Abstract
Idiopathic hypocitraturia (IH) is a risk factor for urolithiasis. IH is associated with vitamin D receptor (VDR) gene single nucleotide polymorphisms (SNPs) in a Chinese Han population. However, this association between VDR SNPs and IH has not been recapitulated in a Chinese Bai population. The aim of this study is to investigate the association between VDR SNPs and IH in a Chinese Bai population. A total of 320 participants comprising of 200 Chinese Bai patients with IH and 120 Chinese Bai control participants with normal urinary citrate level were enrolled for this study. The VDR SNPs rs7975232, rs2228570, rs731236 and rs1544410 were detected by Sanger sequencing, and the association between these SNPs and the presence of IH in the Chinese Bai population was analyzed. The prevalence of VDR SNPs rs7975232 allele A and rs2228570 genotype TT was significantly higher in patients than in controls (p < 0.0125, after Bonferroni correction). The haplotype TCGC was a protective factor in the Chinese Bai population who otherwise might suffer from IH, while the haplotype TTGA was a risk factor. VDR SNPs rs731236 and rs1544410 have a linkage disequilibrium value of 0.811. VDR SNPs rs7975232, rs2228570, and haplotypes TCGC, TTGA are associated with IH in a Chinese Bai population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urolithiasis is one of the most common diseases among urological disorders. Urolithiasis prevalence varies depending on geographical environment [1,2,3]. The disease is marked by a high recurrence rate, up to 50% in Europe and the United States [2] and 74% in Asian countries [3].
Calcium oxalate and calcium phosphate stones account for about 75% of total urinary calculi [4]. Some risk factors for the formation of calcium oxalate and calcium phosphate stones include hyperoxaluria, hyperphosphatemia, hypocitraturia, hypercalciuria, hyperuricemia, deficiency of inhibitors of stone formation, and low urine volume [5]. Importantly, urine citric acid is the strongest inhibitor of the formation of urine calcium oxalate and calcium phosphate crystals [6]. It is estimated that calcium stones and hypocitaturia are comorbid in about 19–63% of patients [7]. These individuals, excluding those with chronic diarrhea, high-protein diets, and incomplete distal renal tubular acidosis, are considered as having idiopathic hypocitraturia (IH).
Urolithiasis is a multifactorial disease caused by environmental, hormonal, and genetic factors [8,9,10]. The formation of urinary calcium stones is a disease of disordered calcium metabolism [11, 12]. Various genetic factors are associated with urinary calcium stone formation, including single nucleotide polymorphisms (SNPs) in calcium-sensing receptor (CaSR), progestin and adipoQ receptor 6 (PAQR6), calcitonin receptor (CTR), and vitamin D receptor (VDR) genes [13,14,15,16]. Several VDR SNPs are associated with the formation of urinary calcium stones and metabolic disease, including rs731236 and rs2228570, rs1544410, and rs7975232 [17]. SNPs rs1544410 and rs7975232 are located on the ninth intron of the 3′ terminal, rs731236 is located on the ninth exon of the 3′ terminal, and rs2228570 is located on the promoter of the 5′ terminal. Urinary calcium level is an important factor of calcium stone formation. However, it is reported that urinary citrate level is a major protective factor of urinary calcium stone formation [18]. In the renal tubule citrate forms complexes with calcium, thus increasing its solubility and reducing the concentration of free calcium in the urine [5, 7]. As a result, we focus on the study of urinary citrate level in our study.
Currently the association between VDR SNPs and IH remains unclear [19]. The association between VDR SNPs and IH varies among different races and ethnic populations [20]. The association between VDR SNPs and IH in minority populations has not been studied. Yunnan is a province with a high incidence of urinary stones in China [21]. The Chinese Bai population is the 15th largest Asian Chinese minority ethnic group that mainly resides in the Yunnan province. Its population is about 1,860,000, accounting for 0.15% of the total Chinese population. Urinary calcic incidence rates are almost 10% in this Chinese Bai population [22]. The prevalence of hypocitraturia and hypercalciuria in the Chinese Bai population is 8 and 7%, respectively [23]. Studying IH in Chinese Bai populations could help develop better IH diagnostic and therapeutic strategies. This study examined the association between VDR SNPs rs731236, rs2228570, rs1544410, and rs7975232 and IH in a Chinese Bai population.
Materials and methods
Subjects
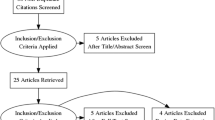
This study was performed on 200 patients with IH and 120 controls with normal urinary citrate level in a Chinese Bai population from October 2013 to December 2015 at the Urology Department of our hospital and Affiliated Hospital of Dali University (Fig. 1). IH was defined as a condition in which patients had a urinary citrate excretion below 320 mg/24 h. Subjects in the two groups excluded those with renal failure, urinary tract infection, renal tubular acidosis (RTA), hypomagnesemia, chronic gastrointestinal disease, gout, primary or secondary hyperparathyroidism metabolic diseases and history of recently taking thiazide diuretics, glucocorticoids, and estrogen. Controls were matched by gender. Age, weight and body mass index (BMI) between 200 cases (100M, 100F) with IH and 120 controls (60M, 60F) did not differ significantly between groups.
Ethics
The study was approved by the medical ethical committee of the Kunming Medical University of China. The parents or guardians of each patient authorized their inclusion in the study and their voluntary participation by informed consent. The identity of the selected subjects was kept strictly confidential.
Methods
All subjects were maintained on a standard constant diet with a daily composition of 1000 mg calcium, 100 mEq sodium, 900 mg phosphorus, 300 mg carbohydrates, 70 g proteins, and without any citric acid products (total 2200 calories) for 7 days. On day 8, a fasting urinary sample was collected 3 h after the first morning void, and its pH was determined using an electrochemical pH meter (Denver Instruments, USA). Additionally, 24-h urine samples were collected and analyzed for citrate urate, and electrolytes. Fasting venous blood samples were obtained to determine serum parathyroid hormone (PTH), urate, creatinine, and electrolyte levels. Urinary citrate levels were determined using citric acid enzymatic kits (Abcam Company, England) with an ultraviolet spectrophotometer (ABI Company, USA). Serum PTH levels were measured by immunochemiluminometric assay (Magic Lite, Ciba Corning Diagnostic, Medfield, MA). RTA was defined by the normal anion gap (normal AG) and a urine pH > 6.0, in addition to a urine K+ (UK+) concentration > 35 mEq/l, excluding hypokalaemia. AG = [Na+] − {[Cl−] + [HCO3−]}. Other blood and urinary parameters were obtained using a Hitachi 7180 automatic analyzer (ABI Company, USA). Genomic DNA of all subjects was extracted from fasting venous blood samples using the DNA extraction kit (BBI Company, Canada). Four VDR SNP site primers (Sangon Biotech Co. Ltd. Shanghai, China) were designed using Primer Premier 5.0 software (Stable 1). The polymerase chain reaction (PCR) for VDR SNPs rs731236, rs2228570, rs1544410, and rs7975232 was performed for 1 cycle of 3 min at 95 °C, then for 35 cycles of 30 s at 94 °C, 35 s at 55 °C, and 50 s at 72 °C, followed by a final 35 cycles for 8 min at 72 °C for extension. Purified PCR products were submitted for direct sequencing using a ready reaction mix (ABI Company, USA) and the same primers as used for PCR. Sequencing products were generated during 1 cycle for 1 min at 96 °C, followed by 25 cycles for 10 s at 96 °C, 5 s at 50 °C, and 4 min at 60 °C. The sequencing products were kept at 4 °C and purified using CleanSweep PCR Purification Reagent (Thermo Fisher Scientific, USA). The 3730xl Genetic Analyzer (ABI Company, USA) was used for capillary electrophoresis. The stones were analyzed using Fouriertransform infrared spectroscopy and high-resolution X-ray diffraction (ABI Company, USA). Stones with a calcium oxalate content > 70% and a calcium apatite content < 5% were considered calcium oxalate stones. Calcium phosphate-containing stones may be brushite, apatite, or less well-defined crystal forms.
Statistical analysis
Statistical analysis was performed with SPSS ver. 17.0 statistical software (SPSS, IL, USA). A Chi-square test was applied for categorical variables of subjects and a Student’s t test was used to compare continuous variables of subjects. All data are presented as mean ± standard deviation or percentages whenever appropriate. A p value < 0.0125 (after Bonferroni correction) was considered to be statistically significant. Bonferroni correction means that the cutoff divides n, and n represents the times of comparison. And n = 4 was applied in the correction due to four times of comparison between groups.
According to the Hardy–Weinberg equilibrium, statistical differences in genotype frequencies between the actual and the expected were assessed by using the Chi-square test. A p value > 0.05 indicated that the population was in genetic equilibrium and the data were from the same Mendel group.
The relative risk of IH was estimated by calculating the odds ratio (OR) and its 95% confidence interval (95% CI). The VDR gene haplotype blocks and linkage disequilibrium between four SNPs were estimated using Haploview 4.1 software (http://www.broad.mit.edu/mpg/haploview). A p value < 0.0125 (after Bonferroni correction) was considered to be statistically significant.
Results
Wave crest maps of 4 SNPs are shown in Fig. 2. Urine citrate excretion between 200 cases with IH and 120 controls in a Chinese Bai population was statistically significant (239.58 ± 80.41, 593.65 ± 86.33, p < 0.001), but there were no other significant disparities of urine and serum biochemistry between IH and control groups (Table 1). The Hardy–Weinberg equilibrium of four VDR SNP site genotypes (p = 0.138, p = 0.115, p = 0.625, p = 0.413) was estimated, indicating that the population was in genetic equilibrium, and the data were from the same Mendel group (STable 2). Genotype and allele frequency at rs2228570 was significantly different between cases and controls (p = 0.005, p = 0.005), while allele frequency at rs7975232 was different (p = 0.008). Genotype and allele frequency at rs731236 and rs1544410 was not significantly different in cases and controls (Table 2). Using the Haploview 4.1 software to estimate the VDR gene haplotype blocks and linkage disequilibrium between SNPs, there was a linkage disequilibrium between VDR SNPs rs731236 and rs1544410 (D′ = 0.811, r2 = 0.392, STable 3). Also, the VDR haplotypes of TCGC and TTGA were associated with IH in the Chinese Bai population. The haplotype TCGC (p = 0.001, OR = 0.559) was a protective factor for IH in Chinese Bai populations, whereas the haplotype TTGA (p = 0.006, OR = 1.929) was a ri sk factor (Table 3).
Discussion
Accumulating evidence shows that urolithiasis presents a genetic trait [24]. As a result, the genetic etiology of urinary stones needs to be further explored. IH also has a genetic predisposition [25]. Recent findings suggest that citrate and calcium metabolism are associated with genetic variations [26]. Nevertheless, associations between VDR SNPs and IH have not been reported [19, 20].
VDR is located on the long arm of chromosome 12 (12q13–q14), the cDNA of which is 65 kb, and composed of 10 exons and 9 introns [27]. Exon 1f–1c is located in the 5′ terminal promoter region, and the 3′ terminal is a non-translation region. SNPs rs1544410 and rs7975232 do not affect the VDR amino acid sequence, but may affect the transcriptional efficiency of VDR or mRNA stability [28]. The rs731236 SNP does not affect the VDR amino acid sequence. Rs2228570 is a functional SNP that disrupts the VDR initiation codon. As a result, the translation of the amino acid sequence starts from the second codon [29]. The VDR gene product is a nuclear receptor protein belonging to the steroid/thyroid hormone receptor superfamily, which 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] binds to and exerts downstream effects. Deregulation of VDR directly affect the efficacy of 1,25(OH)2D3 [30]. The main biological function of VDR includes regulating calcium and phosphorus metabolism, as well as cell proliferation and differentiation. VDR SNPs are closely related to obesity, type II diabetes, rickets, multiple sclerosis, breast cancer, prostate cancer, colorectal cancer, and various other metabolic diseases [31,32,33,34,35,36,37]. VDR SNPs can affect VDR expression [32]. Consequently, 1,25(OH)2D3 does not exert its normal biological activity in people with certain VDR SNPs, resulting in the occurrence of various disease [31].
At its core, IH is a metabolic disease [38]. VDR and 1,25(OH)2D3 play critical roles in regulating citric acid metabolism [39]. Na+-dicarboxylate cotransporter (NaDC1) regulates citric acid reabsorption in human renal proximal tubule epithelial cells (HK-2 cells) [40, 41]. Crucially, VDR SNPs affect NaDC1 expression and function, leading to dysfunctional citric acid reabsorption [7]. In addition, calcium affects citrate transport in a way that parallels in vivo studies that demonstrate the regulation of urinary citrate excretion with urinary calcium excretion. In fact, VDR may influence the urinary citrate excretion by regulating urinary calcium excretion indirectly [42].
The genotypes of four SNP sites were in accordance with the Hardy–Weinberg equilibrium, indicating that each of four SNP sites was from the same Mendel group. It was the genetic basis for discussing the statistical significance of these four SNP sites. The differences in genotype and allele frequency of VDR SNP sites rs7975232 and rs2228570 between cases and controls are statistically significant, indicating that the two sites of VDR gene were closely associated with IH in Chinese Bai populations. The genotype frequency of rs2228570 was 19.0% CC (FF), 51.0% CT (Ff), and 30.0% TT (ff), in accordance with studies conducted in China and other countries [43]. However in a study examining the prevalence of IH in Chinese populations, genotype frequency of rs2228570 was 19.0% CC (FF), 29.0% CT (Ff), and 52.0% TT (ff), and frequency of VDR SNP rs7975232 was not significantly different between IH and control patients [20]. The difference was attributed to the false-positive results of the PCR restriction fragment length polymorphisms (PCR-RFLP) technique, which increases the chances of mutations.
No significant difference was observed in the frequency of rs731236 and rs1544410 SNP genotypes and allele frequency between IH and control patients. Our findings contradict those from both Chinese and international studies in which the aforementioned VDR SNP sites were found to be associated with IH [14, 15]. This disparity might be due to differences in race, experimental methods, and sample size of the study subjects. The combined effect of multiple genes on IH might be missing while analyzing the effect of single gene mutation. The linkage disequilibrium between VDR SNPs rs731236 and rs1544410 was week, because the significance of D′ decreased especially when the allele frequencies of subjects were low (2.8 and 4.8%, STable 2).
The VDR rs7975232 allele A and rs2228570 TT genotypes are candidate bio-markers of IH in the Chinese Bai population. A Chinese Bai individual with VDR SNP rs7975232 or rs2228570 is more susceptible to formation of calcium stones due to IH. This finding provides an insight into the prevention of calcium stone formation and possibly offers a new direction for the experimental basis for gene therapy. Even so, the mechanisms associating SNPs rs7975232 and rs2228570 with IH are still unclear. Further research is needed to determine the molecular basis of IH and the pathogenesis of calcium stones.
There were several limitations within this study that need further exploration in the future. The first limitation is that the sample size was small. Future effort should replicate the present study’s findings in larger and independent Chinese Bai populations. In addition, future research will investigate the predictive efficacy of more SNP sites for IH in a larger sample size.
Conclusions
In conclusion, this study was the first to investigate the association between VDR SNPs and IH in a Chinese minority ethnic group. We found VDR rs7975232 allele A and rs2228570 TT genotypes were associated with IH in the Chinese Bai population. The results provide the experimental basis for studying candidate genes for IH in the Chinese Bai population.
References
Theka T, Rodgers AL, Webber D et al (2014) Variability in kidney stone incidence between black and white South Africans: AGT Pro11Leu polymorphism is not a factor. J Endourol 28(5):577–581
Turney BW, Appleby PN, Reynard JM et al (2014) Diet and risk of kidney stones in the Oxford cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur J Epidemiol 29(5):363–369
Bae SR, Seong JM, Kim LY et al (2014) The epidemiology of reno-ureteral stone disease in Koreans: a nationwide population-based study. Urolithiasis 42(2):109–114
Jiang D, Geng H (2017) Primary hyperoxaluria. N Engl J Med 376(15):e33
Arrabal-Polo MA, Arias-Santiago S, Giron-Prieto MS et al (2012) Hypercalciuria, hyperoxaluria, and hypocitraturia screening from random urine samples in patients with calcium lithiasis. Urol Res 40(5):511–515
Rez P (2017) What does the crystallography of stones tell us about their formation? Urolithiasis 45(1):11–16
Rendina D, De Filippo G, Gianfrancesco F et al (2017) Evidence for epistatic interaction between VDR and SLC13A2 genes in the pathogenesis of hypocitraturia in recurrent calcium oxalate stone formers. J Nephrol 30(3):411–418
Haymann JP (2015) Metabolic disorders: stones as first clinical manifestation of significant diseases. World J Urol 33(2):187–192
Letendre J, Cloutier J, Villa L et al (2015) Metabolic evaluation of urinary lithiasis: what urologists should know and do. World J Urol 33(2):171–178
Kan WC, Chou YH, Chiu SJ et al (2014) Study of the association between ITPKC genetic polymorphisms and calcium nephrolithiasis. Biomed Res Int 2014:397826
Skolarikos A, Straub M, Knoll T et al (2015) Metabolic evaluation and recurrence prevention for urinary stone patients: EAU guidelines. Eur Urol 67(4):750–763
Arrabal-Martin M, Poyatos-Andujar A, Cano-Garcia Mdel C et al (2015) The importance of calciuria as lithogenic factors in patients with osteopenia/osteoporosis. Int Urol Nephrol 47(3):445–449
Rungroj N, Nettuwakul C, Sudtachat N et al (2014) A whole genome SNP genotyping by DNA microarray and candidate gene association study for kidney stone disease. BMC Med Genet 15:50
Vezzoli G, Terranegra A, Rainone F et al (2011) Calcium-sensing receptor and calcium kidney stones. J Transl Med 9:201
Bid HK, Chaudhary H, Mittal RD (2005) Association of vitamin-D and calcitonin receptor gene polymorphism in paediatric nephrolithiasis. Pediatr Nephrol 20(6):773–776
Basiri A, Shakhssalim N, Houshmand M et al (2012) Coding region analysis of vitamin D receptor gene and its association with active calcium stone disease. Urol Res 40(1):35–40
Ferreira LG, Pereira AC, Heilberg IP (2010) Vitamin D receptor and calcium-sensing receptor gene polymorphisms in hypercalciuric stone-forming patients. Nephron Clin Pract 114(2):c135–c144
Zuckerman JM, Assimos DG (2009) Hypocitraturia: pathophysiology and medical management. Rev Urol 11(3):134–144
Mossetti G, Vuotto P, Rendina D et al (2003) Association between vitamin D receptor gene polymorphisms and tubular citrate handling in calcium nephrolithiasis. J Intern Med 253(2):194–200
Zhu C, Ye Z, Chen Z et al (2010) Association between vitamin D receptor gene polymorphisms and idiopathic hypocitraturia in the Chinese population. Urol Int 85(1):100–105
Alatab S, Pourmand G, El Howairis Mel F et al (2016) National profiles of urinary calculi: a comparison between developing and developed worlds. Iran J Kidney Dis 10(2):51–61
Zeng G, Mai Z, Xia S et al (2017) Prevalence of kidney stones in China: an ultrasonography based cross-sectional study. BJU Int 120(1):109–116
Wu W, Yang D, Tiselius HG et al (2014) The characteristics of the stone and urine composition in Chinese stone formers: primary report of a single-center results. Urology 83(4):732–737
Lieske JC, Turner ST, Edeh SN et al (2014) Heritability of urinary traits that contribute to nephrolithiasis. Clin J Am Soc Nephrol 9(5):943–950
Ferraro PM, Robertson WG, Johri N et al (2015) A London experience 1995–2012: demographic, dietary and biochemical characteristics of a large adult cohort of patients with renal stone disease. QJM J Med 108(7):561–568
Rull MO, Ochoa-Hortal MA, Cano-Garcia MC, Martin MA et al (2015) Calcium and phosphorus metabolism and lithogenic factors in patients with osteoporotic fracture. Actas Urol Esp 39(5):279–282
Uitterlinden AG, Fang Y, van Meurs JBJ et al (2004) Genetics and biology of vitamin D receptor polymorphisms. Gene 338:143–156
Mostowska A, Lianeri M, Wudarski M et al (2013) Vitamin D receptor gene BsmI, FokI, ApaI and TaqI polymorphisms and the risk of systemic lupus erythematosus. Mol Biol Rep 40(2):803–810
Zhan Y, Liu M, You Y et al (2015) Genetic variations in the vitamin-D receptor (VDR) gene in preeclampsia patients in the Chinese Han population. Hypertens Res 38(7):513–517
Carvalho AY, Bishop KS, Han DY et al (2013) The role of Vitamin D level and related single nucleotide polymorphisms in Crohn’s disease. Nutrients 5(10):3898–3909
Ferrarezi DA, Bellili-Munoz N, Nicolau C et al (2012) Allelic variations in the vitamin D receptor gene, insulin secretion and parents’ heights are independently associated with height in obese children and adolescents. Metabolism 61(10):1413–1421
Al-Daghri NM, Al-Attas OS, Alkharfy KM et al (2014) Association of VDR-gene variants with factors related to the metabolic syndrome, type 2 diabetes and vitamin D deficiency. Gene 542(2):129–133
Mao S, Huang S (2014) Vitamin D receptor gene polymorphisms and the risk of rickets among Asians: a meta-analysis. Arch Dis Child 99(3):232–238
Bermúdez-Morales VH, Fierros G, Lopez RL et al (2017) Vitamin D receptor gene polymorphisms are associated with multiple sclerosis in Mexican adults. J Neuroimmunol 306:20–24
Shahbazi S, Alavi S, Majidzadeh AK et al (2013) BsmI but not FokI polymorphism of VDR gene is contributed in breast cancer. Med Oncol 30(1):393
Liu S, Cai H, Cheng W et al (2017) Association of VDR polymorphisms (Taq I and Bsm I) with prostate cancer: a new meta-analysis. J Int Med Res 45(1):3–10
Bai YH, Lu H, Hong D et al (2012) Vitamin D receptor gene polymorphisms and colorectal cancer risk: a systematic meta-analysis. World J Gastroenterol 18(4):1672–1679
Amaro CR, Goldberg J, Damasio PC et al (2015) An update on metabolic assessment in patients with urinary lithiasis. World J Urol 33(1):125–129
Domrongkitchaiporn S, Ongphiphadhanakul B, Stitchantrakul W et al (2000) Risk of calcium oxalate nephrolithiasis after calcium or combined calcium and calcitriol supplementation in postmenopausal women. Osteoporos Int 11(6):486–492
Pajor AM (2014) Sodium-coupled dicarboxylate and citrate transporters from the SLC13 family. Pflugers Arch 466(1):119–130
Ohana E, Shcheynikov N, Moe OW et al (2013) SLC26A6 and NaDC-1 transporters interact to regulate oxalate and citrate homeostasis. J Am Soc Nephrol 24(10):1617–1626
Hering-Smith KS, Schiro FR, Pajor AM et al (2011) Calcium sensitivity of dicarboxylate transport in cultured proximal tubule cells. Am J Physiol Renal Physiol 300(2):F425–F432
Medina-Escobedo M, Gonzalez-Herrera L, Villanueva-Jorge S et al (2014) Metabolic abnormalities and polymorphisms of the vitamin D receptor (VDR) and ZNF365 genes in children with urolithiasis. Urolithiasis 42(5):395–400
Funding
This study was funded by National Natural Science Foundation of China (Grant number 81460141), by Science and Technology Department of Yunnan Province Uniting Kunming Medical University Specialized Foundation (Grant number 2013FB134, 2017FE468(-029) and 2017FE467(-042)), by Doctoral Scientific Research Fund of the First Affiliated Hospital of Kunming Medical University (Grant number 2015BS026 and 2017BS016) and by the scientific research funding of Yunnan Provincial Department of Education (Grant number 2018JS208).
Author information
Authors and Affiliations
Contributions
KL and YL contributed equally to the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consents
Informed consent forms were signed by all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, K., Luo, Y., Mo, Y. et al. Association between vitamin D receptor gene polymorphisms and idiopathic hypocitraturia in a Chinese Bai population. Urolithiasis 47, 235–242 (2019). https://doi.org/10.1007/s00240-018-1069-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-018-1069-3