Abstract
Nephrolithiasis is thought to be governed by urinary thermodynamic and kinetic risk factors. However, identification of one or more of these factors which consistently and unambiguously differentiates between healthy subjects (N) and calcium oxalate (CaOx) renal stone patients (SF) remains elusive. The present study addresses this challenge. 24 h urines were collected from 15 N and 10 SF. Urine compositions were used to compute thermodynamic risk indices including urinary ratios, quotients and supersaturation (SS) values, while CaOx metastable limits (MSL) were determined experimentally. Crystallisation kinetics was determined by measuring rates of particle formation (number, volume, size) using a Coulter counter multisizer (CC) and a Coulter flow cytometer (FC). Particle shapes were qualitatively differentiated by FC and were viewed directly by scanning electron microscopy. Several urinary composition ratios and risk quotients were significantly different between the groups. However, there were no significant differences between CaOx MSL or SS values. Using transformed FC data, the rate of CaOx crystallisation in SF was significantly greater than in N. This was not supported by CC measurements. There were no significant differences between the groups with respect to particle size or CaOx crystal growth rates. Single and aggregated CaOx dihydrate crystals were observed in both groups with equal frequency and there were no differences in the kinetic properties of these deposits. A few CaOx monohydrate crystals were observed in SF. Although several risk factors were found to be significantly different between the groups, none of them were consistently robust when compared to other cognate factors. Arguments were readily invoked which demonstrated inter-factor inconsistencies and conflicts. We suspect that a unique discriminatory factor, such as any of those which we investigated in the present study, may not exist.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For over 50 years, researchers have attempted to identify or develop a single crucial urinary risk index to discriminate between normal controls (N) and calcium oxalate (CaOx) stone formers (SF). During this time, a wide variety of so-called “risk indices” has been proposed, tested and reviewed [1–3]. These can be broadly divided into categories involving (1) single urine parameters, (2) simple ratios of two components, (3) quotients of several parameters and (4) ion activity product ratios yielding approximations of supersaturation (SS) values of urinary salts. The upper limit of CaOx metastability (MSL) is an experimentally determined crystallisation risk indicator associated with SS, which has also been used in previous studies in attempts to discriminate between N and SF.
These indices are governed by thermodynamic principles. However, other urinary risk factors which involve inhibitors and promoters of crystal nucleation, growth and aggregation are driven by kinetic principles [4]. These too have been used in attempts to distinguish between the urines of the two groups.
Frequently, indices which discriminate in some studies fail to do so in others because results are parochial in terms of patient groups, laboratory protocols and other variables. To overcome this inherent limitation for assessing discriminatory efficacy, standardisation of experimental variables is required. Such an approach dictates that all the risk factors under scrutiny are derived from the same group of N and the same group of SF, and that the same urine collections are used for all determinations in each group. The present study was undertaken to address this challenge.
Subjects and methods
Subjects and urine collection
Fifteen healthy male controls (N) without prior history of stone disease and 10 recurrent CaOx stone-forming male patients (SF) participated in the study. Control subjects were recruited from the staff and postgraduate student cohorts at the University of Cape Town. Patients were recruited from the Departments of Urology at Groote Schuur and Tygerberg Hospitals in Cape Town. N and SF were age matched (range 25–50 years). SF had passed at least one CaOx stone in the preceding year. All patients had been advised to increase their fluid intake. Stone recurrence information was available for only six of the SF group: 2 stones in past year, 2 stones in past 2 years, 3 stones in past 2 years, 2 stones in past 10 years, 5 stones in past 10 years, 3 stones in past 20 years. Stone composition was verified by ourselves as CaOx mono-or dihydrate, using X-ray powder diffraction. None of SF was taking anti-stone medication at the time of the study.
Each subject collected a single 24-h urine sample without preservative in a 2.5 l glass bottle under normal dietary conditions. Volume and pH were recorded. Samples were checked for blood and infection and were discarded if positive. Urines were stored at 4 °C for 1–2 days prior to analysis. However, crystallisation experiments were performed on the day of urine collection.
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the University of Cape Town Research Ethics Committee and with the 1964 Helsinki declaration and its amendments or comparable ethical standards. Informed consent was obtained from all participants.
Urine analyses, simple ratios, risk quotients and relative supersaturation
Urines were analysed for Na, K, Ca, Mg, Phos, Urate, Cl and creatinine using standard laboratory techniques. Oxalate was determined using oxalate decarboxylase [5]. Citrate was determined by conversion to oxaloacetate using citrate lyase [6]. Combinations of these parameters were used to compute risk ratios and quotients. The concentrations of ionised species, and SS of CaOx and calcium phosphate (CaP) salts, as well as uric acid (UA), were calculated using the speciation programme JESS [7].
CaOx metastable limit (MSL) and CaOx crystallisation kinetics
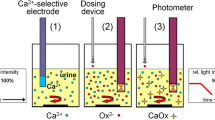
Urine samples were passed through 0.75 µm pre-filters and 0.45 µm filters to remove cellular debris and particulate matter. Filtered urines were used for the determination of MSLs and crystallisation kinetics. MSL is the supersaturation level above which spontaneous crystallisation will occur. It was determined in each sample by the addition of 0.15 mL of increasing concentrations of sodium oxalate (range 0.01–0.20 mol/L) to a series of small containers, each containing 15 mL of urine [8]. CaOx crystallisation kinetics were determined in each urine by dosing with an aliquot of sodium oxalate at a concentration corresponding to that of the MSL and measuring the ensuing crystallisation as a function of time [8]. For both determinations, crystallisation was followed by measuring the increase in particle numbers using a Coulter Counter Multisizer I particle counter and an Epics Profile Flow Cytometer equipped with an argon laser.
Crystal deposits in the urine of some subjects (6 × N, 4 × SF) were viewed by scanning electron microscopy at the final time point in the kinetics experiments.
Instrument settings
Coulter counter (CC)
The instrument was fitted with a 140 µm orifice. It was set to aspirate a volume of 500 µL and to count particles in 256 channels over the size range 2.8–56 µm. Coulter data were divided into four size ranges: “small” (<4 µm), “medium” (4–5 µm), “large” (5–8 µm) and “very large” (>8 µm) [9].
Flow cytometer (FC)
The principles and operation of this instrument for urine analysis in the context of stone formation have been described previously [10]. Briefly, in a FC, particles in liquid suspension are passed through a laser beam. The scattered light is detected and provides data concerning particle size, number and morphology. The light scatter in the forward direction is correlated with particle size and refractive index, whereas the light scatter in the side direction is correlated with surface morphology and internal structure. Instrument settings were: sample volume 100 µL, flow rate 50 µL/min, sheath pressure 7.5 psi, laser power 15 mW, photomultiplier tube voltage for side scatter 212 V, amplifier gains for forward scatter 30, for side scatter 30, and discriminator for forward scatter 70. Particle subpopulations were defined at different forward and side scatter values, thereby enabling zones with different sizes and particle shapes, respectively, to be isolated. Four size zones, identical to those for CC experiments, were defined. Two morphology zones were defined as type 1 and type 2, corresponding to relative qualitative differences in physical structure.
Scanning electron microscopy (SEM)
Deposited crystals were captured on 0.22 μm cellulose acetate filters. These were pasted onto aluminium stubs which were then coated with Au/Pd. The stubs were examined using a Cambridge S200 scanning electron microscope (specimen tilt 35°, accelerating voltage 10 kV, working distance 9–14 mm, resolution 9–10, aperture 30 μm).
Instrument protocols for determining crystallisation kinetics
For CC experiments, 2 mL sodium oxalate was added to 200 mL filtered urine. For FC experiments, 10 mL filtered urine and 0.1 mL sodium oxalate were used. In some cases, only one instrument was used due to time constraints and lack of availability. Irrespective of the instrument, urine aliquots were withdrawn at 10 min intervals over a period of 120–130 min and analysed for particle number.
CC was also used to record particle volume-size distributions at 30, 60 and 90 min for each urine sample. The diameter matching the peak in these plots corresponds to the average size of the particles precipitating in those samples [8]. Since particle volume and discrete particle size cannot be measured by FC, similar distributions could not be determined with this instrument.
Data treatment
Data collected with both instruments were used to construct two graphs for each urine sample: particle number versus the concentration of added sodium oxalate for the determination of MSL, and particle number versus time for the determination of crystallisation kinetics. Additional graphs of particle volume versus time curves were constructed using data obtained from CC measurements. Slope and R 2 values were calculated for the linear section of the kinetics curves. The slopes equate to the rate of CaOx crystallisation. The particle count vs time curves for both instruments were also transformed to their linear forms using a logit function [11]. This allowed more data points to be included in the calculation of the gradient of the linear section of the sigmoidal curves.
Statistical analysis
Mean and standard error values were calculated. The mean values for groups (N versus SF) were compared by analysis of variance and two-tailed t tests (GraphPad InStat 3.06, La Jolla, USA; p < 0.05 statistically significant).
Results
Urine composition: thermodynamic factors
Mean urinary excretions are given in Table 1. Completeness of 24 h collections was confirmed by checking that urinary excretion of creatinine was within the normal reference range in each sample. Significantly higher excretions of calcium, urate, chloride and creatinine, and higher urinary volumes occurred in SF. Calculated concentrations of ionised species (Table 2) indicated that only Ca2+ was significantly different (SF > N, p = 0.0469). For simple urinary ratios, Ca/Cr, Ca/Mg, Ca2+/Mg2+, Ca/Ox and Na/K were significantly higher in SF (Table 3). Values for quotients and risk formulae are given in Table 4. Significant discrimination between the groups was achieved by the Discriminant Scores Sm1 and Sm2 [15], the Crystallisation Risk Index [19], the AP(CaP) standardised index [2] and the Citrate–Magnesium–Calcium Ratio [21]. There was no significant difference between the CaOx MSL values in N and SF (Table 4). There were no significant differences between N and SF for supersaturation values for any of the stone-forming salts (Table 5).
Crystallisation experiments: kinetic factors
Crystallisation kinetics plots of particle number vs time for data from both instruments and of particle volume vs time (CC only) were constructed for each urine sample. A representative plot of number and volume vs time for SF, obtained from CC data, is shown in Fig. 1. Logit of particle number vs time plots for transformed data from both instruments was also constructed (not shown here). Crystallisation rates were determined from the gradients of the total number vs time and the logit of particle number vs time plots for both instruments (Table 6). The only statistically significant difference between the groups occurred in the transformed FC data which showed that the rate of CaOx crystallisation was greater in SF than in N (p = 0.019).
It is noted that crystallisation rates determined using the two instruments differ by an order of magnitude (Table 6). This is due to the different operational modes in CC and FC; while absolute particle numbers per se are measured by CC, laser dispersion in FC is only correlated with particle number without necessarily providing a direct measure thereof.
Combined plots of total particle number and total particle volume as functions of time in N and SF such as that shown in Fig. 1, demonstrated a general trend in which the rate of increase in particle number was greater than that at which particle volume increased. This occurred in 8 of 12 subjects in N (66.7 %) and in 5 of 10 subjects in SF (50 %). There were only two urines (SF) in which the reverse trend was observed while in the remaining urines (4 × N and 3 × SF), rates were equal.
Mean particle diameters at different time intervals, determined from the mode of volume-size distribution curves, varied between 4.08 and 4.66 µm across both groups of subjects (Table 7). Representative curves from two N and two SF subjects are shown in Fig. 2. There were no significant inter-group differences. Growth rates were calculated (diameter × time−1) in each urine sample using the modes at 30 and 90 min. These were not statistically different. It should be noted that these values represent the change in particle diameter as a function of time and as such are deemed as being measures of growth kinetics, whereas values derived from the gradients of particle number vs time plots are measures of nucleation kinetics.
Crystallisation rates in the different size ranges followed the same trend (small ≥ medium > large > very large) in N and SF (both instruments), respectively. Absolute values are not given here but typical SF plots (CC data) showing these trends are given in Fig. 3.
SEM revealed the presence of numerous COD single crystals and COD aggregates in all the urines from both groups (not shown here). In addition, a few COM crystals were observed in 3 of 4 SF urines. None occurred in urines from N.
FC side scatter plots in the urines of both groups (not shown here) demonstrated the formation of two different crystal shapes (qualitatively designated as “Type 1” and “Type 2”) during the crystallisation process. Crystallisation rates of these two types were followed by measuring the forward scatter data as a function of time. This revealed that Type 1 crystals formed at a faster rate in both groups. However, there were no inter-group significant differences. Representative examples of the change in particle number as a function of time for both crystal types in SF are shown in Fig. 4.
Discussion
Interrogation of numerous published studies shows that individual urinary parameters do not consistently discriminate between N and SF. Thus, although our observations of a significantly higher Ca, Ur, Cl and Cr excretion in SF might be regarded as discriminatory, and although the reasons for these relatively higher excretions are not obvious to us, the absence of significant differences between other widely regarded individual lithogenic risk factors such as pH and citrate, and others, casts doubt on the likelihood of identifying a unique discriminator among single urine parameters.
Of note is the significantly greater urinary volume in SF compared to N. This observation is probably a manifestation of the advice given to members of the former group to increase their fluid intake. As with other individual urinary variables when considered in isolation, its implication on relative stone risk cannot be predicted.
The concentration of Ca2+ rather than Ox2− emerged as being significantly higher in SF in the present study. This is surprising since Ox2− is widely regarded as the limiting factor in CaOx urolithiasis [22]. Furthermore, none of the CaOx crystal types had higher SS values in this group of patients despite the higher concentration of Ca2+. We also calculated SS for several CaP salts to fully explore the possibility of them being potential discriminators. Significant differences were not detected here either. These findings support the notion that SS is not a critical risk factor in urinary stone formation [23]. The absence of a significant difference between the urinary CaOx MSL values of N and SF provides further evidence in support of this notion.
The significantly higher concentration of total Ca in SF directly influences the values of the ratios Ca/Cr, Ca/Mg, Ca2+/Mg2+ and Ca/Ox all of which were significantly higher in SF, thereby raising the question whether these ratios per se are independent discriminators or not. On the other hand, the ratio Na/K whose magnitude is independent of total Ca proved to be discriminatory (SF > N, p = 0.0182). This finding agrees with reported values for early morning spot urines in N and SF [24]. Inspection of the factors in the ratio itself shows that the mean concentrations of Na in N and SF are not themselves significantly different but those of K are significantly lower in the latter group (p = 0.005). Thus, the significantly lower concentration of K in SF causes the higher Na/K ratio in this group. Whether it is the lower K alone or the ratio itself which triggers a pathophysiological mechanism (as yet unidentified) for increasing the risk of stone formation is a challenge for the future.
In the present study, several quotients were significantly higher in SF relative to N (Sm1 and Sm2, CRI and AP(CaP) index-standardised) while one ratio (CMC) was significantly lower in this group. As was the case with the simple ratios, these differences can be attributed to the higher total Ca in SF. (In the CMC ratio, total Ca appears in the denominator of the expression). Thus, for a ratio or quotient to be convincingly discriminatory, it would have to provide an outcome which is not predictable by mere arithmetic comparison of individual urinary parameters in N and SF. For example, it would be compelling if none of the individual urinary parameters in N and SF were significantly different, yet a risk quotient using these parameters demonstrated discrimination between the groups. Another scenario would be one in which a risk ratio or quotient successfully discriminates between N and SF despite the presence of two or more significantly different urinary parameters with conflicting clinical risk implications. Neither of these scenarios occurred in the present study.
Conversely, the question arises of how to interpret the efficacy of a risk formula which contains one or more individual urinary parameters which are significantly different in N and SF, yet fails to discriminate between them. In the present study, SFRI contains two parameters (Ca and Ur), each of which was significantly higher in SF, yet no discrimination was achieved by the index itself. Interpreting the physicochemical or pathological implications of this finding on stone risk is difficult, if not impossible. The dilemma for the stone researcher is to decide whether to attach greater weight to individual parameters or to complex quotients which take synergistic effects into account, and to have sound scientific reasons for doing so.
Similar arguments apply to the Tiselius formulae [2, 12–14, 16, 17] all of which contain Ca in the numerator. Despite this, none of them discriminated between N and SF, except for AP(CaP) Standardized Index [2]. The latter finding is intriguing since this index provides a risk assessment for CaP stone formation, yet the patient group in the present study comprised CaOx stone formers. Furthermore, SS values for all the CaP salts in the present study were not significantly different between N and SF. These findings again create a dilemma for the researcher who has to reconcile these apparently conflicting results. On the other hand, the results could be interpreted as indicating that AP(CaP) Standardised Index is indeed a discriminator between CaOx SF and N. Such a conclusion would be in agreement with Tiselius’s contention that CaP is a precursor of CaOx stone formation [25].
Despite these concerns, it could be argued that the ultimate effect of high Ca on “risk” can be moderated by concomitantly high Cit or Mg levels, so the ratios Ca/Cit and Ca/Mg (as well as those involving their ionised species) do indeed have physicochemical gravitas even though Cit and Mg themselves might not be significantly different in the two groups. In the more complex quotients, it could be argued that their rigour lies in their holistic formulation which often includes five or more urinary parameters, each one of which may interact with some or all of the other parameters in subtle and nuanced ways to influence the chemical speciation which ultimately determines supersaturation. However, herein lies the crucial point, namely that all the risk quotients, irrespective of whether they are simple ratios or complex formulae, provide a measure of supersaturation in one way or another. Since the capacity of this physicochemical parameter to discriminate consistently between N and SF has been challenged previously [23], the case in support of finding a universal thermodynamic discriminator in the urine of N and SF has come full circle. It remains weak. We suspect that it probably does not exist. A study which follows the same philosophy as described in the present paper, involving much larger numbers of N and SF, is needed to support or rebut this notion.
With regard to the kinetic risk factors, the only statistically significant difference between N and SF was demonstrated by the transformed FC data which showed a faster rate of CaOx crystallisation in the latter group. This can be interpreted in two different but conflicting ways. On the one hand, it could be argued that a faster rate of crystallisation increases the risk of stone formation because the presence of a greater number of crystals increases the probability of aggregation and/or crystal retention [26]. On the other hand, a fast crystallisation rate can be regarded as a protective mechanism because it reduces supersaturation quickly [27]. Although we have presented here crystallisation plots in the different size zones for SF only (Fig. 3), we can report that comparison with similar plots in N showed that at any given time, the number of very small particles in SF is indeed greater than that in N. Thus, while this supports the notion that a fast crystallisation rate produces a greater number of smaller crystals and increases the risk of stone formation, it contradicts the hypothesis that this mechanism is protective against stone formation, as this effect was observed in the urine of SF rather than in N.
The well-known crystallisation mechanisms of nucleation, growth and aggregation could all be occurring simultaneously and at different rates. As such, they are very difficult to disentangle. In a simplistic approach, increases in particle number are indicative of nucleation while increases in particle volume are indicative of growth and/or aggregation. If one accepts the commonly held view that large crystals (caused by growth or aggregation) are a risk factor for crystal retention and stone formation, a situation in which particle number increases at a faster rate than particle volume is preferable. This effect was observed in 66 % of N and 50 % of SF, thereby suggesting that this urinary property could discriminate between the two groups. However, this is speculative, since the number of participants in both groups was small. Nevertheless, we believe that it is worth reporting here as it may be of interest to stone researchers.
We suspect that the two different crystal morphologies (Types 1 and 2) detected by FC correspond to single and aggregated COD crystals, as SEM revealed that these morphologically distinct deposits were consistently present in all urines. Crystallisation rates of these single and aggregated deposits showed the same trends in N and SF. Importantly, there were no inter-group differences in the other kinetic characteristics for these deposits, such as lag times for crystallisation. Furthermore, neither single nor aggregated crystals were more dominant in one group compared to the other. Thus, as with our investigation of thermodynamic risk factors, no compelling discriminatory kinetic risk factors emerged in the present study. Further exploration is warranted, but our results are not encouraging.
The presence of a few COM crystals in SF but not in N is noteworthy yet counterintuitive, as this crystal type has been associated with relatively lower urinary calcium levels [28], which was not the case in the present study. On the other hand, crystal adhesion with renal cells, which increases the risk of crystal retention and stone formation, has been shown to occur more readily with COM than with COD [29]. As such, the formation of the former crystal type in SF but not in N may be a discriminatory feature of their urines. Unfortunately, the conflicting observations highlight the difficulty of identifying a consistent, uncompromised differentiator.
Limitations of the present study include the relatively small number of N and SF participants, the collection of only one 24 h urine sample from each of these and the assumption on our part that crucial differences are to be found in 24 h samples as opposed to spot urine collections. All these shortcomings might be redressed in future studies seeking to identify a universal discriminator between the two groups. It should also be recognised that our findings are for idiopathic calcium oxalate stone formers. Clearly, other outcomes might emerge with other types of stone formers. The final limitation of our study is common to most other investigations of urinary stone formation risk factors, namely that urines from SF were collected at various times after the actual stone episodes, when stone formation activity might have been dormant and/or when patients might have been following some strategy for reducing their risk for stone formation.
In conclusion, despite our finding of several thermodynamic and kinetic risk factors which differed significantly between N and SF, arguments are easily invoked which challenge their validity as true discriminators. It is apparent that identification of a universal risk factor which consistently differentiates between the urines of the two groups remains elusive. We suggest that such a factor may not exist. Other lithogenic mechanisms such as those which involve urinary macromolecules [30] or Randall’s plaque [31], among others, may harbour such a discriminator and deserve continued exploration.
References
Robertson WG, Peacock M (1983) Review of risk factors in calcium oxalate urolithiasis. World J Urol 1:114–118
Tiselius HG (1997) Risk formulas in calcium oxalate urolithiasis. World J Urol 15:176–185
Laube N, Kleinen L (2011) Risk indices. In: Rao PN, Preminger GM, Kavanagh JP (eds) Urinary tract stone disease. Springer-Verlag Limited, London, pp 355–368
Hess B, Kok DJ (1996) Nucleation, growth, and aggregation of stone-forming crystals. In: Coe FL, Favus MJ, Pak CYC, Parks JH, Preminger GM (eds) Kidney stones: medical and surgical management. Lippincott-Raven Publishers, Philadelphia, pp 3–32
Chiriboga J (1963) Some properties of an oxalic oxidase purified from barley seedlings. Biochem Biophys Res Commun 11:277–282
Gruber W, Mollering H (1966) Citrat-lyase und bestimmung von citrate. Biochemische Zeitschrift 346:85–88
May PM, Murray K (1991) JESS, a joint expert speciation system—1. Talanta 38:1409–1417
Ryall RL, Hibberd CM, Marshall VR (1985) A method for measuring inhibitory activity in whole urine. Urol Res 3:285–289
Markovic M, Komunjer L (1979) A new method to follow crystal growth by coulter counter. J Cryst Growth 46:701–705
Rodgers A, Hibbert B, Probyn T (1995) Determination of urinary calcium oxalate crystallisation mechanisms and kinetics using flow cytometry. Urol Int 55(2):93–100
Bewick V, Cheek L, Ball J (2005) Statistics review 14: logistic regression. Crit Care 9(1):112–118
Tiselius HG (1982) An improved method for the routine biochemical evaluation of patients with recurrent calcium oxalate stone disease. Clin Chim Acta 122(3):409–418
Tiselius HG (1983) Different estimates of the risk of calcium oxalate crystallization in urine. Eur Urol 9:231–234
Tiselius HG (1984) A simplified estimate of ion-activity product of calcium phosphate in urine. Eur Urol 10:191–195
Parks JH, Coe FL (1986) A urinary calcium-citrate index for the evaluation of nephrolithiasis. Kidney Int 30:85–90
Tiselius HG (1989) Standardized estimate of the ion activity product of calcium oxalate in urine from renal stone formers. Eur Urol 16:48–50
Tiselius HG (1991) Aspects on estimation of the risk of calcium oxalate crystallization in urine. Urol Int 47:255–259
Esen T, Akinci M, Tellaloglu S (1994) Stone formation risk index (SFRI): a possible prognostic factor governing the need for metaphylaxy. In: Ryall RL, Bais R, Marshall VR, Rofe AM, Smith LH, Walker VR (eds) Urolithiasis 2 Proceedings 7th International Symposium on Urolithiasis. New York: Plenum Press, pp 410
Daudon M, Labrunie M, Ivaldi A, et al (1996) A new crystallization risk index (CRI) for calcium oxalate (CaOx) stone formers. In: Pak CYC, Resnick MI, Preminger GM (eds) Urolithiasis 1996: Proceedings of the 8th International Symposium on Urolithiasis. Dallas: Millet the Printer Inc, pp 357–358
Porile JL, Asplin JR, Parks JH et al (1996) Normal calcium oxalate crystal growth inhibition in severe calcium oxalate nephrolithiasis. J Am Soc Nephrol 7(4):602–607
McConnell N, Campbell S, Gillanders I et al (2002) Risk factors for developing renal stones in inflammatory bowel disease. BJU Int 89(9):835–841
Robertson WG, Hughes H (1993) Importance of mild hyperoxaluria in the pathogeness of urolithiasis—new evidence from studies in the Arabian peninsula. Scanning Micros 7:391–401
Rodgers AL (2014) Urinary saturation: casual or causal risk factor in urolithiasis? BJU Int 114(1):104–110
Cirillo M, Laurenzi M, Panarelli W, Stamler J (1994) Urinary sodium to potassium ratio and urinary stone disease. The Gubbio Population Study Research Group. Kidney Int 46(4):1133–1139
Tiselius HG (2011) A hypothesis of calcium stone formation: an interpretation of stone research during the past decades. Urol Res 39:231–243
Kok DJ, Khan SR (1994) Calcium oxalate nephrolithiasis, a free or fixed particle disease. Kidney Int 46:847–854
Kavanagh JP (1992) Methods for the study of calcium oxalate crystallization and their application to urolithiasis research. Scanning Micros 6:685–705
Ryall RL, Chauvet MC, Grover PK (2001) A space oddity. In: Kok DJ, Romijn HC, Verhagen P, Verkoelen CF (eds) Eurolithiasis 9th European Symposium on Urolithiasis. Shaker Publishing, Maastricht, pp 273–274
Wesson J, Worcester E, Weissner J et al (1998) Control of calcium oxalate crystal structure and cell adherence by urinary macromolecules. Kidney Int 33:952–957
Ryall RL (2011) The possible roles of inhibitors, promoters and macromolecules in the formation of calcium kidney stones. In: Preminger GM, Kavanagh JP (eds) Rao NP. Urinary Tract Stone Disease, London, pp 31–60
Daudon M, Bazin D, Letavernier E (2015) Randall’s plaque as the origin of calcium oxalate kidney stones. Urolithiasis 43(suppl 1):S5–S11
Acknowledgments
The authors wish to thank the South African National Research Foundation, the South African Medical Research Council and the University of Cape Town for financial support.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodgers, A.L., Webber, D. & Hibberd, B. Experimental determination of multiple thermodynamic and kinetic risk factors for nephrolithiasis in the urine of healthy controls and calcium oxalate stone formers: does a universal discriminator exist?. Urolithiasis 43, 479–487 (2015). https://doi.org/10.1007/s00240-015-0802-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-015-0802-4