565, p = 0.012) were positively associated with increased risk of urolithiasis, while tea intake (OR = 0.473, 95% CI = 0.299-0.784, p = 0.001) was positively associated with reduced risk of urolithiasis. Mediation analysis identifies blood metabolites capable of mediating the causal relationship between cigarettes smoked per day, tea intake and urolithiasis. We have come to the conclusion that blood metabolites serve as potential causal mediators of urolithiasis, underscoring the importance of early lifestyle interventions and metabolite monitoring in the prevention of urolithiasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urolithiasis is a common ailment with a steadily increasing incidence and an exceptionally high recurrence rate [1]. The probability of an individual developing urolithiasis during their lifetime is estimated to be 5–10%. After the initial treatment of urolithiasis, the recurrence rate can reach 50% and 80–90% within 5 and 10 years, respectively [2]. While treatment methods for this condition have improved in terms of efficacy and safety, managing urolithiasis and its complications imposes a significant economic burden on society [3]. The widely used medication, hydrochlorothiazide, which carries a high level of evidence for preventing urinary stone formation, has been proven ineffective in kidney stone prevention [4]. There is a pressing need for further research to explore preventive measures for this condition, to alleviate the burden on patients and society. Considering the exorbitant costs associated with conducting clinical trials and the substantial expenses involved in pharmaceutical development, identifying potential factors related to the prevention and treatment of urolithiasis before embarking on clinical trials is paramount.
Considerable evidence has established a link between lifestyle factors and urolithiasis incidence [5, 6]. Yuan et al. recently reported from a randomized study that increased coffee and caffeine consumption could potentially lower the risk of urinary stone formation [7]; however, they did not explore the potential underlying mechanisms. Urolithiasis is regarded as a chronic metabolic disorder [8], and Khamaysi et al. demonstrated that the presence of succinate metabolism in the blood can alter the risk of calcium oxalate stone formation [9]. Metabolomics is a systematic study of small molecules or metabolites associated with metabolic processes in organisms, including cells, tissues, and biological fluids, providing unique chemical fingerprints [10, 11]. This technique is utilized to discover biomarkers, thereby identifying specific metabolites associated with environmental exposures and diseases, further elucidating the mechanisms of disease [12, 13]. In recent years, with advancements in genome-wide association studies (GWAS) regarding genetic determinants in human metabolism [14,15,16], new biological targets for preventing urolithiasis can be accurately identified at the metabolomic levels through Mendelian randomization (MR) analysis. The use of MR analysis holds promise in advancing our understanding of urolithiasis prevention by pinpointing novel biological pathways and targets.
MR analysis utilizes strongly correlated single nucleotide polymorphisms (SNPs) as substitutes for exposure factors to estimate causal relationships with the phenotype of interest [17]. It follows Mendel’s Second Law, the law of independent assortment, akin to clinical randomized controlled trials (RCTs) [18]. Hence, helping to mitigate possible confounding variables and addressing the reverse causality problems commonly encountered in observational studies [19].
We conducted univariable Mendelian randomization (UVMR) to assess the causal link between lifestyle factors and urolithiasis formation. Additionally, we performed multivariable Mendelian randomization (MVMR) to account for potential biases arising from confounding factors. Furthermore, we delved into the mechanisms linking lifestyle factors, urolithiasis, and blood metabolites. Subsequently, a two-step MR analysis was employed to evaluate whether these blood metabolites, which exhibited a significant causal relationship with urolithiasis, could mediate the influence of lifestyle factors on the risk of urolithiasis formation, and this effect was quantified.
Methods and materials
Study design
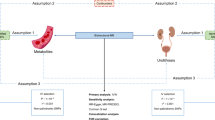
The data utilized for MR analysis were publicly sourced from databases that have obtained ethical approval. This study primarily followed several steps. Initially, we used MR analysis to evaluate the causal impact of lifestyle factors on urolithiasis. Selecting ‘cigarettes smoked per day,’ ‘frequency of alcohol intake,’ and ‘tea consumption’ as lifestyle factors was based on numerous epidemiological studies identifying them as potential risk factors for urolithiasis [5, 20,21,22,23,24]. However, it was crucial to highlight that the connections between these factors and the risk of urinary stone formation are debated in the literature. Our aim was to study factors that have both clinical significance and solid genetic foundation. Subsequently, we conducted Univariable Mendelian Randomization (UVMR) to evaluate the individual effects of three lifestyle factors, including cigarettes smoked per day, alcohol intake frequency, and tea intake, after mutual adjustment. Next, from a pool of 249 blood metabolites, we selected three potential mediators in the association between lifestyle factors and urolithiasis: phenylalanine, tyrosine, and phospholipids to total lipids ratio in very large very-low-density lipoprotein (VLDL) (XL_VLDL_PL_pct). Finally, we conducted mediation analysis to quantify the mediating effects of these three metabolites on the causal link between lifestyle factors and the risk of urolithiasis. (Fig. 1).
Study flowchart illustrating the Mendelian randomization (MR) analysis process. SNPs related to lifestyle factors, blood metabolites, and kidney and ureter calculus were selected for MR analysis. Due to adherence to MR principles, MR can assess causal relationships without interference from confounding factors. Furthermore, this study employed MR to estimate whether blood metabolites mediate the causal relationship between lifestyle factors and kidney and ureter calculus. VLDL, very-low-density lipoprotein; XL_VLDL_PL_pct, phospholipids to total lipids ratio in very large VLDL
Screening process for SNPs
The selection of SNPs correlated with the phenotype adhered to the three core assumptions of MR: (1) SNPs are significantly associated with lifestyle factors; (2) SNPs are unrelated to confounding factors; (3) SNPs affect urolithiasis solely through the lifestyle factors [25]. The threshold for SNPs with GWAS significance was set at p < 5 × 10-8, and for SNP independence, the threshold was r2 < 0.001, with a clump distance of 10,000 kb to mitigate potential biases introduced by linkage disequilibrium (LD). LD levels were computed using data from the European sample in the 1000 Genomes Project [26]. Palindromic SNPs and incompatible SNPs were excluded based on allele frequencies. Additionally, we calculated the F-statistic to gauge the statistical robustness of the chosen SNPs, and SNPs with an F-statistic of less than 10 were excluded due to insufficient statistical power. We employed PhenoScanner (www.phenoscanner.medschl.cam.ac.uk) [27, 28] To eliminate the risk of our selected SNPs associated with lifestyle factors being confounded by other phenotypes that could influence the onset of urolithiasis (e.g., infections, genetic defects, adverse drug effects, including increased levels of vitamin D, cystinuria, xanthinuria, ureteropelvic junction obstruction, ureteral stricture, high-temperature work environments, etc.), evaluated at a threshold of p < 5 × 10-8. We also employed MR pleiotropy residual sum and outlier (MR-PRESSO) methods to identify and eliminate SNPs exhibiting pleiotropic outliers (p < 0.05) [29]. SNP information and F-statistics for the GWAS datasets used in this study can be found in Additional file 1: Table S1–7.
Data sources for lifestyle factors
In the initial stages of our research, our criteria for selecting GWAS summary data related to lifestyle factors were focused on ensuring that the data were recently updated and obtainable in a reasonable manner. These GWAS summaried incorporate a larger sample size. This method aimed to bolster the statistical power of our analysis, fortify the robustness of study results, and mitigate the impact of random noise. GWAS summary data for “cigarettes smoked per day” were extracted from the GWAS and Sequencing Consortium of Alcohol and Nicotine use, which includes 249,752 individuals of European ancestry with 12,003,613 genetic variants [30]. This data is publicly accessible on the Medical Research Council Integrative Epidemiology Unit (MRC-IEU) open GWAS database, available at https://gwas.mrcieu.ac.uk/datasets/ieu-b-142/. We sourced the GWAS summary data for ‘alcohol intake frequency’ from the MRC-IEU consortium, which includes 462,346 individuals of European ancestry with 9,851,867 genetic variants. This data can be publicly accessed from the MRC-IEU open GWAS database (https://gwas.mrcieu.ac.uk/datasets/ukb-b-5779/). Similarly, the GWAS summary data for “tea intake” comprises 447,485 individuals of European ancestry and encompasses 9,851,867 genetic variants, which is openly accessible through the MRC-IEU open GWAS database (https://gwas.mrcieu.ac.uk/datasets/ukb-b-6066/).
Data sources for blood metabolites
The UK Biobank (UKB) is a biomedical database comprising approximately 500,000 participants [31]. The blood metabolites data utilized in the present study originated from an analysis of approximately 120,000 UK volunteers’ baseline EDTA plasma samples recruited by the Nightingale Health platform in 2020 within the UKB database. These volunteers had a baseline age range of 37 to 73 years, and the blood samples were collected approximately 4 hours after their last meal, with females accounting for 54% of the cohort. These data encompassed 168 absolute level measures and 81 ratio measures, covering 249 indicators such as lipoprotein lipids, fatty acids, and small molecules, representing various metabolic pathways in the human body. You can find comprehensive data on these blood metabolites in the MRC-IEU GWAS database (https://gwas.mrcieu.ac.uk/) [32].
Data sources for urolithiasis
The GWAS summary data related to urolithiasis (including kidney and ureter calculus) were sourced from the FinnGen consortium [33]. Data from the eighth version of the database were utilized, which included 8597 cases and 333,128 controls, with an analysis covering 20,175,454 variants. This GWAS dataset was adjusted for covariates, including gender, age, the top 10 principal components, genetic batch, and genetic relatedness. You can access detailed data from the FinnGen database at https://www.finngen.fi/en/access_results. All populations used for analysis shared the same European ancestry. The decision to utilize the summary of the urinary tract stone GWAS from FinnGen was motivated by two reasons. Firstly, FinnGen provides a larger number of urinary tract stone cases. Secondly, Given that our data sources for blood metabolites were from the UKBiobank, continuing to use the UKBB’s Urolithiasis Summary in the two-sample MR analysis might introduce sample overlap, potentially biasing our results [34]. After careful consideration, we made the decision to use FinnGen’s Urolithiasis Summary.
Statistical analysis
Step 1: Identification of independent causal effects of lifestyle factors on urolithiasis using UVMR and MVMR
Three common UVMR methods, namely inverse variance weighted (IVW), MR-Egger, and weighted median (WM), were employed to examine the causal relationship between lifestyle factors and urolithiasis. The IVW method assumes that all instrumental variables used in the analysis are valid. The WM method relies on the assumption that a minimum of 50% of the analyzed SNPs are valid. The MR-Egger method assumes that all genetic variants used in the analysis have no impact on the exposure and outcome. However, the differing fundamental assumptions of the three methods may introduce bias into the results. The IVW method was the primary analytical approach used [35], and the MR-Egger regression and WM method served as supplementary analyses to validate the results’ reliability and stability. If the IVW p-value was < 0.05 and the direction of the odds ratio (OR) aligned with the direction of the other two analysis methods, we proceeded with further assessment. Subsequently, a set of sensitivity analyses were carried out, such as the Cochran’s Q test, to estimate heterogeneity in the causal relationship between lifestyle factors and urolithiasis (Heterogeneity in MR analysis results was indicated by a p-value < 0.05 for Cochran’s Q test). MR-Egger intercept was utilized to assess whether there was genetic pleiotropy in the causal relationship between lifestyle factors and urolithiasis (a p-value of < 0.05 for intercept indicated pleiotropy); the regression intercept was used to assess the magnitude of pleiotropy [36]. A leave-one-out analysis was conducted to assess the influence of each SNPs on the causal relationship, identifying strongly correlated SNPs. To elucidate the independent causal impact of lifestyle factors on urolithiasis, the least absolute shrinkage and selection operator (LASSO) method was first used to exclude potential collinearity between lifestyle factors. Furthermore, we primarily used MVMR-IVW as the multivariate analysis method and employed MVMR-Egger for sensitivity analysis of the multivariate results. All statistical analyses were performed using MendelianRandomization (Version 0.7.0), TwoSampleMR (Version 0.5.6), and MVMR (Version 0.3) packages.
Step 2: Selection of potential mediators
The IVW method was the primary MR approach for assessing the causal effects of 249 blood metabolite levels on urolithiasis, resulting in the identification of 15 metabolites with causal effects on this condition. We further assessed heterogeneity among the SNPs used in the primary analysis using Cochran’s Q statistic. We also employed MR-Egger intercepts to detect directional pleiotropy. Metabolites exhibiting heterogeneity and pleiotropy were eliminated, resulting in the selection of three metabolites: tyrosine, XL_VLDL_PL_pct, and phenylalanine, which were considered to have a causal impact on urolithiasis. Subsequently, we employed the same methods to evaluate the causal associations between lifestyle factors and the three metabolites. If there was causal evidence indicating that lifestyle factors influenced metabolites, which in turn affected the risk of urolithiasis, these metabolites were chosen as candidate mediators for the respective lifestyle factors.
Step 3: Quantification of the mediating effects of different blood metabolites in the causal associations between lifestyle factors and urolithiasis.
A two-step MR analysis was conducted to quantify the mediating effects of different metabolites in the causal relationship between various lifestyle factors and urolithiasis. First, univariate MR was applied to calculate the causal effect (β1) of each lifestyle factor on blood metabolites with causal impacts. Second, MVMR was employed to calculate the causal effect (β2) of each metabolite on urolithiasis risk while accounting for various lifestyle factors. The mediation proportion of blood metabolites in mediating the relationship between lifestyle factors and urolithiasis was calculated by dividing the mediation effect (β1 × β2) by the total effect [37,38,39]. Confidence intervals (CIs) were calculated using the delta method [40, 41].
Results
MR and MVMR analyses of independent causal effects of lifestyle factors on urolithiasis
According to our screening criteria for SNPs related to lifestyle factors, no SNPs requiring exclusion were identified during the use of PhenoScanner. Using the IVW method, our analysis revealed that cigarettes smoked per day (OR = 1.159, 95% CI = 1.004–1.338, p = 0.044) and alcohol intake frequency (OR = 1.286, 95% CI = 1.056–1.565, p = 0.012) were causally associated with an elevated risk of urolithiasis, while tea intake was causally associated with a lower risk of urolithiasis (OR = 0.473, 95% CI = 0.299–0.748, p = 0.001). A LASSO analysis of the three lifestyle factors showed no potential collinearity among them. Further MVMR analysis revealed that smoking (OR = 1.189, 95% CI = 1.010–1.399, p = 0.038), alcohol consumption (OR = 1.35, 95% CI = 1.071–1.702, p = 0.011), and tea consumption (OR = 0.352, 95% CI = 0.219–0.567, p < 0.001) independently influenced the risk of urolithiasis (Fig. 2).
Impact of blood metabolites on the risk of urolithiasis
In the MR analysis of 249 blood metabolites with urolithiasis, 15 blood metabolites showed significant results using the IVW method. We further assessed the results for heterogeneity using Cochran’s Q statistic and examined directional pleiotropy with the MR-Egger intercept. Any blood metabolites exhibiting heterogeneity and pleiotropy were eliminated (Fig. 3a). Results showed that tyrosine (OR = 1.201, 95% CI = 1.023–1.411, p = 0.025) and phenylalanine (OR = 1.404, 95% CI = 1.056–1.886, p = 0.019) increased the risk of urolithiasis, while XL_VLDL_PL_pct (OR = 0.840, 95% CI = 0.741–0.952, p = 0.006) reduced the risk of urolithiasis (Fig. 3b).
Selection process of metabolites causally related to kidney and ureter calculus among the 249 blood metabolites. a Blood metabolite (√) causally related to kidney and ureter calculus selected based on the primary inverse-variance weighted (IVW) analysis method and sensitivity analysis methods. The intensity of brown color indicates the significance of blood metabolites under the respective analysis method (p < 0.05), while the intensity of blue color suggests the lack of significance for blood metabolites under this method (p > 0.05). A Cochran-Q method with a p-value > 0.05 indicates no heterogeneity in MR analysis, and an Egger-intercept method with a p-value > 0.05 indicates the absence of directional pleiotropy in the analysis. b Genetic susceptibility of blood metabolites associated with kidney and ureter calculus. Results were obtained from the IVW median method with a random-effects model. A P-value < 0.05 indicates a causal effect of the blood metabolite on kidney and ureter calculus. VLDL, very-low-density lipoprotein; XL_VLDL_PL_pct, phospholipids to total lipids ratio in very large VLDL; OR odds ratio, CI confidence intervals, SNPs single nucleotide polymorphisms
Impact of lifestyle factors on blood metabolites
Using two-sample UVMR analysis, it was found that cigarettes smoked per day had a causal impact on XL_VLDL_PL_pct (β = 0.066, 95% CI = 0.025–0.106, p = 0.001) and phenylalanine (β = 0.037, 95% CI = 0.007–0.068, p = 0.017). Tea intake had a causal impact on tyrosine (β = 0.124, 95% CI = 0.025–0.224, p = 0.015) and XL_VLDL_PL_pct (β = 0.115, 95% CI = 0.026–0.204, p = 0.011). Alcohol intake frequency had a causal impact on tyrosine (β = − 0.050, 95% CI= − 0.097 to 0.003, p = 0.037) and XL_VLDL_PL_pct (β = 0.069, 95% CI = 0.020–0.118, p = 0.006) (Fig. 4). By combining previous analyses, we identified causal relationships as follows: “cigarettes smoked per day → urolithiasis,” “cigarettes smoked per day → phenylalanine,” “phenylalanine → urolithiasis,” “tea intake → urolithiasis,” “tea intake → XL_VLDL_PL_pct,” and “XL_VLDL_PL_pct → urolithiasis.” Therefore, it was inferred that phenylalanine and XL_VLDL_PL_pct mediate the impact of cigarettes smoked per day and tea intake on the risk of urolithiasis. No blood metabolites were identified as mediators of the relationship between alcohol intake and the risk of urolithiasis. Consequently, the mediating effects of phenylalanine and XL_VLDL_PL_pct on the association between lifestyle factors and the risk of urolithiasis were further analyzed.
Causal associations between lifestyle factors and blood metabolites. Results are obtained from the inverse variance-weighted median method with a random effects model. XL_VLDL_PL_pct, phospholipids to total lipids ratio in very large VLDL; beta, effect size; CI confidence intervals, SNPs single nucleotide polymorphisms
Mediation effects of blood metabolites on urolithiasis
In further mediation analyses, the total effects of different lifestyle factors on urolithiasis were denoted as “c” and the indirect effects mediated through blood metabolites due to lifestyle factors were expressed as “a × b” [39] (Fig. 5a). In the mediation analysis involving cigarettes smoked per day—phenylalanine—urolithiasis, after adjusting for cigarettes smoked per day in the MVMR analysis, the direct effect of phenylalanine on urolithiasis was estimated with an OR of 1.467 (95% CI = 1.106–1.945). Therefore, the percentage of the causal effect of cigarettes smoked per day on urolithiasis mediated by phenylalanine was 9.7% (4.2–15.2%) (Fig. 5b). In the mediation analysis involving tea intake—XL_VLDL_PL_pct—urolithiasis, after adjusting for tea intake in the MVMR analysis, the direct effect of XL_VLDL_PL_pct on urolithiasis was estimated with an OR of 0.870 (95% CI = 0.753–1.004). Therefore, the proportion of the causal effect of tea intake on urolithiasis mediated by XL_VLDL_PL_pct was 2.1% (1.0–3.3%) (Fig. 5b).
Mediating effect of selected blood metabolites on the association between lifestyle factors and kidney and ureter calculus. a Illustration of the total effect and the direct effect of lifestyle factors on kidney and ureter calculus. b Estimates of the impact of tea intake explained by XL_VLDL_PL_pct and the impact of cigarettes smoked per day explained by phenylalanine on kidney and ureter calculus. PM proportion mediated, VLDL very-low-density lipoprotein, XL_VLDL_PL_pct phospholipids to total lipids ratio in very large VLDL, CI confidence intervals
Complementary and sensitivity analyses
Based on the significant results from IVW, we further validated our main analysis using weighted median and MR-Egger as supplementary analytical approaches. The three MR analytical methods employed in the primary analysis consistently showed the same direction of OR values (Additional file 1: Table S8).
Regarding MR sensitivity analyses, although they may reduce the precision of the results, they did not alter the direction of causality. Some MR results showed heterogeneity (Additional file 1: Table S9) but with no evidence of directional pleiotropy (Additional file 1: Table S10). The leave-one-out analysis was performed to evaluate the impact of each SNPs on the primary MR analysis’s causal effect, and no strongly correlated SNPs were identified. The results for leave-one-out analysis are presented in Additional file 2: Figure S1-8.
Discussion
The current study was designed to assess potential associations between lifestyle factors and urolithiasis incidence and whether these associations were mediated by blood metabolites. This is the first study to utilize MR to explore the role of blood metabolites in mediating the influence of lifestyle factors on urolithiasis incidence. Our genetic analysis revealed causal relationships between cigarettes smoked per day, tea intake, and alcohol intake frequency and the risk of urolithiasis development. Further analysis showed that phenylalanine mediated 9.7% of the causal relationship between cigarettes smoked per day and urolithiasis, while XL_VLDL_PL_pct mediated 2.1% of the causal association between tea intake and urolithiasis.
This marks the inaugural MR investigation to assess the causal and distinct impact of cigarettes smoked per day on the risk of urolithiasis. It identified phenylalanine as the metabolic mediator of the association between cigarettes smoked per day and urolithiasis and quantified its mediating effect in this pathway. Currently, there is significant controversy surrounding the relationship between cigarettes smoked per day and urolithiasis risk. Some studies found no correlation between smoking and urolithiasis [42,43,44]. However, alternative research has identified a noteworthy link between smoking and urolithiasis. The discrepancies in these study results could potentially be attributed to confounding factors. Hamano et al. conducted a case-control study and noted that a smoking habit significantly increased the risk of urolithiasis (OR = 4.41, 95% CI = 2.85–6.84, p < 0.0001) [45]. Liu et al. conducted a case-control study and reported that cigarette smoking significantly elevated the risk of calcium urolithiasis (OR = 1.66; 95% CI = 1.11–2.50; p = 0.014) [21]. Soueidan and colleagues observed a significantly higher smoking rate among participants with urolithiasis compared with those without urolithiasis (7% vs. 21%, p = 0.02) [46]. Regarding the relationship between phenylalanine and urolithiasis, Prié et al. found that patients with mutations leading to the substitution of 48 amino acids by phenylalanine were prone to recurrent urolithiasis [47], a case report also described urinary stones in patients with untreated phenylketonuria [48]. Duan et al. also established a close association between phenylalanine metabolism abnormalities and urolithiasis patients. These studies provide strong supporting evidence for our study on the reliability of phenylalanine mediating the increased risk of urolithiasis due to cigarettes smoked per day.
Although several observational studies have confirmed the association between tea intake and urolithiasis, research conclusions are inconsistent due to the inevitable interference of confounding factors. Goldfarb et al. found that tea prevented stone disease [49], which is analogous to Littlejohns et al.’s conclusion [24]. However, Haghighatdoost et al. found that drinking more than 4 cups of tea per day increased the risk of calcium oxalate kidney stones [50]. To obtain more reliable research conclusions, subsequent studies employed MR methods to evaluate the causal relationship between tea intake and urolithiasis. Liu et al. conducted an MR analysis to examine the causal association between tea intake and urolithiasis, revealing an inverse association between tea consumption and kidney stones [51]. The present study used a larger sample size and established the causal association between tea intake and urolithiasis. More importantly, our study discovered for the first time that XL_VLDL_PL_pct mediates the connection between tea intake and urolithiasis.
Similarly, observational research has produced inconsistent results regarding the causal relationship between frequency of alcohol consumption and urolithiasis. Some studies reported a negative correlation [20, 52, 53], while others found no association between the two parameters [21, 49]. The discrepancy in conclusions is also attributed to the inevitable confounding factors in traditional observational studies, such as recall bias during questionnaire surveys. Subsequently, Yang et al. and coworkers performed an MR analysis and found that alcohol intake frequency increased the risk of urolithiasis [54]. While our method aligns with theirs, our MR results cannot be considered an independent replication of their findings. First, we employed a dataset with a larger sample size, adding more confidence to our study. Second, there was no sample overlap between our study cases and controls, eliminating weak instrument bias and Type I error rates. Lastly, we further explored the causality between alcohol intake frequency and urolithiasis. Although we did not identify any substances mediating their relationship in blood metabolism, this may direct future investigations. There is a need to broaden the search scope for blood metabolites or explore alternative approaches to clarify the potential mechanisms behind their causal relationship.
The strengths of this study include the use of MR analysis, which introduces a novel approach to exploring the causal link between lifestyle factors and urolithiasis while reducing bias caused by confounding factors. Additionally, we employed MR-PRESSO to remove SNPs that might introduce bias, ensuring that they are significantly associated with exposure but unrelated to the outcome. Furthermore, the SNPs used in our analysis had F-statistics greater than 10 to ensure statistical power, and we performed several sensitivity analyses to further confirm the consistency of causal associations. In addition, we selected 249 blood metabolites to explore potential mechanisms underlying the causal link between lifestyle factors and urolithiasis. Nevertheless, this study does come with several limitations. Firstly, despite employing various methods to control bias during the analysis, complete elimination of bias is unlikely. Secondly, our study exclusively relied on GWAS data from individuals of European ancestry, potentially constraining the generalizability of our findings. Thus, future studies should include individuals from diverse ethnic backgrounds. Thirdly, our analysis did not differentiate the composition of stones, which might affect the associations between lifestyle factors and urolithiasis and warrants further investigation.
Conclusion
In summary, this study revealed the associations between cigarettes smoked per day, alcohol intake frequency, and tea intake and the risk of urolithiasis. Additionally, phenylalanine was identified as a causal mediator for the increased risk of urolithiasis due to smoking and XL_VLDL_PL_pct was identified as a causal mediator for the reduced risk associated with tea intake. These findings hold significant implications for precise interventions targeting lifestyle-related metabolites to prevent urolithiasis. Furthermore, the study findings deepen our understanding of the potential mechanisms underlying the connection between lifestyle factors and urolithiasis, providing additional targets for future prevention and intervention strategies for urolithiasis.
Data availability
The data used to support the findings of this study are available from the corresponding authors upon reasonable request.
References
Stamatelou KK, Francis ME, Jones CA, Nyberg LM et al (2003) Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney Int. 5:1817–1823. https://doi.org/10.1046/j.1523-1755.2003.00917.x
Skolarikos A, Straub M, Knoll T, Sarica K et al (2015) Metabolic evaluation and recurrence prevention for urinary stone patients: EAU guidelines. Eur Urol. 4:750–763. https://doi.org/10.1016/j.eururo.2014.10.029
Ruhayel Y, Tepeler A, Dabestani S, MacLennan S et al (2017) Tract sizes in miniaturized percutaneous nephrolithotomy: a systematic review from the European Association of Urology Urolithiasis Guidelines Panel. Eur Urol 2:220–235. https://doi.org/10.1016/j.eururo.2017.01.046
Alexander RT (2023) Do thiazides reduce the risk of kidney-stone recurrence? N Engl J Med 9:841–842. https://doi.org/10.1056/NEJMe2300120
Peerapen P, Thongboonkerd V (2023) Kidney stone prevention. Adv Nutr 3:555–569. https://doi.org/10.1016/j.advnut.2023.03.002
D’Ambrosio V, Ferraro PM, Lombardi G, Friso S et al (2022) Unravelling the complex relationship between diet and nephrolithiasis: the role of nutrigenomics and nutrigenetics. Nutrients. https://doi.org/10.3390/nu14234961
Yuan S, Larsson SC (2022) Coffee and caffeine consumption and risk of kidney stones: a Mendelian randomization study. Am J Kidney Dis 1:9-14.e11. https://doi.org/10.1053/j.ajkd.2021.04.018
Zhang XZ, Lei XX, Jiang YL, Zhao LM et al (2023) Application of metabolomics in urolithiasis: the discovery and usage of succinate. Signal Transduct Target Ther. 1:41. https://doi.org/10.1038/s41392-023-01311-z
Khamaysi A, Anbtawee-Jomaa S, Fremder M, Eini-Rider H et al (2019) Systemic succinate homeostasis and local succinate signaling affect blood pressure and modify risks for calcium oxalate lithogenesis. J Am Soc Nephrol. 3:381–392. https://doi.org/10.1681/asn.2018030277
Griffin JL, Atherton H, Shockcor J, Atzori L (2011) Metabolomics as a tool for cardiac research. Nat Rev Cardiol 11:630–643. https://doi.org/10.1038/nrcardio.2011.138
Onuh JO, Qiu H (2021) Metabolic profiling and metabolites fingerprints in human hypertension: discovery and potential. Metabolites. https://doi.org/10.3390/metabo11100687
Johnson CH, Ivanisevic J, Siuzdak G (2016) Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol 7:451–459. https://doi.org/10.1038/nrm.2016.25
Wang Y, Liu F, Sun L, Jia Y et al (2023) Association between human blood metabolome and the risk of breast cancer. Breast Cancer Res 1:9. https://doi.org/10.1186/s13058-023-01609-4
Shin SY, Fauman EB, Petersen AK, Krumsiek J et al (2014) An atlas of genetic influences on human blood metabolites. Nat Genet. 6:543–550. https://doi.org/10.1038/ng.2982
Kettunen J, Demirkan A, Würtz P, Draisma HH et al (2016) Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat Commun. https://doi.org/10.1038/ncomms11122
Michailidou K, Lindström S, Dennis J, Beesley J et al (2017) Association analysis identifies 65 new breast cancer risk loci. Nature 7678:92–94. https://doi.org/10.1038/nature24284
Ebrahim S, Davey Smith G (2008) Mendelian randomization: can genetic epidemiology help redress the failures of observational epidemiology? Hum Genet 1:15–33. https://doi.org/10.1007/s00439-007-0448-6
Sleiman PM, Grant SF (2010) Mendelian randomization in the era of genomewide association studies. Clin Chem 5:723–728. https://doi.org/10.1373/clinchem.2009.141564
Smith GD, Ebrahim S (2003) ’Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 1:1–22. https://doi.org/10.1093/ije/dyg070
Wang H, Fan J, Yu C, Guo Y et al (2021) Consumption of tea, alcohol, and fruits and risk of kidney stones: a prospective cohort study in 0.5 million Chinese adults. Nutrients. https://doi.org/10.3390/nu13041119
Liu CC, Huang SP, Wu WJ, Chou YH et al (2009) The impact of cigarette smoking, alcohol drinking and betel quid chewing on the risk of calcium urolithiasis. Ann Epidemiol. 8:539–545. https://doi.org/10.1016/j.annepidem.2009.02.006
Aizezi X, Xie L, Xie H, Li J et al (2022) Epidemiological and clinical characteristics of stone composition: a single-center retrospective study. Urolithiasis. 1:37–46. https://doi.org/10.1007/s00240-021-01274-2
Turney BW, Appleby PN, Reynard JM, Noble JG et al (2014) Diet and risk of kidney stones in the Oxford cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur J Epidemiol 5:363–369. https://doi.org/10.1007/s10654-014-9904-5
Littlejohns TJ, Neal NL, Bradbury KE, Heers H et al (2020) Fluid intake and dietary factors and the risk of incident kidney stones in UK biobank: a population-based prospective cohort study. Eur Urol Focus. 4:752–761. https://doi.org/10.1016/j.euf.2019.05.002
Emdin CA, Khera AV, Kathiresan S (2017) Mendelian randomization. JAMA. 19:1925–1926. https://doi.org/10.1001/jama.2017.17219
Abecasis GR, Altshuler D, Auton A, Brooks LD et al (2010) A map of human genome variation from population-scale sequencing. Nature. 7319:1061–1073. https://doi.org/10.1038/nature09534
Staley JR, Blackshaw J, Kamat MA, Ellis S et al (2016) PhenoScanner: a database of human genotype-phenotype associations. Bioinformatics. 20:3207–3209. https://doi.org/10.1093/bioinformatics/btw373
Kamat MA, Blackshaw JA, Young R, Surendran P et al (2019) PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. 22:4851–4853. https://doi.org/10.1093/bioinformatics/btz469
Verbanck M, Chen CY, Neale B, Do R (2018) Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 5:693–698. https://doi.org/10.1038/s41588-018-0099-7
Liu M, Jiang Y, Wedow R, Li Y et al (2019) Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet 2:237–244. https://doi.org/10.1038/s41588-018-0307-5
Sudlow C, Gallacher J, Allen N, Beral V et al (2015) UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 3:e1001779. https://doi.org/10.1371/journal.pmed.1001779
Julkunen H, Cichońska A, Tiainen M, Koskela H et al (2023) Atlas of plasma NMR biomarkers for health and disease in 118,461 individuals from the UK Biobank. Nat Commun. 1:604. https://doi.org/10.1038/s41467-023-36231-7
Kurki MI, Karjalainen J, Palta P, Sipilä TP et al (2023) FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 7944:508–518. https://doi.org/10.1038/s41586-022-05473-8
Cai J, Wei Z, Chen M, He L et al (2022) Socioeconomic status, individual behaviors and risk for mental disorders: a Mendelian randomization study. Eur Psychiatry 1:e28. https://doi.org/10.1192/j.eurpsy.2022.18
Liu N, Tan JS, Liu L, Li H et al (2023) Roles of obesity in mediating the causal effect of attention-deficit/hyperactivity disorder on diabetes. Epidemiol Psychiatr Sci. https://doi.org/10.1017/s2045796023000173
Burgess S, Thompson SG (2017) Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol 5:377–389. https://doi.org/10.1007/s10654-017-0255-x
VanderWeele TJ (2016) Mediation analysis: a practitioner’s guide. Annu Rev Public Health. https://doi.org/10.1146/annurev-publhealth-032315-021402
Relton CL, Davey Smith G (2012) Two-step epigenetic Mendelian randomization: a strategy for establishing the causal role of epigenetic processes in pathways to disease. Int J Epidemiol 1:161–176. https://doi.org/10.1093/ije/dyr233
Carter AR, Sanderson E, Hammerton G, Richmond RC et al (2021) Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur J Epidemiol 5:465–478. https://doi.org/10.1007/s10654-021-00757-1
MacKinnon DP, Fairchild AJ, Fritz MS (2007) Mediation analysis. Annu Rev Psychol. https://doi.org/10.1146/annurev.psych.58.110405.085542
Wang Y, Ye C, Kong L, Zheng J et al (2023) Independent associations of education, intelligence, and cognition with hypertension and the mediating effects of cardiometabolic risk factors: a Mendelian randomization study. Hypertension 1:192–203. https://doi.org/10.1161/hypertensionaha.122.20286
Lee YH, Huang WC, Lu CM, Tsai JY et al (2003) Stone recurrence predictive score (SRPS) for patients with calcium oxalate stones. J Urol 2(Pt 1):404–407. https://doi.org/10.1097/01.ju.0000072365.22948.30
Słojewski M, Czerny B, Safranow K, Droździk M et al (2009) Does smoking have any effect on urinary stone composition and the distribution of trace elements in urine and stones? Urol Res 6:317–322. https://doi.org/10.1007/s00240-009-0221-5
Marić I, Kizivat T, Smolić M, Smolić R et al (2019) Lifestyle risk factors and bone mass in recurrent stone-forming patients: a cross-sectional study in 144 subjects. Acta Clin Croat. 3:439–445. https://doi.org/10.20471/acc.2019.58.03.06
Hamano S, Nakatsu H, Suzuki N, Tomioka S et al (2005) Kidney stone disease and risk factors for coronary heart disease. Int J Urol 10:859–863. https://doi.org/10.1111/j.1442-2042.2005.01160.x
Soueidan M, Bartlett SJ, Noureldin YA, Andersen RE et al (2015) Leisure time physical activity, smoking and risk of recent symptomatic urolithiasis: survey of stone clinic patients. Can Urol Assoc J 7–8:257–262. https://doi.org/10.5489/cuaj.2879
Prié D, Huart V, Bakouh N, Planelles G et al (2002) Nephrolithiasis and osteoporosis associated with hypophosphatemia caused by mutations in the type 2a sodium-phosphate cotransporter. N Engl J Med 13:983–991. https://doi.org/10.1056/NEJMoa020028
Hou JW, Wang TR (1995) Methylmalonic aciduria and urolithiasis in a Chinese boy with untreated phenylketonuria. J Inherit Metab Dis 1:79–80. https://doi.org/10.1007/bf00711379
Goldfarb DS, Fischer ME, Keich Y, Goldberg J (2005) A twin study of genetic and dietary influences on nephrolithiasis: a report from the Vietnam Era Twin (VET) Registry. Kidney Int. 3:1053–1061. https://doi.org/10.1111/j.1523-1755.2005.00170.x
Haghighatdoost F, Sadeghian R, Abbasi B (2021) The associations between tea and coffee drinking and risk of calcium-oxalate renal stones. Plant Foods Hum Nutr 4:516–522. https://doi.org/10.1007/s11130-021-00933-4
Liu D, Wang J, Chen Y, Liu F et al (2023) Tea intake and risk of kidney stones: a mendelian randomization study. Nutrition. https://doi.org/10.1016/j.nut.2022.111919
Ferraro PM, Taylor EN, Gambaro G, Curhan GC (2013) Soda and other beverages and the risk of kidney stones. Clin J Am Soc Nephrol 8:1389–1395. https://doi.org/10.2215/cjn.11661112
Kim SY, Yoo DM, Bang WJ, Choi HG (2022) Obesity is positively associated and alcohol intake is negatively associated with nephrolithiasis. Nutrients. https://doi.org/10.3390/nu14194122
Yang S, Tan W, Wei B, Gu C et al (2023) Association between alcohol and urolithiasis: a mendelian randomization study. Urolithiasis 1:103. https://doi.org/10.1007/s00240-023-01472-0
Acknowledgements
We thank Kettunen et al, FinnGen consortium, the UK Biobank and Nightingale Health Ltd for providing GWAS data.
Funding
This study was supported by the Key Research and Development Program of Hubei province (2023BCB001), the Translational Medicine and Multidisciplinary Research Project of Zhongnan Hospital of Wuhan University (ZNJC202217, ZNJC202232).
Author information
Authors and Affiliations
Contributions
XW, SL and TL: were responsible for the concept and design. ZL and HW: collected the data and the data analysis. ZL: interpreted the results and wrote the manuscript. XW, SL, TL, HW and XT: assisted in revising the manuscript. All authors have read and approved this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Z., Wei, H., Tang, X. et al. Blood metabolites mediate the impact of lifestyle factors on the risk of urolithiasis: a multivariate, mediation Mendelian randomization study. Urolithiasis 52, 44 (2024). https://doi.org/10.1007/s00240-024-01545-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00240-024-01545-8