Abstract
The aim of this study was to compare the clinical characteristics of uric acid stones and their potential risk for chronic kidney disease (CKD). A total of 401 patients (196 with uric acid stone and 205 without) were enrolled from our database of patients with urolithiasis. We analyzed the clinical demographic features, stone location, urine chemistries, and renal function. There was a significant difference (p < 0.001) between the two groups in terms of age, with the higher mean age in the uric acid group. Patients with uric acid stones had much lower pH of urine (p < 0.001) and higher serum uric acid level (p = 0.002). Notably, those with uric acid stones had worse eGFR than those with non-uric acid stones. Multivariate analysis confirmed that age over 60 years (ORs = 9.19; 95% CI 3.5–24.3), female sex (ORs = 4.01; 95% CI 1.8-9.0), hyperuricemia (ORs = 8.47; 95% CI 1.6–43.5), and uric acid stone (OR = 2.86; 95% CI 1.2–6.7) were the independent predictors of poor prognoses in CKD. Therefore, an association exists between uric acid stones and higher prevalence of CKD. Patients with uric acid stones may need close monitoring of renal function during follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urolithiasis is not an uncommon disease. It affects 12% of men and 6% of women in their lifetime and is considered a vital public health issue with a substantial burden on human health and considerable national economic consequences [1, 2]. The majority of these patients develop calcium-containing stones, with approximately half of them developing a combination of calcium oxalate and calcium phosphate stones. Among non-calcium-containing stones, uric acid stones comprise 8–10% of all kidney stones in the USA [3]. The formation of uric acid stones has been identified in patients with high urine uric acid excretion and acidic urine. Uric acid stones may contain pure uric acid, sodium urate, ammonium urate, or other purine. Since uric acid stones are radiolucent, the diagnosis may be missed on plain radiography. Currently, non-contrast-enhanced computerized tomography (CT) scan is the preferred radiologic test to establish the presence of such a stone. Previous reports have showed that the two major determinants of uric acid stone formation were climate and diet [4]. Higher fluid losses in hot and dry weather may decrease urinary volume and pH. The incidence of uric acid stone may increase as a result of global warming. Protein-rich food also causes a fall in urinary pH and increased uric acid excretion. Moreover, uric acid stone formation is frequently associated with obesity, metabolic syndrome (MetS), and type 2 diabetes mellitus, which were postulated to reduce ammoniagenesis and result in low urine pH [5, 6]. A recent review article [7] concluded that urolithiasis may cause chronic kidney disease (CKD) and suggested that the assessment of renal function is mandatory in renal stone disease. Moreover, even after the resolution of the stone, patients may continue to suffer from higher cystatin C levels and proteinuria that may increase the long-term risk of CKD [8]. Till now, our understanding of the pathophysiologic mechanisms accounting for the decrease in renal outcomes and urolithiasis remains limited. Previous investigations have shown a significant risk of urolithiasis and CKD among patients with any type of renal stone independent of other risk factors; however, less amount of data focuses on the risk attributable to the specific stone types [9, 10]. It is difficult to analyze the composition of stones or urine in population-based studies. Previous studies have demonstrated that patients with uric acid, calcium oxalate, apatite, and struvite stones had lower creatinine levels than the normal population [11]. We previously showed that the patients with uric acid stones had the lowest estimated glomerular filtration rate among others [10]. Despite evidence of CKD in patients with uric acid stones, less data exist regarding the clinical implications. Therefore, we aim to evaluate the change in renal function and the risk of developing CKD in patients with uric acid stones compared with those without uric acid stones.
Materials and methods
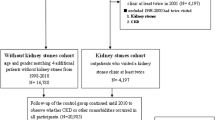
The present study was approved by the Institutional Review Board of the Kaohsiung Medical University Hospital. Our hospital database contained records of patients with urolithiasis from March 2013 to May 2016. Patients were separated into two groups: the uric acid stone group and the non-uric acid stone group. All patients had radiological evidence of urinary stones. Clinical data were retrospectively collected. Renal function was evaluated with estimated glomerular filtration rage (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine-based formula [12]. Pre-operation and post-operation follow-up renal functions were recorded. CKD was defined as an eGFR less than 60 ml/min/1.73 m2. We excluded patients with solitary kidneys, congenital renal anomalies, severe urinary obstruction, and autosomal dominant polycystic kidney disease. Stone compositions were analyzed by Fourier transform infrared spectroscopy. Images were analyzed and reported separately by two different doctors.
Statistical analysis
All values are expressed as a mean ± standard deviation (SD), rate, or median (range). Differences between categorical parameters were assessed using a χ2 or Fisher’s exact test, whichever was appropriate. Fisher’s exact test was used when the amount of data was small. Continuous parameters were assessed using a t test or Mann–Whitney–Wilcoxon test. Risk factors for CKD were derived using univariate testing. Only those variables for which p < 0.05 were considered for the model. Once we identified these potential risk factors, a multivariate stepwise logistic regression analysis was used to identify independent prognostic factors. Clinical data were analyzed separately in the univariate analysis. Significant factors were used for the multivariate analysis. The independent prognostic factors in the final model are presented with odds ratios (ORs). The odds ratio predicts the magnitude of the influence of the risk factor on developing CKD when present, compared with its absence. Statistical significance was set at p < 0.05. SPSS 20.0J (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
Results
We included 196 patients with uric acid stones (uric acid group) and 205 with non-uric acid stones (non-uric acid group). The average age of patients was 57.2 ± 14.0 years and 40% of the patients were older than 60 years. There was a significant difference (p < 0.001) between the two groups in terms of age, with an older mean age in the uric acid group. The majority of the patients (77.1%) were male. Patients with uric acid stones had much lower pH of urine (p < 0.001) and higher serum uric acid level (p = 0.002). Hypertension was present in 35.4% of the patients; 15.2% of the patients had diabetes, 6.7% had a history of dyslipidemia, 27.9% had a history of gout, and 21.2% of the patients had hyperuricemia. The average pre-operation and post-operation eGFR were 81.4 ± 19.8 and 85.5 ± 20.9 ml/min/1.73 m2, respectively. The average follow-up time was 6.6 ± 4.3 months. Notably, those with uric acid stones had worse eGFR than those with non-uric acid stones (p < 0.001; Table 1, 2). We further compared the basic characteristics across post-operation follow-up eGFR level. Univariate analysis showed that age over 60 years, female sex, diabetes, hyperuricemia, and uric acid stone were the risk factors for CKD. Multivariate analysis confirmed that age over 60 years (ORs = 9.19; 95% CI 3.5–24.3), female sex (ORs = 4.01; 95% CI 1.8–9.0), hyperuricemia (ORs = 8.47; 95% CI 1.6–43.5), and uric acid stone (OR = 2.86; 95% CI 1.2–6.7) were the independent predictors of poor prognoses in CKD (Table 3). Within non-uric acid stones group, 135 (65.9%) patients had calcium oxalate mixed with calcium phosphate stones, while 44 (21.5%) had calcium oxalate stones, 21 (10.2%) had calcium phosphate stones, 3 (1.5%) had brushite stones, and 2 (1.0%) had non-calcium struvite stones. The eGFR values were 90.2 ± 21.2, 87.1 ± 25.3, 88.6 ± 31.0, 76.0 ± 25.6, and 72.0 ± 32.6 ml/min/1.73 m2 in patients with calcium oxalate mixed with calcium phosphate stones, calcium oxalate stones, calcium phosphate stones, brushite stones, and struvite stones, respectively (Supplementary table).
Discussion
A recent population-based, retrospective study revealed that urolithiasis itself significantly increases the risk of hypertension and chronic kidney disease, with a hazard ratio of 1.42 for hypertension and 1.82 for chronic kidney disease [13]. Due to the heterogeneous nature of renal stones and their formation, the link between urolithiasis and CKD is likely multifactorial [14]. The reasons for impaired kidney function in patients with renal stones include obstruction, infection, and stone burden [15]. Ureteral obstruction and subsequent hydronephrosis may lead to vasoconstriction and increased intratubular pressure [16], which causes decline in GFR and renal blood flow. Furthermore, the obstruction may be associated with matrix deposition related to interstitial volume expansion, fibroblast proliferation, and monocyte infiltration [17, 18].
The risk of CKD also varies by the composition of the renal stone [9,10,11]. It is difficult to analyze the effects of different stones on renal function using population-based studies, which often lack data of granular details, stone analyses, or urine chemistry [9]. Previous reports have demonstrated that patients with uric acid stones had a much lower creatinine clearance than those with other types of stones or healthy patients [10, 11, 19]. Kadlec et al. showed that eGFR was significantly divergent from the stone composition, with uric acid stones associated with a lower eGFR and calcium phosphate stones associated with a greater Egfr [19]. In our results, uric acid stones were predominantly seen in patients with eGFR < 60 ml/min/1.73 m2 (the prevalence of eGFR < 60 ml/min/1.73 m2 in the uric acid stone vs. non-uric acid stone group: 48.2 vs. 25.2%; p < 0.001). We also demonstrated that the prevalence of uric acid stones was higher in patients with CKD (eGFR < 60 ml/min/1.73 m2; uric acid stone vs. non-uric acid stone: 14.3 vs. 4.9%). Previous studies have reported that uric acid stone formation is frequently associated with obesity, metabolic syndrome (MetS), and type 2 diabetes mellitus (T2D) [5, 6]. MetS is becoming more common due to the increasing prevalence of obesity across the globe and the burden of MetS is expected to rise rapidly. It is biologically plausible to understand the relationship between MetS and CKD [20]. Insulin resistance was often observed among patients with visceral obesity, who are also at risk for diminished filtration [21]. Therefore, visceral obesity, not overweight or obesity by itself, may be a more sensitive predictor of kidney disease than BMI [22]. The interaction between adipocytes, a significant origin of inflammatory and immunomodulatory factors, and macrophages may contribute to insulin resistance and many of the features that characterize MetS. The development of eGFR < 60 (ml/min/1.73 m2) and microalbuminuria or overt proteinuria was proved to be associated with MetS [23, 24]. Another longitudinal study with focus on the association between Mets and CKD in a non-Asiatic cohort of patients with T2D showed 41% incidence and 33% increased risk for the development of low eGFR and albuminuria, respectively, among patients with Mets [25]. Obviously, patients with more comorbid conditions may suffer from worse renal dysfunction, which suggests that a proportion of the loss of renal function may be contributed to a comorbidity. Though several previous studies have showed a relationship between urolithiasis and CKD, it is still being debated if the stone is the primary cause of CKD or just a contributing risk factor. Kidney stones are not directly thought to be the primary cause of decreased renal function, but they may be important contributing factors, and further studies are still needed [9]. One possible hypothesis concerning the uric acid stone could be an explanation for our results. For patients with uric acid stone, the incidence of hyperuricemia is much higher than those with non-uric acid stone (ORs = 2.02; 95% CI 1.3–3.0). Chou et al. reported that patients with progressively elevated level of serum uric acid had significantly higher risk of developing CKD compared to subjects with persistently low serum uric acid level after adjustment for potential confounders [26]. Uric acid may play a much devastating role in the deterioration of kidney function compared to other stone components. Finally, other possible reasons accounting for the excess risk of loss of kidney function are the surgical or percutaneous treatments used (rather than the stones themselves). Sairam et al. revealed that those with more advanced CKD tended to have experienced a greater proportion of previous operations, particularly previous percutaneous nephrolithotomy (PCNL) [27]. Clearly, a complex interaction between stone disease, interventions, chronic infections, and poor drainage impacts renal function [27].
There are several limitations to our study. First, this was a retrospective analysis. As with most retrospective studies, data may be subject to incomplete, missing, or inaccurate reporting of events. Second, a relatively lower proportion of patients with renal stones who could be treated conservatively and different stone burdens may have diverse effects on subsequent CKD. The database did not record stone size and the numbers of episodes; therefore, we did not analyze their impact on CKD. Third, other predisposing factors, such as smoking, family history, and dietary habits, were not adjusted in our study. Fourth, selection bias may occur during identification of the study population. Fifth, the serum creatinine was not determined by the standard isotope dilution-liquid chromatography–mass spectrometry (IDLCMS) method, interference may occur in plasma creatinine assays. Despite these limitations, this study is based on one of the largest databases of uric acid stones in the world.
Conclusion
An association exists between uric acid stones and higher prevalence of CKD. Patients with uric acid stones were older, predominantly male, and had higher serum uric acid level and had lower urine pH. The policy of monitoring renal function during follow-up of uric acid stone formers is a general policy in stone formers as suggested by the Consensus statement [28].
References
Curhan GC (2007) Epidemiology of stone disease. Urol Clin N Am 34:287e93
Sakhaee K (2008) Nephrolithiasis as a systemic disorder. Curr Opin Nephrol Hypertens 17:304–309
Sakhaee K (2014) Epidemiology and clinical pathophysiology of uric acid kidney stones. J Nephrol 27:241–245
Trinchieri A, Montanari E (2017) Prevalence of renal uric acid stones in the adult. Urolithiasis. https://doi.org/10.1007/s00240-017-0962-5
Daudon M, Traxer O, Conort P, Lacour B, Jungers P (2006) Type 2 diabetes increases the risk for uric acid stones. J Am Soc Nephrol 17:2026–2033
Maalouf NM, Cameron MA, Moe OW, Adams-Huet B, Sakhaee K (2007) Low urine pH: a novel feature of the metabolic syndrome. Clin J Am Soc Nephrol 2:883–888
Gambaro G, Croppi E, Bushinsky D, Jaeger P, Cupisti A, Ticinesi A, Mazzaferro S, D’Addessi A, Ferraro PM (2017) The risk of chronic kidney disease associated with urolithiasis and its urological treatments: a review. J Urol 198:268–273
Haley WE, Enders FT, Vaughan LE, Mehta RA, Thoman ME, Vrtiska TJ, Krambeck AE, Lieske JC, Rule AD (2016) Kidney function after the first kidney stone event. Mayo Clin Proc 91:1744–1752
Rule AD, Krambeck AE, Lieske JC (2011) Chronic kidney disease in kidney stone formers. Clin J Am Soc Nephrol 6:2069–2075
Chou YH, Li CC, Hsu H, Chang WC, Liu CC, Li WM, Ke HL, Lee MH, Liu ME, Pan SC, Wang HS (2011) Renal function in patients with urinary stones of varying compositions. Kaohsiung J Med Sci 27:264–267
Worcester EM1, Parks JH, Evan AP (2006) Renal function in patients with nephrolithiasis. J Urol 176:600–603
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612
Denburg MR, Jemielita TO, Tasian GE, Haynes K, Mucksavage P, Shults J, Copelovitch L (2015) Assessing the risk of incident hypertension and chronic kidney disease after exposure to shockwave lithotripsy and ureteroscopy. Kidney Int 89:185–192
Coe FL, Evan AP, Worcester EM, Lingeman JE (2010) Three pathways for human kidney stone formation. Urol Res 38:147–160
Worcester E, Parks JH, Josephson MA, Thisted RA, Coe FL (2003) Causes and consequences of kidney loss in patients with nephrolithiasis. Kidney Int 64:2204–2213
Gaudio KM, Siegel NJ, Hayslett JP, Kashgarian M (1980) Renal perfusion and intratubular pressure during ureteral occlusion in the rat. Am J Physiol 238:F205–F209
Klahr S (2001) Urinary tract obstruction. Semin Nephrol 21:133–145
Klahr S, Morrissey J (2003) Obstructive nephropathy and renal fibrosis: the role of bone morphogenic protein-7 and hepatocyte growth factor. Kidney Int Suppl 87:105–112
Kadlec AO, Greco KA, Fridirici ZC, Gerber D, Turk TM (2011) Effect of renal function on urinary mineral excretion and stone composition. Urology 78:744–747
Thomas G, Sehgal AR, Kashyap SR, Srinivas TR, Kirwan JP, Navaneethan SD (2011) Metabolic syndrome and kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol 6:2364–2373
Viazzi F, Garneri D, Leoncini G, Gonnella A, Muiesan ML, Ambrosioni E et al (2014) Serum uric acid and its relationship with metabolic syndrome and cardiovascular risk profile in patients with hypertension: insights from the I-DEMAND study. Nutr Metab Cardiovasc Dis 24:921–927
Pinto-Sietsma SJ, Navis G, Janssen WM, de Zeeuw D, Gans RO, de Jong PE (2003) A central body fat distribution is related to renal function impairment, even in lean subjects. Am J Kidney Dis 41:733–741
Bagby SP (2004) Obesity-initiated metabolic syndrome and the kidney: A recipe for chronic kidney disease? J Am Soc Nephrol 15:2775–2791
Schelling JR, Sedor JR (2004) The metabolic syndrome as a risk factor for chronic kidney disease: more than a fat chance? J Am Soc Nephrol 15:2773–2774
Viazzi F, Piscitelli P, Giorda C, Ceriello A, Genovese S, Russo G, Guida P, Fioretto P, De Cosmo S, Pontremoli R, AMD-Annals Study Group (2017) Metabolic syndrome, serum uric acid and renal risk in patients with T2D. PLoS One 12:e0176058
Chou YC, Kuan JC, Yang T, Chou WY, Hsieh PC, Bai CH, You SL, Chen CH, Wei CY, Sun CA (2015) Elevated uric acid level as a significant predictor of chronic kidney disease: a cohort study with repeated measurements. J Nephrol 28:457–462
Sairam K, Scoffone CM, Alken P, Turna B, Sodha HS, Rioja J, Wolf JS Jr, de la Rosette JJ, CROES PCNL Study Group (2012) Percutaneous nephrolithotomy and chronic kidney disease: results from the CROES PCNL Global Study. J Urol 188:1195–1200
Gambaro G, Croppi E, Coe F, Lingeman J, Moe O, Worcester E, Buchholz N, Bushinsky D, Curhan GC, Ferraro PM, Fuster D, Goldfarb DS, Heilberg IP, Hess B, Lieske J, Marangella M, Milliner D, Preminger GM, Reis Santos JM, Sakhaee K, Sarica K, Siener R, Strazzullo P, Williams JC; Consensus Conference Group (2016) Metabolic diagnosis and medical prevention of calcium nephrolithiasis and its systemic manifestations: a consensus statement. J Nephrol 29: 715–734
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare no conflict of interest.
Ethical approval
All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, formal consent is not required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, CC., Chien, TM., Wu, WJ. et al. Uric acid stones increase the risk of chronic kidney disease. Urolithiasis 46, 543–547 (2018). https://doi.org/10.1007/s00240-018-1050-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-018-1050-1