Abstract
The aim of the study was to investigate clusterin expression in the kidney and evaluate the urine clusterin level in the kidney stone formers. (1) In vitro, we treated the Madin-Darby canine kidney (MDCK) cell line with different concentrations of calcium oxalate (CaOx), and then the clusterin protein expression in the cells was evaluated by Western blotting. (2) Kidney stone patients who received percutaneous nephrolithotomy were enrolled in our study. Urine samples were collected before surgery, the kidney punctured to obtain kidney tissue guided by ultrasound intraoperatively. Clusterin expression in the human kidney tissue was evaluated by immunochemistry. The urine clusterin level was determined by enzyme-linked immunosorbent assay. Non-kidney disease subjects were chosen as controls. In vitro, the clusterin expression was up-regulated in the MDCK cells induced by CaOx. The study included 49 patients and 41 non-kidney disease subjects. All calculi were composed of calcium oxalate monohydrate or calcium oxalate dihydrate and a few also contained protein or uric acid. Mean ± SD urine clusterin level was 17.47 ± 18.61 μg/ml in patients, and 3.31 ± 5.42 μg/ml in non-kidney disease subjects, respectively (p < 0.001). Immunohistochemistry revealed the clusterin was located in the cytoplasm of the renal distal and collecting tubular epithelial cells. Also the tissue clusterin expression increased significantly in the kidney stone formers compared to the control groups (p = 0.001). CaOx could induce clusterin expression in renal tubular cells, and increase clusterin levels in the kidney and urine from the kidney stone formers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urolithiasis has been cited as the third most common affliction of the urinary tract, characterized by an increased incidence and frequent recurrence of calcium oxalate (CaOx) stone in occidental societies [1, 2].
The development of kidney stones requires formation of crystals from supersaturated urine followed by their retention in the kidney. Crystal nucleation, growth, and aggregation all occur in renal tubular lumens and renal pelvises. In 1937, Randall observed small subepithelial plaques of calcium salts localized in the renal papillae [3]. In the human autopsy studies, researchers found calcium deposits in the renal papillae (Randall plaques) of 1/3 patients who had the nephrolithiasis history [4]. However, the origin of Randall plaques themselves remains controversial. In one recent study published by Evan et al. [5], researchers found that the crystal was not only deposited in the interstitium but also between individual urothelial cells. Therefore, the hypothesis of Randall plaque cannot comprehensively explain all the kidney stone formation. Renal tubular epithelial and basement membrane could play an important role in the kidney stone formation [6]. Saeed R. Khan suggested that oxalate, calcium oxalate crystals of Randall plaque could provoke renal cell reactive oxygen species production, which could in turn mediate inflammation response, and induce expression of inflammation-related molecule, such as α-1-microglobulin, e-cadherin, and osteopontin [7]. Clusterin is another inflammation-related protein, and could be up-regulated by reactive oxygen species which may play a protective role in responding to epithelial cell injury [8].
In 1996, Lieske et al. [9] described the adherence of CaOx crystals to African green monkey renal epithelial (BSC-1) cells and wild-type Madin-Darby canine kidney (MDCK) cells. Crystals like as calcium phosphate (CaP) and CaOx in the urine could induce the chemical injury in the renal epithelial cells, in turn may lead to cell death and subsequently cell regeneration. Then the crystals could adhere to the injured renal tubules [10]. Moreover, without tubular injury/regeneration, the crystals could not retain and grow up in the renal tubular lumen. Both animal models, as well as tissue culture studies, indicate that exposure to high levels of oxalate and CaOx crystals is injurious to renal epithelial cells. Some study with renal tubular epithelial cells in culture showed that confluent monolayers of distal tubule/collecting duct-like MDCK-I cells are non-adherent to calcium oxalate monohydrate crystals [11]. In contrast, crystals bind to cells in subconfluent cultures and in confluent monolayers recovering from mechanical injury [12]. In vivo crystals seen in the renal tubules of hyperoxaluric rats are always associated with cellular degradation products [13, 14]. Besides, crystals were found adhered to the luminal surface of hyaluronan-, osteopontin-, and CD44-expressing injured/regenerating cells [15]. These data, therefore, strongly suggest that crystal retention in the kidney needs tubular epithelial injury. Therefore, renal epithelial cell injury is an initiation event in the stone formation. Actually the damaged renal tubular cells could produce some macromolecules, which could play an important role in the crystal retention. So these protein molecules could be used as a biomarker to predict the risk of kidney stone occurrence or recurrence, but also indicates the renal tubular cell injury in the kidney stone formers. Recently, in one study on the human matrix of calcium oxalate monohydrate (COM) stones, Canales BK et al., investigated the solubilizing proteins in the kidney stone by reversed phase, high-performance liquid chromatography (RP-HPLC), and tandem mass spectrometry, and some proteins were identified, including immunoglobulins, defensin 3, clusterin, complement C3a, kininogen, and fibrinogen [16]. Furthermore, The Food and Drug Administration (FDA) and the European Medicines Agency (EMEA) endorsed clusterin as one of 7 urinary biomarkers of nephrotoxicity in non-clinical settings in 2008 [17].
Clusterin (449 amino acids) is a heterodimeric disulfide-linked glycoprotein. In human, clusterin gene is located on chromosome 8p21-p12, where it is organized into nine exons, and clusterin is expressed in nearly all tissues [18]. The gene encodes two isoforms: a conventional ubiquitous secretory heterodimeric disulfide-linked glycoprotein and a truncated nuclear form. Clusterin has been functionally implicated in several physiologic processes, including cell adhesion, morphologic transformation, membrane recycling, and cell–cell interactions. In the kidney, it is highly expressed during early stages of development [19, 20]. In the healthy mature kidney, clusterin messenger RNA (mRNA) and protein are not detectable [21], but are up-regulated in response to renal tubular injury and a variety of renal diseases [22]. It has been suggested that clusterin expression is up-regulated in rats following nephrectomy [23], unilateral ureteral obstruction [24], renal ischemia–reperfusion [25], or nephrotoxicity [26, 27]. Although increased expression of clusterin (mRNA and/or protein) is seen in a variety of renal disorders, to date there has been no clinical study or primary research demonstrating the role of clusterin in kidney stone formers. In this study, we evaluated the clusterin expression in the kidney tissue and urine of kidney stone formers. Also, we evaluated the clusterin expression in the renal tubular epithelial cell lines (MDCK) treatment with calcium oxalate. The results suggested that clusterin expression was up-regulated in MDCK cells after treatment with calcium oxalate. Also, clusterin expression was up-regulated in the distal renal epithelial tubular cell and the urine samples of kidney stone formers.
Materials and methods
Study protocols involving human materials were approved by HeBei General Hospital institutional ethics committee. Patients with kidney stones without a family history of urinary stone disease and non-kidney disease control subjects were included in the study.
Non-kidney disease controls were patients who were involved in trauma and subsequently died. In this study, a total of 41 non-kidney disease controls were involved, whose middle-stream urine was obtained when the patients entered the emergency and signed the consent documents, while normal kidney tissue samples were obtained at autopsy within 3-h after death, and archived at the pathology department in our institution. All the statutory agents of these subjects have signed the consent forms to participate in this study.
Physical examination, abdomen CT, and urinalysis were performed to guarantee that those included controls had no urinary microscopic hematuria, urinary infection, chronic kidney disease, or renal calcification. Also to exclude the controls who had renal calcification, paraffin slides of kidneys were stained by H&E from the same block for clusterin staining. After those slides are stained by H&E [28], they were reviewed by two pathologists to determine whether there was renal calcification. The control cases which had renal calcification in the kidney were excluded.
All the kidney stone patients were free of hypercalcemia, hyperoxaluria, and hyperuricuria (based on the clinical guideline: guidelines for the management of asymptomatic primary hyperparathyroidism: from the third international workshop (2008); and European association of urology (EAU) urolithiasis guideline [update February 2012)]. Other potential causes of kidney stones and other kidney disease had been excluded, including primary hyperparathyroidism, gout, renal tubular acidosis, inflammatory bowel disease, medullary sponge kidney, sarcoidosis, vitamin D intoxication, urinary infection, and hydronephrosis. All patients underwent percutaneous nephrostolithotomy. Before the surgery, the urine of patients will be collected for the clusterin detection. At the end of the procedure, ultrasound-guided puncture biopsy was performed to acquire medulla tissue of kidney.
Middle stream Urine samples of preoperative patients and non-kidney disease subjects were collected. Once the samples were collected, the urine was centrifuged, separated, and both supernatant and pellets were stored at −80 ℃ for the test. All the urine samples were collected using the same protocol. Urinary clusterin level was measured in each participant using enzyme-linked immunosorbent assay (R&D, cat. DCLU00). Samples were assayed in the same batches to avoid any possible bias produced by inter-assay variation. Intra-assay coefficients were less than 10 % for all assays.
Stones were analyzed with the Vector 22 Fourier transformation infrared spectrometer (Bruker, Karlsruhe, Germany) according to the manufacturer's protocol. 1 mg of renal calculi from kidney stone patients was ground and mixed with 200-mg potassium bromide (Sigma). The mixture was filtered by 0.074-mm strainer, and compressed into a pellet (diameter: 13 mm). The pellets were put into infrared spectrometer (Spectrum 4000–800 cm−1) to be analyzed, and the infrared spectroscopy of renal calculi was collected. The components of renal calculi were identified based on the standard Infrared Spectroscopy.
Immunohistochemical assays were performed in formalin fixed, paraffin embedded sections. In immunohistochemical studies, we used mouse monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, California, sc-166907). Sections (4 µm) on glass slides were deparaffinized in Hemo-D (Fisher Scientific, Waltham, Massachusetts) and rehydrated in graded alcohol, followed by endogenous peroxidase block in 3 % H2O2 and antigen retrieval in boiling 10 % citrate buffer. Slides were incubated with monoclonal antibody against clusterin (1:200 dilution) overnight at 4 °C. They were then incubated with horseradish peroxidase-labeled dextran polymer coupled to anti-mouse antibody (EnVision™—System horseradish peroxidase) for 30 min at room temperature after 3 washes in tris-buffered saline-Tween 20 (Dako, Glostrup, Denmark, pH 7.6). Finally, slides were developed with diaminobenzidine for 10 min and counter stained with hematoxylin after 3 washes in tris-buffered saline-Tween20. Staining specificity was confirmed by processing sections from the same paraffin block and omitting the primary antibody as a negative control. As a positive control, we used reactions with renal cell carcinoma sections archived at the pathological department at our hospital. Cytoplasmic staining clearly distinguishable from the background was considered positive.
We evaluated at least 500 epithelial cells per area showing positive immunoreactivity in the kidney tissue of patients with nephrolithiasis and controls. We analyzed the percent of cells with 0-no, 1-weak, 2-moderate, and 3-intense staining by visual inspection under 400× magnification, and calculated a staining score using an overall H score with a range of 0–300. The scoring system accounted for staining intensity and the percent of cells showing clusterin staining. Clusterin expression was graded semiquantitatively according to staining score results. We also classified clusterin staining into over expression, defined as moderate or strong staining in any renal epithelium, and normal expression, defined as weak or negative clusterin staining. Analysis was done using an E-400 microscope (Nikon®) with a computer-aided image analysis system. Slides were evaluated twice at different times by 3 blinded investigators, and the mean was used for statistical analysis [29].
Madin-Darby canine kidney cells were purchased from the American type culture collection (USA), which were the MDCK cell line. The cells were grown at 37 °C in Dulbecco’s modified essential medium and F-12 (DMEM/F-12 at 1:1 ratio; Gibco BRL) medium containing 10 % fetal calf serum (FCS) in 75-cm2 corning flasks, 5 % CO2 air atmosphere.
The MDCK cells were seeded at 106/well in 6-well plates and adhering for 12 h, respectively, in turn the cells were placed overnight in a serum and sodium pyruvate-free media (Gibco BRL), hydrocortisone (Sigma), L-triiodothyronine (Sigma), and prostaglandin E1 (Sigma) to arrest the growth. Then the MDCK cells in each well of the 6-well plate were treated with different CaOx (Sigma) concentrations (300, 500, and 900 μg/ml) for 24 h. The activity concentration of CaOx of distal renal tubule in normal subjects ranged from 179 to 820 μg/ml; while a range of 128–974 μg/ml was observed in the kidney stone patients [30]. Therefore, we treated MDCK cells with concentration of 300–900 μg/ml, respectively. After that, all the cells were harvested for Western blot, while the supernatants were collected, respectively, for clusterin level assay by ELISA. The cells were treated with PBS as a negative control.
All cells were washed with ice-cold PBS twice, extracted, and solubilized with lysis buffer (50 mMTris-HCl, 150 mMNaCl, 5 mM EDTA, 1 mM PMSF, 1 % Triton X-100, 0.5 % sodium deoxycholate, 0.2 % SDS). Then protease inhibitors (50 μg/ml leupeptin, 10 μM pepstatin, 1 mM phenylmethylsulfonyl fluoride, and 20 μg/ml aprotinin) were added to the cell lysates and incubated for 1 h at 4 °C. Lysates were centrifuged at 12,000 rpm for 10 min. After the concentration of protein was determined, equal amounts of protein (10 µg/lane) were separated on a 12 % SDS–polyacrylamide gel and transferred to nitrocellulose membranes, using standard electroblotting procedures. The membranes were blocked with 5 % skim milk in Tris–Cl-buffered saline (TBS-T, 0.1 % Tween-20) at 4 °C overnight. Blots were incubated with primary antibodies of clusterin (1:800, Santa Cruz, sc-166907). Immunoblots were washed three times with 0.05 % Tween-20 in PBS, then incubated with the secondary antibody conjugated to anti-mouse (1:3000, Santa Cruz) at room temperature for 1 h, then subsequently processed for enhanced chemiluminescence (ECL) detection using Super Signal Substrate (Pierce). Signals were detected by chemiluminescence detection system (Bio-Rad, USA).
Statistics
We calculated descriptive statistics for each variable. Before the study, we examined each variable for its distributional characteristics. All data are shown as the mean ± SD. We compared variations in clusterin levels in the 2 groups, and differential renal clusterin expression in patients with renal stones and controls using the Student t test. Statistical analysis of these hypothesis tests was 2-sided with 2-tailed, p < 0.05 considered statistically significant. Calculation was made using SAS® 9.1.
Results
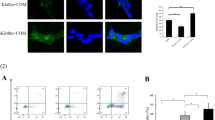
Madin-Darby canine kidney cells were analyzed by Western blotting with the antibody that detected the expression of clusterin (Fig. 1), after treatment with different concentrations of CaOx (300, 500, and 900 μg/ml) for 24 h. The results showed that CaOx activated the expression of clusterin. We found that the expression of clusterin in the cells exposed to CaOx was much higher than their negative controls.
After the MDCK cells were treated for 24 h, the supernatants of culture media were collected, respectively, and clusterin level in the supernatant was determined by ELISA. After treatment with different concentrations of CaOx 300, 500, and 900 μg/ml, the clusterin levels in the culture media were 241.33 ± 59.28 pg/ml, 247.67 ± 22.48 pg/ml, and 270.67 ± 43.50 pg/ml, respectively, which were much higher than the clusterin level (78.67 ± 39.88 pg/ml) in the culture media without CaOx treatment (p = 0.002).
A total of 49 patients 23–58 years old with kidney stones were included in this study and 41 non-kidney disease subjects 21–53 years old served as controls (Table 1). All calculi were composed of calcium oxalate monohydrate or calcium oxalate dihydrate. A few calculi also contained protein or uric acid (Fig. 2).
Stone Composition analysis by FTIR. Renal calculi were collected from kidney stone patients, ground and mixed with potassium bromide. Then the mixture was analyzed by infrared spectrometer (Spectrum 4000–800 cm−1). a Infrared spectrum of calcium oxalate monohydrate of the calculi; b infrared spectrum of calcium oxalate monohydrate and protein. Program copyright 1992 Bruker Analytische Messtechnik GmbH
We noted a predominantly cytoplasmic clusterin staining pattern in renal distal and collecting tubular epithelial cells. A total of 49 samples from patients with kidney stones and 41 autopsy samples were available for study. Clusterin immunoreactivity was normal (negative or weak) and over-expressed in 14 and 35 nephrolithiasis samples, and in 24 and 17 autopsy samples, respectively. The mean clusterin score in nephrolithiasis and autopsy samples was 223.04 ± 50.03 and 187.39 ± 50.55, respectively. The figure shows representative photomicrographs of clusterin expression in different kidney tissues (Fig. 3). We further analyzed clusterin staining scores in different kidney tissues using the Student's t test. Patients with kidney stones had a significantly higher mean clusterin score than controls (p = 0.001). Mean urine clusterin level was 17.47 ± 18.61 µg/ml in patients with kidney stones, and 3.31 ± 5.42 µg/ml in controls, respectively. There was a significant difference in urine clusterin in patients vs. non-kidney disease controls (p = 0.000006) (Table 1). Between two kidney clusterin expression score groups (>200 and ≤200), urine clusterin level was not significantly different. Also there was no significant difference in the urine clusterin level and kidney clusterin expression between the male patients and female patients (Table 2). In the kidney stone group, linear regression analysis indicated that urine clusterin is significantly associated with kidney clusterin expression (p < 0.0001).
The clusterin expression in the kidney tissues (400×). The clusterin was located in the cytoplasm of renal distal tubular epithelial cells (↑cytoplasmic staining of clusterin in renal tubular epithelial cell). a The clusterin expression in the kidney stone formers; b the clusterin expression in the controls; c quantitative analysis of immunohistochemistry (asterisks, p = 0.001; the error bars represent SD, generated as described in Materials and methods)
Discussion
In this study, we found that CaOx could induce the clusterin up-regulated expression in the MDCK renal tubular cells. In the human CaOx kidney stone formers, the clusterin was expressed in the renal distal tubular epithelial cells and the clusterin expression was much higher than the normal controls. Besides, the urine clusterin level was much higher than the controls.
Plaque of calcium salts deposited in the interstitial tissue plays an important role in the renal calculi formation [4]. High osmolality of tip of the renal papilla is almost 10-fold higher than the cortex, which could promote the inflammation, in turn inflammatory cytokines and proteins can accumulate, which would contribute renal tubular epithelial cell injury, and expose basement membrane [31]. The crystals adhered on the interstitium could keep growing, and could have some interaction with interstitium. In one vitro study, the researchers found that the renomedullary interstitial cells (RMIC) produced hyaluronan and expressed CD44 [32]. In another vivo study on interstitial nephritis of mouse, Sibalic et al. [33] found that hyaluronan, CD44, and osteopontin were up-regulated, which were well-known molecules-related formation of kidney stones. Also hyaluronan could interact with CD44, in turn stimulate the TNF-α expression and inflammation response of macrophages cells [34], which indicated that hyaluronan and CD44 could be involved in the inflammation process. Furthermore, in one study on the stone analysis, some inflammation-related protein including clusterin was found in the stone matrix [16]. However, there are few studies about the interstitial and stone-related molecule, which need to be investigated in future. And in our study, we found the clusterin expressed in the renal tubular cells, instead of interstitial cells, but also MDCK cells could express and secreted clusterin after treatment with CaOx. So we speculated crystals of Randall plaque could have some effect on the renal tubular cell, like as cell injury. Besides, crystals could induce the renal tubular cell injury. Khan et al. [35] concluded that crystal–cell communication is an essential element in the development of urinary stone disease, and renal tubular injury promotes crystal retention and the development of a stone nidus on the renal papillary surface. In addition, renal tubular injury enhances crystal nucleation at low supersaturation. And the injured renal tubular epithelial cells would develop necrosis, apoptosis, and regeneration [10], in which some cell injury related proteins expression would be up-regulated. Clusterin is one of the renal tubular injury-induced macromolecules, which had been proven as an apoptosis-related protein, expressed in kinds of renal disease, including nephrotoxicity and renal ischemia [25–27].
In vitro, our results had shown that the expression of clusterin was up-regulated after the MDCK cells were treated with CaOx, which indicated that CaOx could induce the renal tubular cells injured, and promoted the clusterin expression. Clusterin is known as one of the heterodimeric glycoprotein. Clusterin has been thought to be involved in many important biological processes, including apoptosis, cell–cell interaction, and tissue remodeling in response to the cytotoxic injuries or to the degenerative diseases [35, 36]. If the clusterin expression was deleted in the kidney, Zhou et al. [37] found that the renal tubular epithelial cells apoptosis increased significantly. Clusterin has the ability to promote renal epithelial cell communication, whereas the maintenance of cell–cell and cell-substratum contacts appears to be particularly relevant to acute renal injury. In the process of calculi formation, renal tubular cells could be injured by CaOx, and then clusterin expression could be initiated and play a protective or reparative role by enhancing cell interactions, and scavenging necrotic cell debris and toxic denaturated macromolecules. Some related results revealed that the clustein could inhibit the cells apoptosis via up-regulation of NF-κB pathway [38].
Previous studies had indicated that the expression of clusterin in the kidney is markedly induced in a variety of renal tubular injury states, which has been proved in many studies. Aiko Ishiii et al. established obstructed kidney model using the Wistar rat model, and they recommended that clusterin mRNA expression of the obstructed kidney was 60-fold higher than the controls. Furthermore, immunochemistry revealed that the clusterin expression was located in the renal tubular epithelial cells [24]. In another study of the Wistar rats with cisplatin treatment, the nephrotoxicity was induced, and clusterin was detected in the urine and up-regulated [39]. Vinken et al. [40] also suggested that urinary clusterin could be one of the most sensitive biomarkers for detection of cisplatin-induced kidney damage. They found that clusterin could be secreted in the urine and 13-fold increase in the urine at the day 5 of treatment with cisplatin, thence clusterin is a glycoprotein produced by renal tubules in response to injury. In our study, we found that the clusterin expression in the kidney tissue of the nephrolith patients was much higher than the controls. Furthermore, linear regression indicated that urine clusterin level was associated with clusterin expression in the distal renal tubular cells. So urinary clusterin levels could serve as a valuable biomarker for the severity of tubular damage in kidney stone formers. Besides, molecular size prevents filtration of clusterin (76–80 kDa) in the kidney, thus rendering its urinary levels specific to kidney injury. We speculated that the reciprocal effects of clusterin functions might contribute to the protection against renal tubular injury. The crystals could induce the injury of renal tubular cells, in turn the cells produce the clusterin to response the injury. Also, the clusterin was secreted or leaked in the urine by the renal tubular cells, so we found the urine clusterin level was increased.
There are some limitations in this study. There are two isoforms of clusterin, secreted clusterin and nuclear clusterin. Secreted form of clusterin was suggested to be a novel stress-induced heat shock protein (HSP) and an extra-cellular molecular chaperone, participating in clearing extra-cellular debris, while nuclear clusterin has been suggested to translocate into the nucleus of cells where it might act as a pro-death factor. Functions for these intracellular clusterin isoforms are still debated. In further study, we will discuss in detail the effect of different isoform clusterin in the renal tubular injury. More studies are needed to elucidate clearly the functions and signal pathway of clusterin in the renal tubular cells of kidney stone formers. In addition, whether there is some relation between the clusterin and some other stone-related molecules, like as Tamm-Horsfall protein, Osteopontin, and more experiments are needed to be performed to clarify.
In summary, CaOx could promote the clusterin expression in the renal tubular cells. Clusterin expression was up-regulated in the distal and collecting renal tubular cells, and urine clusterin level increased in the kidney stone formers.
References
Scales CD Jr, Smith AC, Hanley JM, Saigal CS (2012) Prevalence of kidney stones in the United States. Eur Urol 62:160–165
Stoller ML, Bolton DM (1995) Urinary stone disease. In: Tanagho EA, McAninch JW (eds) Smith’s general urology, 14th edn. Appleton and Lange, Los Altos, p 298
Randall A (1937) The origin and growth of renal calculi. Ann Surg 105:1009–1027
Randall A (1940) Papillary pathology as a precursor of primary renal calculus. J Urol 44:580
Evan AP, Lingeman JE, Coe FL, Parks JH, Bledsoe SB, Shao Y, Sommer AJ, Paterson RF, Kuo RL, Grynpas M (2003) Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest 111:607–616
Bagga HS, Chi T, Miller J, Stoller ML (2013) New insights into the pathogenesis of renal calculi. Urol Clin North Am 40:1–12
Khan SR (2014) Reactive oxygen species, inflammation and calcium oxalate nephrolithiasis. Transl Androl Urol 3:256–276
Kim JH, Kim JH, Jun HO, Yu YS, Min BH, Park KH, Kim KW (2010) Protective effect of clusterin from oxidative stress-induced apoptosis in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 51:561–566
Lieske JC, Leonard R, Swift H, Toback FG (1996) Adhesion of calcium oxalate monohydrate crystals to anionic sites on the surface of renal epithelial cells. Am J Physiol 270:F192–F199
Asselman M, Verkoelen CF (2002) Crystal-cell interaction in the pathogenesis of kidney stone disease. Curr Opin Urol 12:271–276
Verkoelen CF, van der Boom BG, Kok DJ, Houtsmuller AB, Visser P, Schröder FH, Romijn JC (1999) Cell type-specific acquired protection from crystal adherence by renal tubule cells in culture. Kidney Int 55:1426–1433
Verkoelen CF, van der Boom BG, Houtsmuller AB, Schroder FH, Romijn JC (1998) Increased calcium oxalate monohydrate crystal binding to injured renal tubular epithelial cells in culture. Am J Physiol 274:F958–F965
Khan SR, Glenton PA, Byer KJ (2006) Modeling of hyperoxaluric calcium oxalate nephrolithiasis: experimental induction of hyperoxaluria by hydroxy-L-proline. Kidney Int 70:914–923
Miyazawa K, Suzuki K, Ikeda R, Moriyama MT, Ueda Y, Katsuda S (2005) Apoptosis and its related genes in renal epithelial cells of the stone-forming rat. Urol Res 33:31–38
Asselman M, Verhulst A, De Broe ME, Verkoelen CF (2003) Calcium oxalate crystal adherence to hyaluronan-, osteopontin-, and CD44-expressing injured/regenerating tubular epithelial cells in rat kidneys. J Am Soc Nephrol 14:3155–3166
Canales BK, Anderson L, Higgins L, Slaton J, Roberts KP, Liu N, Monga M (2008) Second prize: comprehensive proteomic analysis of human calcium oxalate monohydrate kidney stone matrix. J Endourol 22:1161–1167
http://www.fda.gov/drugs/developmentapprovalprocess/drugdevelopmenttoolsqualificationprogram/ucm284076.htm. Accessed 8 April 2015
Falgarone G, Chiocchia G (2009) Chapter 8; Clusterin: a multifacet protein at the crossroad of inflammation and autoimmunity. Adv Cancer Res 104:139–170
Kujiraoka T, Takano M, Hattori H (2004) Apolipoprotein J (apo J). Nihon Rinsho Suppl 12:117–120
Jung GS, Kim MK, Jung YA, Kim HS, Park IS, Min BH, Lee KU, Kim JG, Park KG, Lee IK (2012) Clusterin attenuates the development of renal fibrosis. J Am Soc Nephrol 23:73–85
Harpur E, Ennulat D, Hoffman D, Betton G, Gautier JC, Riefke B, Bounous D, Schuster K, Beushausen S, Guffroy M, Shaw M, Lock E, Pettit S, HESI Committee on Biomarkers of Nephrotoxicity (2011) Biological qualification of biomarkers of chemical-induced renal toxicity in two strains of male rat. Toxicol Sci 122:235–252
Adiyanti SS, Loho T (2012) Acute Kidney Injury (AKI) biomarker. Acta Med Indones 44:246–255
Correa-Rotter R, Hostetter TH, Nath KA, Manivel JC, Rosenberg ME (1992) Interaction of complement and clusterin in renal injury. J Am Soc Nephrol 3:1172–1179
Ishii A, Sakai Y, Nakamura A (2007) Molecular pathological evaluation of clusterin in a rat model of unilateral ureteral obstruction as a possible biomarker of nephrotoxicity. Toxicol Pathol 35:376–382
Yoshida T, Kurella M, Beato F, Min H, Ingelfinger JR, Stears RL, Swinford RD, Gullans SR, Tang SS (2002) Monitoring changes in gene expression in renal ischemia-reperfusion in the rat. Kidney Int 61:1646–1654
Kharasch ED, Schroeder JL, Bammler T, Beyer R, Srinouanprachanh S (2006) Gene expression profiling of nephrotoxicity from the sevoflurane degradation product fluoromethyl-2,2-difluoro-1-(trifluoromethyl)vinyl ether (“compound A”) in rats. Toxicol Sci 90:419–431
Silkensen JR, Agarwal A, Nath KA, Manivel JC, Rosenberg ME (1997) Temporal induction of clusterin in cisplatin nephrotoxicity. J Am Soc Nephrol 8:302–305
Wang YP, Chen X, Zhang ZK, Cui HY, Wang P, Wang Y (2014) Effects of a restricted fetal growth environment on human kidney morphology, cell apoptosis and gene expression. J Renin Angiotensin Aldosterone Syst. 1470320314543808, (Epub ahead of print)
Li JY, Zhou T, Gao X, Xu C, Sun Y, Peng Y, Chang Z, Zhang Y, Jiang J, Wang L, Hou J (2010) Testosterone and androgen receptor in human nephrolithiasis. J Urol 184:2360–2363
Liu Z, Wang T, Yang J, Wang S, Yang W, Liu J, Ye Z (2012) Calcium oxalate monohydrate crystals stimulate monocyte chemoattractant protein-1 and transforming growth factor beta1 expression in human renal epithelial cells. Mol Med Rep 5:1241–1244
Chi T, Miller J, Stoller ML (2012) Randall plaque versus renal stone? Transl Androl Urol 1:66–70
Göransson V, Hansell P, Moss S, Alcorn D, Johnsson C, Hällgren R, Maric C (2001) Renomedullary interstitial cells in culture; the osmolality and oxygen tension influence the extracellular amounts of hyaluronan and cellular expression of CD44. Matrix Biol 20:129–136
Sibalic V, Fan X, Loffing J, Wüthrich RP (1997) Upregulated renal tubular CD44, hyaluronan, and osteopontin in kdkd mice with interstitial nephritis. Nephrol Dial Transpl 12:1344–1353
Lake FR, Noble PW, Henson PM, Riches DW (1994) Functional switching of macrophage responses to tumor necrosis factor-alpha (TNF alpha) by interferons. Implications for the pleiotropic activities of TNF alpha. J Clin Invest 93:1661–1669
Khan SR (2006) Renal tubular damage/dysfunction: key to the formation of kidney stones. Urol Res 34:86–91
Rosenberg ME, Silkensen J (1995) Clusterin: physiologic and pathophysiologic considerations. Int J Biochem Cell Biol 27:633–645
Zhou W, Guan Q, Kwan CC, Chen H, Gleave ME, Nguan CY, Du C (2010) Loss of clusterin expression worsens renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 298:F568–F578
Zoubeidi A, Ettinger S, Beraldi E, Hadaschik B, Zardan A, Klomp LW, Nelson CC, Rennie PS, Gleave ME (2010) Clusterin facilitates COMMD1 and I-kappaB degradation to enhance NF-kappaB activity in prostate cancer cells. Mol Cancer Res 8:119–130
Gautier JC, Riefke B, Walter J, Kurth P, Mylecraine L, Guilpin V, Barlow N, Gury T, Hoffman D, Ennulat D, Schuster K, Harpur E, Pettit S (2010) Evaluation of novel biomarkers of nephrotoxicity in two strains of rat treated with Cisplatin. Toxicol Pathol 38:943–956
Vinken P, Starckx S, Barale-Thomas E, Looszova A, Sonee M, Goeminne N, Versmissen L, Buyens K, Lampo A (2012) Tissue Kim-1 and urinary clusterin as early indicators of cisplatin-induced acute kidney injury in rats. Toxicol Pathol 40:1049–1062
Conflict of interest
Author Jinyi Li has submitted and presented the part of this study in 2013 in the American Urologist Association Annual Conference. The other authors declare that they have no conflict of interest.
Funding
This study was funded by Key Technology Research and Development Program of Hebei Province, China, 2014 (grant number 14277774D).
Author information
Authors and Affiliations
Corresponding author
Additional information
J.-Y. Li and J. Liu are equal study contribution.
Rights and permissions
About this article
Cite this article
Li, JY., Liu, J., Jiang, J. et al. Calcium oxalate calculi-induced clusterin expression in kidney. Urolithiasis 43, 411–418 (2015). https://doi.org/10.1007/s00240-015-0785-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-015-0785-1