Abstract
Purpose
The cranial epidural space (ES) is a potential space and is not generally recognized unless there is underlying pathology. With MRI in newborns, we have frequently observed T2 hyperintense thickening of the ES posterior to the confluence of sinuses, also referred to as “torcular pseudomass” (TP). We aim to identify the frequency of TP and possible associations with delivery.
Methods
Retrospectively, brain MRIs of 194 neonates obtained within the first 2 weeks of life were evaluated. If TP was present, imaging characteristics and thickness were assessed by two observers, using fat-suppressed T2WI/FLAIR, T1WI, and SWI. Exclusion criteria were motion artifact, lack of sagittal T2WI, and lack of clinical data. Medical records were evaluated for demographic and clinical data. Follow-up exams were evaluated if available. Patients with TP and without were compared using Student t and chi-square tests.
Results
TP was present in 64/158 (40%). No difference was found between the groups regarding sex, gestational age, birth weight, delivery type, fetal presentation during delivery, birth difficulty, and neurological sequelae (p > 0.05). Eight patients with TP underwent follow-up imaging, and in 6/8, TP completely resolved. Two patients showed persistent TP, improving from 3.2 to 1 mm in one child and from 3.2 to 2.8 mm in the other within a week.
Conclusion
TP frequently occurs in early newborns. TP does not appear to be associated with factors related to delivery, shows complete resolution in most cases with a follow-up, and is likely of no clinical importance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The brain is covered by several layers, from outside to inside, the scalp, subcutaneous fat tissues, galea aponeurotica, periosteum of the external table of the calvarium, calvaria proper, periosteum of the inner table of the calvarium, and three layers of meninges: dura mater, arachnoid mater, and pia mater. The outermost dura mater has two layers: the inner meningeal and the outer endosteal layers. The main functions of these meninges are to support and protect the brain. In addition, they form potential spaces in the cranial vault. The outer endosteal layer of the dura mater is tightly attached to the periosteum at sutures and at the base of the skull. Due to these tight connections, the epidural space (ES) is a potential space and appears radiologically visible only on pathological conditions as seen in trauma in contrast to the ES in the spinal canal, which contains primarily fat and vascular structures [1,2,3,4,5,6].
We have observed that a substantial number of early neonates who underwent MRI had epidural lesions at the midline, posterior to confluence of sinuses, demonstrating T1 isointensity, T2 hyperintensity, and hyperintense signal on SWI sequence, suggesting proteinaceous fluid or soft tissue lesion rather than a hematoma. These patients were mostly referred to MRI due to hypoxic-ischemic encephalopathy. Other clinical conditions that required MRI exam were hyper/hypotonia, weak or absent neonatal reflexes, seizure or seizure-like activity, apnea, and antenatal or postnatal ultrasound findings such as ventriculomegaly, hemorrhage, corpus callosum agenesis, and physical examination findings that may be related to congenital anomalies or syndromes. As these epidural lesions described above were unexpected, from time to time an attention was raised for evaluation of these lesions in the possible future follow-up exams. To our knowledge, this finding was first described in 2017 by Sampaio et al., who called this radiologic finding “torcular pseudomass” (TP) and thought that it represented “physiologic soft tissue” [7]. As we observed this finding in many infants imaged after a difficult delivery, we suspected that the finding might be related to the process of birth. Therefore, we undertook this study to report the frequency of TP in newborns and ascertain whether TP might be due to labor-related forces on the skull.
Methods
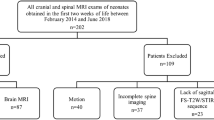
The institutional review board approved this retrospective study. A total of 194 cranial MRI exams of the neonates obtained within 2 weeks of life between February 2014 and June 2018 were detected from our university hospital PACS. All images were acquired with either 3-T Skyra or 1.5-T Aera MRI machines (Siemens, Erlangen, Germany). Our routine infant brain MRI protocol contains a large field-of-view, fat-suppressed sagittal T2W (slice thickness: 2 mm), axial fat-suppressed T2 and FLAIR (slice thickness: 2.5 mm), sagittal 3D-SPACE T1W (slice thickness: 0.9 mm), axial DWI, and axial susceptibility-weighted imaging (SWI). A pediatric neuroradiologist made an initial evaluation and excluded 36 patients due to several reasons: motion (11), lack of sagittal T2W sequence (21), and lack of adequate clinical data (4). A total of 158 MRI exams were included for analysis after exclusion (Fig. 1).
All included exams were reviewed by a radiology resident (AHC) and a fellowship-trained pediatric neuroradiologist (CO). The resident first evaluated the images and acquired the measurements. Subsequently, the pediatric neuroradiologist performed a second session and confirmed or corrected the acquired radiological data. Fat-suppressed axial/sagittal T2W and fat-suppressed axial FLAIR were used to evaluate the presence of TP (categorized into two groups: TP-present vs. TP-absent). If TP was found, the maximum anterior–posterior dimension was measured in the best-seen sequence either in the axial or in the sagittal plane, from the posterior margin of the sinus confluence to the anterior margin of the inner table of the bone. The signal properties of this region were also evaluated using FLAIR (categorized into two groups: suppressed vs. non-suppressed), 3D T1W (categorized into three groups: isointense with cerebrospinal fluid (CSF), mildly more hyperintense than CSF, markedly hyperintense like-blood product), and SWI (categorized into two groups: dark: positive vs. isointense: negative). Additional imaging findings were evaluated, such as the presence of cortical molding and extracranial fluid collection, such as subgaleal edema/hematoma or caput succedaneum (categorized into two groups: present vs. absent). Spinal imaging findings such as the presence of spinal extradural T2 hyperintense thickening and spinal ligament edema were also recorded. Follow-up MRI examinations were evaluated if available to assess the change of TP in time.
Patients’ electronic medical records were evaluated for the following variables by a research fellow and a medical student who were blinded to the MRI findings: patients’ gender, birth date, MRI date, gestational age, categorical gestational age (pre-term, term, post-term), birth weight, categorical birth weight (small for gestational age (SGA), appropriate for gestational age (AGA), large for gestational age (LGA)), the birth method (cesarean section (C/S), normal spontaneous vaginal delivery (NSVD)), the presentation of the fetus on delivery (cranial, breech, transverse, face), presence of difficult delivery per obstetric record, 1-min Apgar score, 5-min Apgar score, neurologic status after birth, and follow-up neurologic status. Delivery was considered “difficult” under many variable circumstances without the possibility of categorization, for instance, any condition which leads to emergent C/S such as placental abruption and failure to descend, in the setting of elective C/S conditions such as placenta previa and breech presentation, or in the setting of NSVD conditions such as vacuum-assisted delivery, failure to descend, chorioamnionitis, and underlying congenital malformation. Two groups, patients with TP and without, were compared. The Student t test is used for numeric variables and the chi-square test is used for categorical values.
Results
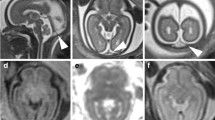
A total of 158 newborns were included in the study. Ninety-two patients (58.2%) were male, 111 patients (71.2%) were full-term with a mean gestational age of 38.3, and 119 patients (79.3%) were AGA. TP was present in 64 (40.5%). In all of those 64 cases, there was mild corresponding T1 hyperintensity—slightly brighter than the CSF, but not as bright as a hemorrhage would appear—and there was no dark signal on SWI to suggest a hemorrhage but favoring edema. The mean thickness of the TP was 3.6 mm (1.3–11.6 mm). A few examples with TP are shown in Figs. 2, 3, and 4.
One-day-old female with a gestational age of 38 weeks, born via normal spontaneous vaginal delivery. There was no history of difficult delivery or complication, but baby had low APGAR scores of 4 and 6, respectively. In the follow-up, the tone was low and the patient developed seizures which prompted MRI. Diffuse subcutaneous edema in the scalp (black asterisk). Arrows point out the torcular pseudomass which is slightly more hyperintense than the CSF on FLAIR and T1 with no evidence of hemorrhage on SWI. Note that patient was found to have symmetrical diffusion restriction in the lenticular nuclei (dashed arrows) and bilateral perirolandic cortex (thin dashed arrows), which confirmed hypoxic-ischemic encephalopathy. This patient also represents copresence of torcular pseudomass and posterior spinal epidural space edema (curved arrow). a Sagittal fat-saturated T2. b Axial fat-saturated FLAIR. c Axial T2. d Axial T1. e Axial SWI. f, g DWI
One-day-old newborn with gestational age of 36 weeks. Born via C/S due to decreased fetal heart rate. Arrows show abnormal T2 hyperintense collection posterior to sinus confluence slightly hyperintense than the CSF on FLAIR with no evidence of hemorrhage on corresponding SWI. a Sagittal fat-saturated T2. b Axial fat-saturated FLAIR. c Axial SWI
Seven-day-old term newborn with a gestational age of 39 weeks, born via planned c-section due to transverse fetal presentation, without complication. APGAR scores were 9/9, respectively. Mother was diabetic. Again, there was a sizeable epidural space-occupying lesion posterior to sinus confluence (arrows). Note that this resolved in the 3-year follow-up MRI. a Axial SWI. b Axial fat-saturated FLAIR. c Sagittal fat-saturated T2. d Sagittal fat-saturated T2, 3 years later
The two groups showed no significant difference in sex, gestational age, categorical gestational age, birth weight, categorical birth weight, the presentation of the fetus on delivery, or birth method (p > 0.05). There was also no significant difference regarding difficult delivery (p = 0.876). Both groups had low APGAR scores, and the “TP-present” group had a significantly lower mean 5-min APGAR score (p = 0.011). The mean 1-min APGAR score was also lower in the “TP-present” group although not statistically significant (p = 0.052) (Table 1).
The majority of the patients had abnormal neurologic status, but there was no statistical difference between the two groups (Table 1). The most common neurologic problems in the “TP-present” and “TP-absent” groups were hypoxic-ischemic encephalopathy (28/27), seizure (16/13), and hypotonia (9/12). In the follow-up, six patients died, and 14 had neurologic sequelae in the “TP-present” group. The most common sequelae were seizure (6) and abnormal muscle tone (3), either hypertonia or hypotonia. In the “TP-absent” group, eight patients died, and 15 patients had sequelae such as seizure (3), cerebral palsy (2), abnormal tone (4), and developmental delay (4). There was no statistical difference between the two groups regarding neurologic sequela in the follow-up (p = 0.305).
The interval between birth and MRI showed a statistically significant difference among the groups. The mean MRIs were 1.6 days earlier in the “TP-present” group than in the “TP-absent” group (Table 1), although the presence of this difference is of unknown clinical or radiologic significance.
Many of the patients had one or more intracranial/extracranial pathologies on MRI; of the 158 patients, 92 (58.2%) were normal on imaging (“TP-Present” group/ “TP-absent” group 60/32, 46.8%/20.2%, p = 0.178). There were various pathologic MRI findings, sorting these findings into several categories in the “TP-present” and “TP-absent” groups: hypoxic-ischemic encephalopathy (12/16), infarct (6/3), subdural hematoma (4/6), congenital anomaly (4/13), and others (parenchymal hemorrhage, hydrocephalus, tumor, etc.). Accompanying cranial molding and extracranial subcutaneous edema/caput succedaneum were more frequent in the “TP-present” group (p < 0.001) (Table 2). Visualized parts of the cervical and upper thoracic spine within the field-of-view were also evaluated and compared. There was statistically significant accompanying “T2 hyperintense thickening of the posterior cervical and upper thoracic spine ES” and spinous ligamentous edema in the “TP-present” group (p < 0.001) (Table 2).
A total of 37 patients had follow-up imaging (TP-present/TP-absent: 8/29, time 5–554 days). Six/8 patients with initial TP showed complete resolution. Two patients showed persistent TP, improving from 3.2 to 1 mm in one child and from 3.2 to 2.8 mm in the other within a week.
Discussion
To our knowledge, Sampaio et al. described this incidental imaging finding for the first time in the literature in 2017 and defined this entity as “torcular pseudomass” [7]. We were not aware of the study by Sampaio et al. until we started writing this manuscript. Sampaio et al. investigated a total of 2283 children under the age of 3 with a TP present in 291 (12.7%). The median age was 4 months, 73.6% were younger than 1-year-old, and 39.9% were between 0 and 2 months of age. In our study, the frequency of TP in imaged newborns was also found to be 40%, supporting their analysis. They reported that incidental TP either disappeared or regressed in follow-up imaging, similar to our study. In addition, Sampaio et al. reported that TP showed enhancement. Contrast agent was given to 65 patients aged 0–2 months, 24 patients aged 3–12 months, and eight patients aged 13–24 months [7]. In our study, considering the patient’s age, contrast agent was almost never administered routinely in neonates unless there was suspicion of a space-occupying tumoral lesion or other pathologies requiring contrast agent. Therefore, we did not assess the contrast enhancement of TP in our study.
TP is not a familiar finding that we can easily relate to some well-known factors. Perhaps, this could be a result of an insult to the skull during delivery. Assuming this was the case, we hypothesized that NSVD might have higher rates of TP because traumatization of neonates would be more likely to occur, and lower rates of TP might be seen with C/S since this would be the least traumatic. However, our analysis failed to show any significant higher rate of TP in babies with NSVD. The delivery itself could be complicated and traumatic for neonates, not only from the delivery type but also other numerous factors. We grouped these multiple factors under an umbrella term as “difficult delivery.” Again, the statistical analysis showed no significant difference in terms of presence and absence of difficult delivery. Fetal presentation on delivery, another complicating factor of delivery, was not significantly different between the groups. It is plausible to think that gestational age and weight might impact this finding since it is well known that pre-term or SGA newborns have higher morbidity and mortality rates than other neonates [8, 9]. We also expected higher rates of TP with LGA babies since they would get more likely entrapped in the birth canal during birth. However, there was no statistically significant difference between the two groups in terms of gestational age and weight.
We found significantly more cranial molding and extracranial fluid collection in the “TP-present” group. Additionally, interspinous ligament edema and edematous thickening of the posterior spinal ES in the cervical and thoracic spine were significantly higher in the “TP-present” group. We investigated these factors, which might support that a trauma-related process may cause TP in these patients. However, our findings lack statistical evidence. Notably, we have previously shown that posterior ES edema in the cervical and upper thoracic spine is a common, reversible finding in newborns, requires no treatment or follow-up, and is not associated with the delivery method [10].
Sampaio et al. suggested that the mechanisms causing TP could be related to embryological developmental pathways [7]. The hypotheses they developed regarding this finding were that it could be associated with the residual connective tissue due to delayed ossification, the periosteal layer of the dura rich in extracellular components, or the involved inner surface of the occiput [7, 10,11,12]. As we described in detail, we hypothesized TP was related to a difficult birth, birth method, or birth-related trauma, but our results failed to support this. Given that Sampaio et al. identified TP not only in early neonates but also in older babies and toddlers, this area likely represents a developmental process and, more likely, a potential loose epidural space. As we speculated in our prior investigation related to posterior spinal epidural space edema (PSES edema) in newborns, PSES edema might be more related to being in prolonged cervical flexion position in the fluid-filled intrauterine space with entrapment of the edema in the stretched posterior ES [13]. We found a statistically significant increased rate of PSES edema in infants with TP, and on imaging, these potential spaces are contiguous with each other. Therefore, this potential ES can be more likely to be captured on MRI in early newborns. Although we did not assess the contrast enhancement in our study, the enhancement of TP as reported by Sampaio et al. could be explained in the setting of loose epidural space theory, as similar widening and enhancement of the ES and adjacent meninges are expected after ventricular shunting and related intracranial hypotension, secondary to lack of blood–brain barrier in these structures.
Regardless of the cause, TP may mimic an epidural hematoma or other space-occupying organic lesions in newborns. It was entirely or almost entirely resolved in the follow-up exams in a limited number of cases in our series. This feature aligns with the study by Sampaio et al. In addition, lack of aggressive features and dark signal on SWI was suggestive of a benign incidental imaging finding. We found that a considerable number of our patients had neurological sequela clinically in the follow-up. However, there was no significant difference between the groups with TP and without TP. Hence, there is no evidence suggesting a worse prognosis with neonates who are positive with this finding. It is likely of no clinical significance and it resolves or regresses in follow-up in neonates.
The limitations of the study are as follows. First, this is a retrospective study. The reliability of the data depends on the accuracy of the electronic medical records and how the provider had recorded the clinical data. Second, the number of patients with follow-up is low. This is mostly secondary to referring clinician’s decision, as many of these infants did not require a follow-up with MRI based on the patients’ current neurologic status and secondly, torcular pseudomass was an underrecognized entity among radiologists in our institution and even when it was recognized, the radiologists thought that this was most likely an incidental unknown benign entity which does not require a definite follow-up MRI based on ALARA principle. Third, the study population is not consisting of entire neonates within the first 2 weeks of life but only those who were referred to MRI for a certain reason. Therefore, the study population contains mostly sick babies with selection bias. Nevertheless, this is an expected limitation and in practice, MRI is not a routine test for every newborn and is only applied in sick babies within 2 weeks of life; hence, a prospective study including normal infants in this kind of exam is practically impossible considering the ALARA principles. Regardless, this is the second study evaluating the presence of TP in neonates, and the first study searching for any possible associations with delivery and outcomes. We think our results are a good resource for the radiologists encountering this similar incidental finding in their routine practice and a guide for future researchers, as we did not find a definite relationship with the birth.
Conclusion
Torcular pseudomass or epidural T2 hyperintense thickening posterior to sinus confluence is a frequently seen MRI finding in newborns within the first 2 weeks of life. We conclude that there is no evidence suggesting a worse prognosis with neonates who have TP. It either resolves or regresses during follow-up in cases with follow-up. Therefore, it might be feasible to suggest that this is a “do not touch” lesion, not requiring further treatment or follow-up.
Data availability
Data and material are available if requested.
Code availability
None.
Abbreviations
- ES:
-
Epidural space
- TP:
-
Torcular pseudomass
- MRI:
-
Magnetic resonance imaging
- C/S:
-
Cesarean section
- NSVD:
-
Normal spontaneous vaginal delivery
- NST:
-
Non-stress test
- SGA:
-
Small for gestational age
- AGA:
-
Appropriate for gestational age
- LGA:
-
Large for gestational age
- CSF:
-
Cerebrospinal fluid
References
Schuenke M, Schulte E, Schumacher U (2010) Thieme atlas of anatomy: head and neuroanatomy. Thieme, New York
Sharman AM, Kirmi O, Anslow P (2009) Imaging of the skin, subcutis, and galea aponeurotica. Semin Ultrasound CT MRI 30:452–464. https://doi.org/10.1053/j.sult.2009.08.001
Hayman AL, Shukla V, Ly C, Taber KH (2003) Clinical and imaging anatomy of the scalp. J Comput Assist Tomogr 27:454–459. https://doi.org/10.1097/00004728-200305000-00027
Greenberg RW, Lane EL, Cinnamon J, Farmer P, Hyman RA (1994) The cranial meninges: anatomic considerations. Semin Ultrasound CT MRI 15:454–465. https://doi.org/10.1016/S0887-2171(05)80017-4
Westbrook JL (2012) Anatomy of the epidural space. Anaesth Intensive Care Med 13:551–554. https://doi.org/10.1016/j.mpaic.2012.08.020
Kemp WJ 3rd, Tubbs RS, Cohen-Gadol AA (2012) The innervation of the cranial dura mater: neurosurgical case correlates and a review of the literature. World Neurosurg 78:505–510. https://doi.org/10.1016/j.wneu.2011.10.045
Sampaio L, Morana G, Severino M, Tortora D, Leão M, Rossi A (2017) Torcular pseudomass: a potential diagnostic pitfall in infants and young children. Pediatr Radiol 47:227–234. https://doi.org/10.1007/s00247-016-3734-4
Xu F, Kong X, Duan S, Lv H, Li Z, Zeng S et al (2019) Care practices, morbidity and mortality of preterm neonates in China, 2013–2014: a Retrospective study. Scie Rep 9:19863. https://doi.org/10.1038/s41598-019-56101-x
American College of Obstetricians and Gynecologists (2013) ACOG Practice bulletin no 134 fetal growth restriction. Obstet Gynecol 121:1122. https://doi.org/10.1097/01.AOG.0000429658.85846.f9
Adeeb N, Mortazavi MM, Tubbs RS, Cohen-Gadol AA (2012) The cranial dura mater: a review of its history, embryology, and anatomy. Childs Nerv Syst 28:827–837. https://doi.org/10.1007/s00381-012-1744-6
Nabeshima S, Reese TS, Landis DM, Brightman MW (1975) Junctions in the meninges and marginal glia. J Comp Neurol 164:127–169. https://doi.org/10.1002/cne.901640202
Mowbray K (2005) Surface bone histology of the occipital bone in humans and chimpanzees. Anat Rec B New Anat 283:14–22. https://doi.org/10.1002/ar.b.20055
Ceylan AH, Özütemiz C, Huang H, Luedemann C, Rubin N, Nascene DR (2021) A common yet undescribed MRI finding in newborns: posterior epidural space edema of the cervical and upper thoracic spine. Neuroradiology 18:1–9. https://doi.org/10.1007/s00234-021-02786-9
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the University of Minnesota Institutional Review Board (IRB #: STUDY00001646). For this type of study, formal consent is not required.
Consent to participate
This study was approved by the University of Minnesota Institutional Review Board (IRB #: STUDY00001646). For this type of study, formal consent is not required and the article is written in HIPAA-compliant fashion. Patients who opted out for research were not included.
Conflict of interest
David Nascene: Consultant for Biogen and World Care Clinical.
Nathan Rubin: Research reported in this publication was supported by NIH grant P30CA077598 utilizing the Biostatistics and Bioinformatics Core shared resource of the Masonic Cancer Center, University of Minnesota, and by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1-TR002494. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The remainder of the authors declares that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The preliminary data of this manuscript was presented as an electronic poster in the 58th annual meeting of the American Society of Neuroradiology in 2020, with the title of “T2 HYPERINTENSE EXTRADURAL THICKENING POSTERIOR TO CONFLUENCE OF SINUSES IN NEWBORNS” by Arda H. Ceylan.
Rights and permissions
About this article
Cite this article
Ceylan, A.H., Nascene, D.R., Huang, H. et al. Torcular pseudomass in newborns and its association with delivery: follow up or leave it alone?. Neuroradiology 64, 2069–2076 (2022). https://doi.org/10.1007/s00234-022-02981-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-022-02981-2