Abstract
The calcium sensing receptor (CaSR) in the distal nephron decreases the propensity for calcium stones. Here we investigate if the apical CaSR in the proximal tubule also prevents stone formation acting via regulation of apical dicarboxylate and citrate transport. Urinary citrate, partially reabsorbed as a dicarboxylate in the proximal tubule lumen, inhibits stone formation by complexing calcium. We previously demonstrated a novel apical calcium-sensitive dicarboxylate transport system in OK proximal tubule cells. This calcium-sensitive process has the potential to modulate the amount of citrate available to complex increased urinary calcium. Using isotope labeled succinate uptake in OK cells along with various pharmacologic tools we examined whether the CaSR alters apical dicarboxylate transport and through which signal transduction pathways this occurs. Our results indicate that in the proximal tubule CaSR adjusts apical dicarboxylate transport, and does so via a CaSR → Gq → PKC signaling pathway. Thus, the CaSR may decrease the propensity for stone formation via actions in both proximal and distal nephron segments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The calcium sensing receptor (CaSR) may play a role in decreasing the propensity for calcium stones. The CaSR is present in many tissues and has many roles. In the kidney, it is located throughout the nephron and has a multitude of functions varying with segment, polarity and cell type [1,2,3,4]. Importantly, some of these functions in distal nephron segments are thought to prevent stone formation [5, 6]. Of note in the proximal tubule the CaSR is located on the apical membrane [7] and is found in the proximal tubule opossum kidney (OK) cell model [3, 8]. CaSR has been found to modulate both fluid reabsorption and H+ secretion in the proximal tubule and gastric gland, perhaps via increases in intracellular calcium levels and ERK activity [9, 10]. The present study in OK cells explores a mechanism whereby CaSR in the proximal tubule may prevent stone formation acting via apical dicarboxylate (and citrate) transport.
Urinary citrate is the most important endogenous inhibitor of calcium nephrolithiasis. Citrate reabsorption in the upstream nephron determines urinary citrate which is available to complex calcium. The bulk of citrate transport is likely via the proximal tubule apical sodium dicarboxylate cotransporter (NaDC1) which reabsorbs dicarboxylic acids/Krebs cycle intermediates from the glomerular filtrate [11,12,13,14]. In previous studies, we used the established OK cell model to study citrate and succinate transport and found inhibition by normal (1.2 mM) levels of extracellular Ca2+ ([Ca2+]o) [15,16,17,18]. (Succinate is used as a test dicarboxylate since its ionic state is not pH sensitive in the normal pH range.) In our prior studies a novel apical calcium-sensitive dicarboxylate transport process was found in OK cells; herein we examine the mechanism of calcium-sensitivity.
Although the initial purpose of the present studies was to determine whether CaSR regulates dicarboxylate/citrate transport, understanding the possible signal transduction mechanisms of this may be important in subsequent efforts to modulate citrate transport and hence stone formation via CaSR. The CaSR is known to couple variously to Gq, Gi and Gs signaling proteins [19, 20] all of which are found in the proximal tubule [21, 22]. Our aim is to establish if calcium-sensitivity of dicarboxylate transport is due to CaSR activation and what roles Gq, Gi and Gs play. Signaling via Gq activates phospholipase C (PLC) resulting in the release of [Ca2+]i stores and activation of PKC [23]. Gi and Gs inhibit and activate adenylate cyclase (AC) respectively [19, 24].
Methods
As previously described, OK cells were maintained in MEM containing 26 mM HCO3− supplemented with 10% FCS (Invitrogen), 25 mM HEPES, 11 mM l-glutamine and 100 IU/ml penicillin in a humidified atmosphere of 5% CO2 at 37 °C [15,16,17]. Cell monolayers were grown on 24-well plates (Corning-Costar), with media changes every 2 days. After reaching confluence, cell monolayers were changed to serum-free media for a minimum of 24 h before experiments were performed.
Dicarboxylate transport was measured by uptake of radiolabeled [14C]-succinate into cell monolayers. Just prior to uptake, cells were rinsed free of media and equilibrated for 2 min at 37 °C in buffer containing either normal (1.2 mM) or low (< 60 µM) [Ca2+]o. The remaining components of the buffer were as follows (in mM): 109 NaCl, 3 KCl, 2 KH2PO4, 1 MgSO4, 5 alanine, 8.3 glucose, 1 sodium acetate, 25 HEPES; osmolality of 290 mosmol/kg H2O and pH 7.4.
Uptake was performed at 37 °C and started by adding 0.4 ml of uptake buffer with 0.626 mCi/ml [1,4-14C]-succinate (Moravek Biochemicals) to individual wells. Uptake buffer also contained [3H] mannitol (Perkin Elmer) to determine the residual extracellular volume. After 3 min, buffer was removed and wells were rinsed 3X with ice cold 0.1 M MgCl2; monolayers were lysed with 1 ml of 0.1 N NaOH and transferred to vials for liquid scintillation counting [15,16,17,18].
Uptake was calculated from measured 14C radioactivity per well; appropriate windows and crossover calculations were used to distinguish between 3H and 14C. Uptake was further factored for residual extracellular volume, not removed by triplicate rinsing, calculated from the residual [3H] mannitol. Because of variability in absolute transport rates, each experiment was performed in a paired fashion with both normal and low [Ca2+]o solutions, with a minimum of four separate passages of OK cells for each experiment. Data are expressed as means ± SE. All statistical analysis was performed using one-way ANOVA. Statistical significance was defined as p < 0.05.
Unless otherwise stated all pharmacologicals were purchased through Sigma Aldrich (St. Louis, MO). Table 1 lists pharmacological agents, vehicle, dose, duration and references.
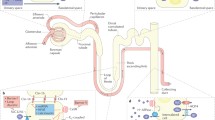
Proximal tubule apical CaSR signaling cascade. The apical proximal tubule CaSR potentially signals via the G-proteins: Gq, Gi or Gs in OK cell monolayers. Figure illustrates an overview of CaSR signaling, the G-protein signaling pathways studied and pharmacologicals used to determine the role of extracellular Ca2+ in apical dicarboxylate transport regulation in OK proximal tubule cell monolayers. Time of treatment, concentration and vehicle controls are outlined in Table 1. Green arrows indicate stimulation, while red bars indicate inhibition by the pharmacological agents used in this study. Black arrows, up arrows, represent stimulation or increases and bars, perpendicular symbols, inhibition of some of the major components of G-protein coupled signaling cascade
A series of colorimetric ELISA cAMP EIA kit competitive binding assays (Cayman Chemical, 581001) were performed on 96 well plates, with standard curves in duplicate and samples in triplicate. As above OK cells were grown on 24 well plates. Buffers containing either normal or low [Ca2+]o were incubated at 37 °C. MgSO4 was not included in low [Ca2+]o solutions as Mg2+ is a CaSR activator and in low [Ca2+]o buffer Mg2+ may be more likely to interfere. All other buffers and pharmacologicals were the same used in uptake experiments. Cells were treated with appropriate buffers and pharmacologicals, buffers were aspirated, 54 µl of 0.1 M HCl was added to each 1.9 cm2 well, and incubated for 20 min at RT. Cells were scraped off, dissociated; lysates were centrifuged at 1000×g for 10 min followed by collection of supernatants. Standard curves and samples were prepared as per kit directions. Samples were diluted 2X and read at 412 nM.
Data are expressed as means ± SE. Each mean is derived from four individual experiments studied on 4 separate days. One-way ANOVA was used for statistical comparison in cAMP assays. Statistical significance was defined as p < 0.05.
Results
Our previous studies determined that normal levels (1.2 mM) of apical [Ca2+]o inhibit apical dicarboxylate transport in OK cell monolayers [15–17]. CaSR is activated only when [Ca2+]o is in the range of 0.5–2 mM [Ca2+]o [8]. Since the CaSR is at the apical membrane of the proximal tubule, CaSR activation may be the basis of the apical calcium-sensitive dicarboxylate transport we previously reported [15,16,18]. If CaSR regulates apical dicarboxylate transport due to changes in luminal [Ca2+]o levels, then apical addition of CaSR agonists may be used to test this hypothesis.
CaSR activation
To assess if CaSR activation is involved in alterations in apical dicarboxylate transport with changes in luminal Ca2+ OK cell monolayers were treated with 1 mM spermine for 3 min during uptake (Table 1). Spermine an orthosteric activator of CaSR [25, 26] directly activates CaSR without requiring the presence of Ca2+ [25]. Spermine activation of CaSR resulted in the inhibition of dicarboxylate transport in both normal and low [Ca2+]o (Fig. 2). As in our previous studies, low [Ca2+]o (vehicle control) transport was increased (174 ± 29.8% of control) compared to normal [Ca2+]o (vehicle control) at 100%. However, CaSR activation by spermine reduced transport both in normal [Ca2+]o (100 ± 0.0 vs 71.6 ± 1.7% of control, p < 0.05) and low [Ca2+]o (174 ± 29.8 vs 123 ± 25.3% of control, p < 0.01). Thus, activation of CaSR reduced dicarboxylate transport at both levels of [Ca2+]o demonstrating the contribution of CaSR in apical dicarboxylate transport regulation.
Apical CaSR activation results in inhibition of apical dicarboxylate transport. We have shown in our previously published studies [16,17,18] and herein that decreasing extracellular Ca2+, [Ca2+]o, results in augmentation of apical dicarboxylate transport (compare bar 3 from left with bar 1) in the OK proximal tubule cell line. Succinate transport increased to 174 ± 29.8% of control in low [Ca2+]o compared to normal [Ca2+]o. Addition of spermine (1 mM, Sp) to activate the apical CaSR resulted in decreasing succinate transport in both normal (bar 2) and low [Ca2+]o (bar 4). Spermine addition decreased succinate transport in normal [Ca2+]o (bar 2) to 71.6 ± 1.7% of control, p < 0.05. In low [Ca2+]o (bar 3, VC) succinate transport was 174 ± 29.8% of that in normal [Ca2+]o; addition of spermine to low [Ca2+]o reduced dicarboxylate transport to 123 ± 25.3% of control, p < 0.01 (bar 4). Taken together the addition of spermine to OK cell monolayers results in decreased dicarboxylate transport indicating the CaSR is involved in the regulation of apical dicarboxylate transport in the proximal tubule. #p < 0.05, *p < 0.01, VC vehicle control
Shown in Fig. 2 the addition of spermine in normal [Ca2+]o resulted in additional inhibition of dicarboxylate transport. The CaSR is generally inactive at [Ca2+]o levels below 0.2 mM with activation threshold that can vary from 0.5 to 2 mM depending on cell type and levels of expression [8]. This additional level of inhibition may be an additive effect in which spermine, an orthosteric activator, increases CaSR activity beyond that which is observed in normal [Ca2+]o.
Gq signaling lowers dicarboxylate transport
Next we sought to determine which G-protein pathway the CaSR signals via in regulation of apical dicarboxylate/citrate transport. As shown in Fig. 1, Gq signaling is known to increase [Ca2+]i via PLC followed by PKC activation. If CaSR activation regulates dicarboxylate transport via Gq signaling, raising [Ca2+]i may alter transport rates. OK cells were pretreated with thapsigargin for 30 min prior to uptake measurements to increase [Ca2+]i. Signaling via Gq activates PLC resulting in increased intracellular Ca2+ and PKC activation (Fig. 1) [33]. In HEK293 cells transfected with human CaSR, PLC activation followed by PKC activation is a Gq-dependent and Gi-independent process [34]. Thapsigargin is often used to increase [Ca2+]i [27].
Thapsigargin (Fig. 3) decreased dicarboxylate transport in both normal (100 ± 0.0 vs 73.5 ± 2.9% of control, p < 0.01) and low [Ca2+]o (134 ± 7.2 vs 100 ± 8.2% of control, p < 0.01). This indicates that CaSR activation followed by increases in [Ca2+]i via Gq signaling results in decreased dicarboxylate transport in both normal and low [Ca2+]o.
Gq signaling is involved in dicarboxylate transport regulation in both normal and low [Ca2+]o. Activation of apical CaSR may result in signaling via one of 3 G-proteins as illustrated in Fig. 1. This series of experiments looks at Gq signaling and used thapsigargin (Thaps) pretreatment (10 µM, 30 min) to increase intracellular calcium [Ca2+]i and thus mimic signaling via Gq. Thaps pretreatment resulted in decreased dicarboxylate transport in both normal (100 ± 0.0 vs 73.5 ± 2.9% of control, p < 0.01) and low [Ca2+]o (134 ± 7.2 vs 100 ± 8.2% of control, p < 0.01). *p < 0.01. VC vehicle control. This indicates that following CaSR activation by calcium, Gq signaling is involved in apical dicarboxylate transport regulation
Similar to the dicarboxylate transport processes we are currently studying, BGT1, the renal betaine/GABA transporter is also inhibited by [Ca2+]o [35]. Figure 3 shows that thapsigargin treatment resulted in dicarboxylate transport inhibition in both normal and low [Ca2+]o. This plus the spermine results (Fig. 2) provides evidence that the CaSR via the Gq pathway regulates dicarboxylate transport and Gq signaling increases [Ca2+]i possibly followed by PKC activation.
PKC activation decreases dicarboxylate transport
The results described above show that both spermine and thapsigargin addition in low extracellular Ca2+ mimics transport inhibition in normal [Ca2+]o and thus links the CaSR to apical dicarboxylate transport regulation. Gq signaling increases [Ca2+]i followed by PKC activation; and PKC is known to regulate many cell membrane transporters [28, 29, 35,36,37]. PKC has also been shown to affect NaDC1 [38]. To investigate if PKC activation regulates apical dicarboxylate transport via Gq, OK cell monolayers were pretreated with the PKC activator, 50 nM phorbol 12-myristate 13-acetate (PMA) for 30 min. Shown in Fig. 4, succinate transport in normal [Ca2+]o was unchanged (100 ± 0.0 vs 100 ± 5.9% of control, p = NS). However, in low [Ca2+]o PMA inhibited dicarboxylate transport (143 ± 7.8 vs 118 ± 6.6% of control, p < 0.01) and reveals that signaling via PKC is involved in apical dicarboxylate transport regulation.
PKC activation. Gq signaling produces increases in intracellular Ca2+ and is known to activate PKC. Since increasing [Ca2+]i with Thaps shown in Fig. 3 is involved; our next step was to directly activate PKC and examine changes in transport. OK cell monolayers were pretreated with phorbol 12-myristate 13-acetate (50 nM PMA) for 30 min to activate endogenous PKC. Succinate transport in normal [Ca2+]o was unchanged (100 ± 0.0 vs 100 ± 5.9% of control, p = NS). However, PMA addition resulted in decreased dicarboxylate transport in low [Ca2+]o (143 ± 7.8 vs 118 ± 6.6% of control, p < 0.01). Thus, when changes/decreases in [Ca2+]o occur, CaSR signaling via Gq → PKC is involved in apical dicarboxylate transport regulation. Thus, PKC activation is likely part of the signaling cascade (Fig. 1) that results in lower levels of apical dicarboxylate transport in normal concentrations of [Ca2+]o. VC vehicle control. *p < 0.01
Gi inhibition
Thus far using spermine to activate CaSR, thapsigargin to increase [Ca2+]i (intracellular calcium), and PMA to activate PKC have demonstrated that dicarboxylate transport regulation likely occurs through CaSR → Gq → PKC signaling at normal [Ca2+]o. However, in the proximal tubule the CaSR may also signal via Gi and Gs. In some cells, CaSR activation is known to signal via Gi or Gs pathways which produce changes in cAMP levels via AC inhibition or activation, respectively [19, 20, 24].
If CaSR activation regulates dicarboxylate transport via Gi signaling, the direct inhibition of Gi would be expected to increase dicarboxylate transport in normal [Ca2+]o. To investigate this we used the direct Gi inhibitor pertussis toxin (PTX). OK cell monolayers were pretreated with PTX 100 ng/ml overnight. Shown in Fig. 5, PTX had little effect on dicarboxylate transport in both normal (100 ± 0.0 vs 107 ± 1.9% of control, p = NS) and low [Ca2+]o (174 ± 24 vs 178 ± 15% of control, p = NS).
Direct inhibition of Gi protein with PTX does not alter dicarboxylate transport. Possible CaSR signaling via the Gi protein can be investigated using pertussis toxin (PTX) to directly inhibit Gi. Signaling via Gi (Fig. 1) results in the inhibition of adenylate cyclase (AC). PTX, used to block the inhibition of AC, should result in an increase of intracellular cAMP. OK cell monolayers were treated overnight with PTX (100 ng/ml) to inhibit Gi. Shown here PTX pretreatment did not alter dicarboxylate transport in either normal (100 ± 0.0 vs 107 ± 1.9% of control, p = NS) or low [Ca2+]o (174 ± 24 vs 178 ± 15% of control, p = NS). VC vehicle control. *p < 0.01
Gi activation
Gi activation inhibits AC and hence cAMP production [19]. To determine if direct pharmacological inhibition of AC results in decreased dicarboxylate transport the AC inhibitor MDL-12, 330A (MDL) 50 nM for 30 min was applied to OK cell monolayers to mimic Gi activation. If Gi signaling regulates apical dicarboxylate transport via AC inhibition, MDL should inhibit apical dicarboxylate transport only in low [Ca2+]o. Shown in Fig. 6, MDL inhibited succinate transport in both normal (100 ± 0.00 vs 53.7 ± 7.4% of control, p < 0.01) and low [Ca2+]o (146 ± 6.4 vs 88.7 ± 9.0% of control, p < 0.01).
Direct inhibition of AC with MDL decreased dicarboxylate transport in both normal and low [Ca2+]o. Another way to investigate Gi signaling is to directly inhibit AC by the addition of MDL-12,330A (MDL). OK cell monolayers were treated with 50 µM of MDL for 30 min before beginning transport measurements. MDL inhibited succinate transport in both normal (100 ± 0.00 vs 53.7 ± 7.4% of control, p < 0.01) and low [Ca2+]o (146 ± 6.4 vs 88.7 ± 9.0% of control, p < 0.01). VC vehicle control. *p < 0.01. The MDL results differ from the results in Fig. 5, where PTX had no effect on dicarboxylate transport; this discrepancy is addressed in the text
These results with MDL (Fig. 6) are unexpected when compared with those of PTX (Fig. 5). MDL was used to directly inhibit AC (i.e., mimic Gi) which resulted in reduced dicarboxylate transport in both normal and low [Ca2+]o. This decrease would be expected if CaSR regulation of dicarboxylate transport was via Gi signaling. It is possible, however, MDL may decrease dicarboxylate transport in some way other than AC inhibition [39,40,41]. Some previous studies indicate MDL may have effects unrelated to AC inhibition such as blocking of L-type Ca2+ channels, altering glycine transport and inhibiting phosphodiesterases [42,43,44].
Gs signaling
If Gi signaling results in dicarboxylate transport regulation by reducing cAMP levels from inhibition of AC (consistent with MDL experiments), then increasing intracellular cAMP by addition of the membrane permeable 8-Br-cAMP (8Br) should result in increased dicarboxylate transport (Fig. 1). Signaling via Gs is known to result in AC activation which in turn increases intracellular cAMP [24]. In contrast to the MDL experiments, if CaSR activity results in decreased dicarboxylate transport in normal [Ca2+]o due to Gs activation of AC, increasing intracellular cAMP via 8Br would be expected to decrease dicarboxylate transport. However, addition of 8Br + MDL (Fig. 7) did not offset the effects of direct AC inhibition via MDL alone (Fig. 6) in normal (100 ± 0.0 vs 100 ± 5.1% of control) or low [Ca2+]o (116 ± 9.2 vs 133 ± 8.7% of control) indicating that MDL may be acting via another mechanism [39,40,41].
Increasing intracellular cAMP does not offset the effects of AC inhibition on dicarboxylate transport. In this series of experiments MDL was used as a direct AC inhibitor to block increases in endogenously generated intracellular cAMP in all four groups of experiments. 8-Br-cAMP (8Br, 100 µM) is a cell membrane permeable form of cAMP [31] and was used to increase intracellular cAMP exogenously. Comparing bars 1 vs 2, increasing intracellular cAMP by the addition of 8Br did not overcome the direct inhibition of AC by MDL in normal [Ca2+]o (100 ± 0.00 vs 100 ± 5.1% of control). Similar results were found in low [Ca2+]o; 8Br had no significant effect in the presence of MDL in low [Ca2+]o (116 ± 9.2 vs 133 ± 8.7% of control) shown in bars 3 vs 4. And when 8Br concentrations were doubled to 200 µM, again there was no significant effect (116 ± 9.2 vs 130 ± 11.3% of control) shown in bars 3 vs 5. This provides further evidence that inhibition of dicarboxylate transport in both normal and low [Ca2+]o (Fig. 6) is not via AC inhibition, indicating that MDL may be acting via another mechanism [39,40,41]
Increasing intracellular cAMP
Because of these varying results, further studies on Gi signaling were needed to resolve the contrasting evidence with PTX and MDL. If CaSR activation results in decreased dicarboxylate transport via Gi inhibition of AC, 8Br would be expected to reverse this inhibition and increase dicarboxylate transport due to rises in intracellular cAMP. However, If CaSR activation decreases transport via Gs resulting in AC activation, 8Br should decrease dicarboxylate transport.
OK cells were pretreated with 100 µM 8Br for 30 min prior to uptake. 8Br (Fig. 8) had no effect on dicarboxylate transport in either normal (100 ± 0.00 vs 94.7 ± 1.7% of control, p = NS) or low [Ca2+]o (154 ± 9.9 vs 131 ± 9.6% of control, p = NS). Thus, neither Gi nor Gs are involved in apical dicarboxylate transport regulation. Importantly, these results align with those of PTX (Fig. 5), where inhibition of Gi had no effect on dicarboxylate transport and thus supports actions of MDL are not likely the result of inhibiting AC, but some other mechanism not involved with Gi [39,40,41].
Increasing intracellular cAMP via 8-Br-cAMP has no effect on dicarboxylate transport. Intracellular cAMP in OK cell monolayers was increased by the addition of 100 µM 8-Br-cAMP (8Br) for 30 min before start of transport measurements. Raising intracellular cAMP had no effect on dicarboxylate transport in either normal (100 ± 0.00 vs 94.7 ± 1.7% of control, p = NS) or low (154 ± 9.9 vs 131 ± 9.6% of control, p = NS) [Ca2+]o. VC vehicle control. *p < 0.01. This suggests that CaSR activation is not regulating dicarboxylate transport via Gs. Importantly, these results align with those in Figs. 5 and 7, indicating that neither Gi nor Gs are involved in regulation of dicarboxylate transport via CaSR activation
cAMP assays
Thus, far the CaSR-Gq signaling studies indicate that CaSR inhibits apical dicarboxylate transport in normal [Ca2+]o via Gq-PKC signaling, but not via Gi or Gs signaling. To verify this, intracellular cAMP assays were performed. Endogenously Gi is an inhibitor of AC and Gs is an activator. If the CaSR signals via Gi in OK cells AC inhibition would reduce cAMP levels; however, signaling via Gs would result in AC activation and increased cAMP levels. Because the CaSR is known for ligand biased signaling (discussed below) it is important to examine the effects of [Ca2+]o and spermine on endogenous cAMP levels to determine if there is a signaling bias.
Shown in Fig. 9 the level of [Ca2+]o had no effect on intracellular cAMP levels (0.335 ± 0.103 vs 0.272 ± 0.095 pmol/ml, p = NS). This further indicates that activation of the CaSR in OK cells does not signal via Gi or Gs. CaSR activator spermine also had little effect on cAMP levels in normal [Ca2+]o compared to normal [Ca2+]o alone (0.335 ± 0.103 vs 0.354 ± 0.136 pmol/ml, p = NS). Thus both spermine and [Ca2+]o likely activate the same CaSR signaling pathway. Forskolin, an AC activator, was used as a control to increase intracellular cAMP. As was expected, forskolin in normal [Ca2+]o resulted in a large increase in cAMP levels compared to normal [Ca2+]o alone (0.335 ± 0.103 vs 1.008 ± 0.228 pmol/ml, p < 0.05).
Assay of Intracellular cAMP Levels. To further examine if there was a role of cAMP in dicarboxylate transport regulation, intracellular levels of cAMP were measured. Shown here, no changes in intracellular cAMP levels were found in low [Ca2+]o (0.335 ± 0.103 vs 0.272 ± 0.095 pmol/ml, p = NS). The CaSR activator spermine (1 mM, 5 min) also had no significant effect on intracellular cAMP levels in normal [Ca2+]o compared to normal control (0.335 ± 0.103 vs 0.354 ± 0.136 pmol/ml, p = NS). This indicates that CaSR activation in OK cell monolayers does not result in AC activation or signal via Gi or Gs which act via changes in intracellular cAMP. As a positive control for the cAMP assays, the AC activator forskolin (10 µM, 30 min) significantly increased endogenous intracellular cAMP in normal [Ca2+]o (0.335 ± 0.103 vs 1.008 ± 0.228 pmol/ml, p < 0.05). These results further indicate that activation of the CaSR to regulate dicarboxylate transport in OK cells is not signaling via Gi or Gs. #p < 0.05
If CaSR activation resulted in Gi signaling, there should be a reduction in intracellular cAMP levels. If signaling is via Gs an increase in intracellular cAMP should result since Gi and Gs inhibit and activate AC respectively. Figure 9 shows there was little change in cAMP levels when comparing normal and low [Ca2+]o indicating that [Ca2+]o is not signaling via Gi or Gs. The CaSR activator spermine also had no effect on cAMP levels. This along with the PTX and 8Br studies is sufficient evidence to conclude that CaSR activation in OK cells does not result in Gi or Gs signaling.
The results with PTX and 8Br on transport, and the measurements of cAMP, indicate that changes in intracellular cAMP are not involved in transducing the CaSR signal involving apical dicarboxylate transport. MDL may be acting via effects unrelated to AC inhibition, explaining the sole discrepant results. MDL has been found to block certain Ca2+ channels, alter glycine transport and inhibit non-cAMP phosphodiesterases [42,43,44].Footnote 1
Therefore, in OK proximal tubule cells CaSR activation results in Gq signaling and not Gi or Gs. Normal [Ca2+]o via activation of the apical CaSR inhibits dicarboxylate transport; spermine, thapsigargin and PMA mimic this regulation in low [Ca2+]o. Thus, dicarboxylate transport inhibition occurs via the CaSR → Gq → PKC pathway.
Discussion
Proximal tubule apical dicarboxylate transport is important from several perspectives, but for considerations here as the main regulator of citrate excretion, a potent inhibitor of nephrolithiasis. Citrate concentration in the urine is determined by proximal tubule reabsorption [45, 46]. Dicarboxylate transporters also reabsorb other filtered Krebs cycle intermediates and their downstream delivery (e.g., succinate and α-ketoglutarate) may be important in downstream receptor signaling which may also impact stone formation [47].
In regards to citrate and stone prevention, teleologically increased urinary or luminal calcium should be matched by increased urinary or luminal citrate to prevent calcium precipitation with anions such as phosphate or oxalate, the complexes of which are not very soluble. Decreased reabsorption of citrate with increased luminal Ca2+ would help achieve this. Our previous studies have established calcium-sensitive dicarboxylate transport at the apical membrane of the OK cell model of the proximal tubule. This calcium-sensitivity may play an important role in preventing stone formation by inhibiting citrate reabsorption in response to increased luminal Ca2+ concentration [15,16,17]. Thus in the apical membrane of the proximal tubule CaSR activation may serve this function and regulate dicarboxylate transport [3, 7, 48]. Importantly (Fig. 2) the addition of the CaSR activator spermine in low [Ca2+]o resulted in inhibition of dicarboxylate transport similar to that observed by increasing extracellular calcium from low to normal [Ca2+]o. This demonstrates that CaSR activation regulates apical dicarboxylate transport.
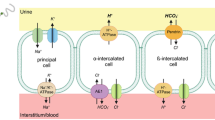
The CaSR prevents Ca2+ stone formation via segment specific functions throughout the nephron. For example, in the distal convoluted and connecting tubules the CaSR and the calcium-selective transient potential vanilloid-subtype 5 channel (TRPV5) co-localize at the luminal membrane and activation of the CaSR results in TRPV5-mediated Ca2+ influx [5]. In the collecting duct, CaSR activation prevents nephrolithiasis by promoting H+-ATPase-mediated H+ excretion and downregulation of Aquaporin 2. This results in urinary acidification and polyuria [6]. Clearly mechanisms in these other nephron segments prevent stone formation via CaSR activation. However, in the proximal tubule such mechanisms have not been described. The present results provide evidence that a mechanism in the proximal tubule may be that calcium activates CaSR which decreases citrate reabsorption, raising urinary citrate and decreasing the propensity for calcium stone formation.
The CaSR in the proximal tubule has been associated with the Gq, Gi or Gs proteins. The G-protein through which it signals depends upon CaSR conformation [25, 49, 50]. Knowing the particular signaling pathway may be useful in the future to modulate citrate excretion. The CaSR has been extensively investigated including its propensity for ligand biased signaling [23, 33, 34, 51,52,53]. Traditionally there was a two-state model where G-protein coupled receptors (GPCRs) isomerize between inactive and active states [54, 55]. New concepts in GPCR signaling, termed ligand biased signaling, considers that various ligands may result in a variety of conformational changes to GPCRs that result in activation of different G-protein signaling pathways. For example the adenine A1 receptor can adopt agonist-specific conformations arising from small changes in ligand structure which result in differential activation of Gi, Gs and Gq [56] The CaSR is known to couple to Gq, Gi and Gs signaling proteins [19, 20] all of which are in the proximal tubule [21, 22] Though many GPCRs, including the CaSR, are known to activate more than one G-protein at a time, ligand biased signaling involves strong bias toward one pathway at the expense of others that may be activated under alternative conditions.
Taken together, the results of spermine, thapsigargin and PMA experiments (Figs. 2, 3, 4) show that apical dicarboxylate transport inhibition in normal [Ca2+]o occurs via the CaSR → Gq → PKC pathway depicted in Fig. 1. Results with PTX and 8Br on transport (Figs. 5, 7, 8), and the measurements of cAMP (Fig. 9), indicate that changes in intracellular cAMP via Gs and Gi are not likely involved in regulation of dicarboxylate transport by the CaSR.
In summary, in OK cells CaSR activation (even by normal [Ca2+]o) inhibits dicarboxylate transport via Gq signaling. Our previous studies have consistently demonstrated calcium-sensitive dicarboxylate transport process in OK proximal tubule cells. Here we offer evidence that CaSR → Gq → PKC signal transduction pathway regulates apical dicarboxylate transport. This pathway provides a mechanism whereby the proximal tubule apical CaSR prevents calcium precipitation by reducing apical dicarboxylate transport.
Notes
Complicating this interpretation, NaDC1 has been considered responsible for all citrate reabsrorption on the apical side of the proximal tube. We have previously shown that NaDC1 ex vivo is not calcium-sensitive. So whether the calcium sensitive transport is NaDC1 or not remains unknown.
References
Egbuna O, Quinn S, Kantham L, Butters R, Pang J, Pollak M, Goltzman D, Brown E (2009) The full-length calcium-sensing receptor dampens the calcemic response to 1alpha,25(OH)2 vitamin D3 in vivo independently of parathyroid hormone. Am J Physiol Renal Physiol 297(3):F720–F728
Quamme GA (1982) Effect of hypercalcemia on renal tubular handling of calcium and magnesium. Can J Physiol Pharmacol 60(10):1275–1280
Riccardi D, Brown EM (2010) Physiology and pathophysiology of the calcium-sensing receptor in the kidney. Am J Physiol Renal Physiol 298(3):F485–F499
Wang WH, Lu M, Hebert SC (1996) Cytochrome P-450 metabolites mediate extracellular Ca(2+)-induced inhibition of apical K+ channels in the TAL. Am J Physiol 271(1 Pt 1):C103–C111
Topala CN, Schoeber JP, Searchfield LE, Riccardi D, Hoenderop JG, Bindels RJ (2009) Activation of the Ca2+-sensing receptor stimulates the activity of the epithelial Ca2 + channel TRPV5. Cell Calcium 45(4):331–339
Renkema KY, Velic A, Dijkman HB, Verkaart S, van der Kemp AW, Nowik M, Timmermans K, Doucet A, Wagner CA, Bindels RJ, Hoenderop JG (2009) The calcium-sensing receptor promotes urinary acidification to prevent nephrolithiasis. J Am Soc Nephrol 20(8):1705–1713
Riccardi D, Hall AE, Chattopadhyay N, Xu JZ, Brown EM, Hebert SC (1998) Localization of the extracellular Ca2+/polyvalent cation-sensing protein in rat kidney. Am J Physiol 274(3 Pt 2):F611–F622
Conigrave AD, Ward DT (2013) Calcium-sensing receptor (CaSR): pharmacological properties and signaling pathways. Best Pract Res Clin Endocrinol Metab 27(3):315–331
Remy C, Kirchhoff P, Hafner P, Busque SM, Müeller MK, Geibel JP, Wagner CA (2007) Stimulatory pathways of the calcium-sensing receptor on acid secretion in freshly isolated human gastric glands. Cell Physiol Biochem 19(1–4):33–42
Capasso G, Geibel PJ, Damiano S, Jaeger P, Richards WG, Geibel JP (2013) The calcium sensing receptor modulates fluid reabsorption and acid secretion in the proximal tubule. Kidney Int 84(2):277–284
Pajor AM (1999) Sodium-coupled transporters for Krebs cycle intermediates. Annu Rev Physiol 61:663–682
Bai XY, Chen X, Sun AQ, Feng Z, Hou K, Fu B (2007) Membrane topology structure of human high-affinity, sodium-dependent dicarboxylate transporter. FASEB J 21(10):2409–2417
Pajor AM (1999) Citrate transport by the kidney and intestine. Semin Nephrol 19(2):195–200
Brennan TS, Klahr S, Hamm LL (1986) Citrate transport in rabbit nephron. Am J Physiol 251(4 Pt 2):F683–F689
Hering-Smith KS, Gambala CT, Hamm LL (2000) Citrate and succinate transport in proximal tubule cells. Am J Physiol Renal Physiol 278(3):F492–F498
Hering-Smith KS, Schiro FR, Pajor AM, Hamm LL (2011) Calcium sensitivity of dicarboxylate transport in cultured proximal tubule cells. Am J Physiol Renal Physiol 300(2):F425–F432
Hering-Smith KS, Mao W, Schiro FR, Coleman-Barnett J, Pajor AM, Hamm LL (2014) Localization of the calcium-regulated citrate transport process in proximal tubule cells. Urolithiasis 42(3):209–219
Law D, Hering-Smith KS, Hamm LL (1992) Citrate transport in proximal cell line. Am J Physiol 263(1 Pt 1):C220–C225
Hofer AM, Brown EM (2003) Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol 4(7):530–538
Hernández-Bedolla MA, González-Domínguez E, Zavala-Barrera C, Gutiérrez-López TY, Hidalgo-Moyle JJ, Vázquez-Prado J, Sánchez-Torres C, Reyes-Cruz G (2016) Calcium-sensing-receptor (CaSR) controls IL-6 secretion in metastatic breast cancer MDA-MB-231 cells by a dual mechanism revealed by agonist and inverse-agonist modulators. Mol Cell Endocrinol 436:159–168
Zhu Y, He Q, Aydin C, Rubera I, Tauc M, Chen M, Weinstein LS, Marshansky V, Jüppner H, Bastepe M (2016) Ablation of the stimulatory G protein α-subunit in renal proximal tubules leads to parathyroid hormone-resistance with increased renal Cyp24a1 mRNA abundance and reduced serum 1,25-dihydroxyvitamin D. Endocrinology 157(2):497–507
Rangel LB, Lopes AG, Lara LS, Carvalho TL, Silva IV, Oliveira MM, Einicker-Lamas M, Vieyra A, Nogaroli L, Caruso-Neves C (2005) PI-PLCbeta is involved in the modulation of the proximal tubule Na+-ATPase by angiotensin II. Regul Pept 127(1–3):177–182
Wu DQ, Lee CH, Rhee SG, Simon MI (1992) Activation of phospholipase C by the alpha subunits of the Gq and G11 proteins in transfected Cos-7 cells. J Biol Chem 267(3):1811–1817
Nasman J, Kukkonen JP, Ammoun S, Akerman KE (2001) Role of G-protein availability in differential signaling by alpha 2-adrenoceptors. Biochem Pharmacol 62(7):913–922
Brennan SC, Thiem U, Roth S, Aggarwal A, Fetahu IS, Tennakoon S, Gomes AR, Brandi ML, Bruggeman F, Mentaverri R, Riccardi D, Kallay E (2013) Calcium sensing receptor signalling in physiology and cancer. Biochim Biophys Acta 1833(7):1732–1744
Petrel C, Kessler A, Dauban P, Dodd RH, Rognan D, Ruat M (2004) Positive and negative allosteric modulators of the Ca2+-sensing receptor interact within overlapping but not identical binding sites in the transmembrane domain. J Biol Chem 279(18):18990–18997
Harriman JF, Liu XL, Aleo MD, Machaca K, Schnellmann RG (2002) Endoplasmic reticulum Ca(2+) signaling and calpains mediate renal cell death. Cell Death Differ 9(7):734–741
Beckman ML, Bernstein EM, Quick MW (1999) Multiple G protein-coupled receptors initiate protein kinase C redistribution of GABA transporters in hippocampal neurons. J Neurosci 19(11):RC9
Forster IC, Traebert M, Jankowski M, Stange G, Biber J, Murer H (1999) Protein kinase C activators induce membrane retrieval of type II Na+-phosphate cotransporters expressed in Xenopus oocytes. J Physiol 517(Pt 2):327–340
Emery AC, Eiden MV, Eiden LE (2013) A new site and mechanism of action for the widely used adenylate cyclase inhibitor SQ22,536. Mol Pharmacol 83:95–105
Yamaguchi T et al (2003) Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int 63:1983–1994
Siebler T et al (1996) Pertussis toxin sensitive G-proteins are not involved in the mitogenic signaling pathway of insulin-like growth factor-I in normal rat kidney epithelial (NRKE) cells. Regul Pept 62:65–71
Wang D, McGiff JC, Ferreri NR (2000) Regulation of cyclooxygenase isoforms in the renal thick ascending limb: effects of extracellular calcium. J Physiol Pharmacol 51(4 Pt 1):587–595
Kifor O, MacLeod RJ, Diaz R, Bai M, Yamaguchi T, Yao T, Kifor I, Brown EM (2001) Regulation of MAP kinase by calcium-sensing receptor in bovine parathyroid and CaR-transfected HEK293 cells. Am J Physiol Renal Physiol 280(2):F291–F302
Kempson SA, Edwards JM, Sturek M (2006) Inhibition of the renal betaine transporter by calcium ions. Am J Physiol Renal Physiol 291(2):F305–F313
Hagos Y, Burckhardt BC, Larsen A, Mathys C, Gronow T, Bahn A, Wolff NA, Burckhardt G, Steffgen J (2004) Regulation of sodium-dicarboxylate cotransporter-3 from winter flounder kidney by protein kinase C. Am J Physiol Renal Physiol 286(1):F86–F93
Wolff NA, Thies K, Kuhnke N, Reid G, Friedrich B, Lang F, Burckhardt G (2003) Protein kinase C activation downregulates human organic anion transporter 1-mediated transport through carrier internalization. J Am Soc Nephrol 14(8):1959–1968
Pajor AM, Sun N (1999) Protein kinase C-mediated regulation of the renal Na(+)/dicarboxylate cotransporter, NaDC-1. Biochim Biophys Acta 1420(1–2):223–230
Collins TP, Terrar DA (2012) Ca(2+)-stimulated adenylyl cyclases regulate the L-type Ca(2+) current in guinea-pig atrial myocytes. J Physiol 590(8):1881–1893
Li X, Guo Q, Gao J, Yang J, Zhang W, Liang Y, Wu D, Liu Y, Weng J, Li Q, Zhang Y (2013) The adenylyl cyclase inhibitor MDL-12,330A potentiates insulin secretion via blockade of voltage-dependent K(+) channels in pancreatic beta cells. PLoS One 8(10):e77934
Dal Molin F, Zornetta I, Puhar A, Tonello F, Zaccolo M, Montecucco C (2008) cAMP imaging of cells treated with pertussis toxin, cholera toxin, and anthrax edema toxin. Biochem Biophys Res Commun 376(2):429–433
Gadea A, López E, López-Colomé AM (1999) The adenylate cyclase inhibitor MDL-12330A has a non-specific effect on glycine transport in Müller cells from the retina. Brain Res 838(1–2):200–204
Lippe C, Ardizzone C (1991) Actions of vasopressin and isoprenaline on the ionic transport across the isolated frog skin in the presence and the absence of adenyl cyclase inhibitors MDL12330A and SQ22536. Comp Biochem Physiol C 99(1–2):209–211
Rampe D, Triggle DJ, Brown AM (1987) Electrophysiologic and biochemical studies on the putative Ca++ channel blocker MDL 12,330A in an endocrine cell. J Pharmacol Exp Ther 243(1):402–407
Brennan S, Hering-Smith K, Hamm LL (1988) Effect of pH on citrate reabsorption in the proximal convoluted tubule. Am J Physiol 255(2 Pt 2):F301-306
Hamm LL, Hering-Smith KS (2002) Pathophysiology of hypocitraturic nephrolithiasis. Endocrinol Metab Clin North Am 31(4):885–893 viii
Peti-Peterdi J (2013) Mitochondrial TCA cycle intermediates regulate body fluid and acid–base balance. J Clin Invest 123(7):2788–2790
Magno AL, Ward BK, Ratajczak T (2011) The calcium-sensing receptor: a molecular perspective. Endocr Rev 32(1):3–30
Chang W, Shoback D (2004) Extracellular Ca2+-sensing receptors—an overview. Cell Calcium 35(3):183–196
Kenakin T (2011) Functional selectivity and biased receptor signaling. J Pharmacol Exp Ther 336(2):296–302
Thomsen AR, Hvidtfeldt M, Bräuner-Osborne H (2012) Biased agonism of the calcium-sensing receptor. Cell Calcium 51(2):107–116
Thomsen AR, Worm J, Jacobsen SE, Stahlhut M, Latta M, Bräuner-Osborne H (2012) Strontium is a biased agonist of the calcium-sensing receptor in rat medullary thyroid carcinoma 6–23 cells. J Pharmacol Exp Ther 343(3):638–649
Davey AE, Leach K, Valant C, Conigrave AD, Sexton PM, Christopoulos A (2012) Positive and negative allosteric modulators promote biased signaling at the calcium-sensing receptor. Endocrinology 153(3):1232–1241
Samama P, Cotecchia S, Costa T, Lefkowitz RJ (1993) A mutation-induced activated state of the beta 2-adrenergic receptor. Extending the ternary complex model. J Biol Chem 268(7):4625–4636
Gether U, Lin S, Kobilka BK (1995) Fluorescent labeling of purified beta 2 adrenergic receptor. Evidence for ligand-specific conformational changes. J Biol Chem 270(47):28268–28275
Cordeaux Y, Ijzerman AP, Hill SJ (2004) Coupling of the human A1 adenosine receptor to different heterotrimeric G proteins: evidence for agonist-specific G protein activation. Br J Pharmacol 143(6):705–714
Acknowledgements
This work was supported by NIH/NIDDK RO1 DK095879 to Dr. Hering-Smith.
Funding
This study was funded by NIH/NIDDK RO1 DK095879 to Dr. Hering-Smith. Dr. Hering-Smith is also supported by the NIH Louisiana Clinical and Translational Sciences Center (LA CaTS) grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Ryan Walker declares that he has no conflict of interest. Dr. Shijia Zhang declares that he has no conflict of interest. Ms. Joycelynn Coleman-Barnett declares that she has no conflict of interest. Dr. L. Lee Hamm declares that he has no conflict of interest. Dr. Kathleen Hering-Smith declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Walker, R.W., Zhang, S., Coleman-Barnett, J.A. et al. Calcium receptor signaling and citrate transport. Urolithiasis 46, 409–418 (2018). https://doi.org/10.1007/s00240-018-1035-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-018-1035-0