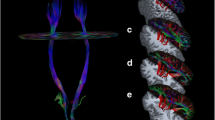
Abstract
Purpose
The ipsilateral hand (ILH) is impaired after unilateral stroke, but the underlying mechanisms remain unresolved. Based on the degeneracy theory of network connectivity that many connectivity patterns are functionally equivalent, we hypothesized that ILH impairment would result from the summation of microstructural white matter (WM) disruption in the motor network, with a task-related profile. We aimed to determine the WM disruption patterns associated with ILH impairment.
Methods
This was a cross-sectional analysis of patients in the ISIS-HERMES Study with ILH and diffusion-MRI data collected 1 month post-stroke. Patients performed three tasks, the Purdue Pegboard Test (PPT), handgrip strength, and movement time. Fractional anisotropy (FA) derived from diffusion MRI was measured in 33 WM regions. We used linear regression models controlling for age, sex, and education to determine WM regions associated with ILH impairment.
Results
PPT was impaired in 42%, grip in 59%, and movement time in 24% of 29 included patients (mean age, 51.9 ± 10.5 years; 21 men). PPT was predicted by ipsilesional corticospinal tract (i-CST) (B = 17.95; p = 0.002) and superior longitudinal Fasciculus (i-SLF) (B = 20.52; p = 0.008); handgrip by i-CST (B = 109.58; p = 0.016) and contralesional anterior corona radiata (B = 42.69; p = 0.039); and movement time by the corpus callosum (B = − 1810.03; p = 0.003) i-SLF (B = − 917.45; p = 0.015), contralesional pons-CST (B = 1744.31; p = 0.016), and i-corticoreticulospinal pathway (B = − 380.54; p = 0.037).
Conclusion
ILH impairment was associated with WM disruption to a combination of ipsilateral and contralesional tracts with a pattern influenced by task-related processes, supporting the degeneracy theory. We propose to integrate ILH assessment in rehabilitation programs and treatment interventions such as neuromodulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hand movements represent a specific and essential function in humans required for everyday life activities. Following stroke, loss of hand functionality is one of the main factors affecting disability and remains a major target of rehabilitation interventions [1, 2]. Parallel to sensorimotor deficits of the paretic hand contralateral to the lesion, the ipsilateral hand (ILH) may show sensorimotor deficits for a large variety of sensorimotor tasks from the acute to chronic periods of stroke [3,4,5,6,7,8]. ILH impairment is frequent in subacute stroke [8] and may compound functional disability since patients require both hands to perform daily life activities [9].
Although several mechanisms have been postulated to account for post-stroke ILH deficits, no consensus has been reached and many aspects remain unresolved [10]. Anatomically, ILH impairment may result from ipsilateral descending motor pathways (i.e., fibers emerging from the damaged hemisphere and descending in the spinal cord without decussating), transcallosal fibers interacting with the undamaged hemisphere, and altered sensorimotor information through fibers crossing in the brainstem such as cerebellar peduncles. Accordingly, a first theory implicates the “uncrossed” ipsilateral corticospinal tract (CST) [11], as 3–15% of the corticospinal fibers descend in the ipsilateral spinal lateral funiculus without decussating in the medullary pyramids [12, 13] (Fig. 1). ILH impairment may also relate to the cerebellar peduncles via the fronto-cerebellar loops and the corticoreticulospinal pathway (CRP) that has bilateral spinal outputs. Another theory relies on bilateral parietal hemispheric control of unilateral movements [6, 10, 14, 15]. The lateralization of motor control has been recently revisited to enhance the role of the contralesional hemisphere, based on increased contralesional activity in the sensorimotor network [16,17,18], and bilateral motor control supported by the posterior parietal cortex [19]. ILH impairment may also relate to interhemispheric transcallosal disconnections [20] as the corpus callosum (CC) coordinates motor function through the balance of excitatory and inhibitory interhemispheric interactions [14]. Finally, the impact of neuropsychological deficits such as apraxia and neglect has also been reported as a factor of ILH impairment [21,22,23].
Schematic representation of crossed and uncrossed fibers of the corticospinal tract (CST). ACR and ALIC fibers emerging from the SMA and PMC and SCR-PLIC fibers emerging from PMC and MI merge in the CP to form the CST, which continue in the pons and medulla. Then, the CST is divided into 2 parts. (1) Crossed CST (solid red and blue lines): most CST fibers decussate in the medullar pyramids to descend in the contralateral spinal cord and terminate in the contralateral anterior spinal horn to distal extremity muscles (direct cortico-motoneurons). (2) Uncrossed CST (dotted red and blue lines): a small proportion of the CST descends in the ipsilateral spinal lateral funiculus without decussating and terminates bilaterally in the ventromedial intermediate zone to propriospinal neurons. In addition, information is shared between the ipsilesional and contralesional hemispheres through transcallosal fibers (CC, dashed dark lines), before travelling through the CST. Abbreviations: CST = corticospinal tract, SCR = superior corona radiata; PLIC = posterior limb of internal capsule, CP = cerebral peduncle. Other ROIs are ACR = anterior corona radiata; ALIC = anterior limb of internal capsule; CC = corpus callosum; RF = reticular formation; PN = propriospinal neurons; MN = motoneurons; i = ipsilesional; and c = contralesional tracts
Such a large variety of mechanisms can be put in perspective with the theory about degeneracy of the connectivity in neural networks [24] proposing that many patterns of neural architecture are functionally equivalent. Indeed, the connectivity pattern for a specified task arises during development in part by a process involving exuberant extension of neuronal processes that compete for targets. In this view, degenerate mechanisms would allow for sensorimotor plasticity and behavioral adaptation [25].
Based on the degeneracy theory, we hypothesized that ILH impairment would result from the summation of several disruptions to the sensorimotor network, with a pattern determined by the motor and visuomotor processes engaged in the ILH tasks. Since diffusion MRI provides reliable measures of white matter microstructure such as fractional anisotropy (FA) reflecting the neural changes related to the stroke lesion and its remote effects [26,27,28,29,30], we aimed to determine microstructure white matter (WM) disruption patterns associated with ILH impairment. WM disruption was assessed with FA measures in the motor WM network and ILH with three behavioral tasks engaging distinct motor and visuomotor processes. To this extent, we performed a cross-sectional analysis of patients in the ISIS-HERMES Study [31] with concomitant ILH and FA measures collected 1 month post-stroke.
Materials and methods
Participants
Patients
We enrolled 31 patients in the randomized controlled stem cell trial (ISIS-HERMES) at the stroke unit of the hospital from October 2010 to 2014 (ClinicalTrials.gov NCT00875654) [31]. In this study, we used the clinical, behavioral, and MRI data collected 1 month post-stroke, corresponding to the baseline visit performed before cell therapy administration. Patients received standard medical care including thrombolysis and thrombectomy when indicated. The ISIS-HERMES study was approved by the Institutional Review Board (CPP: 07-CHUG-25). Written informed consent was obtained from all patients before they participated in the study. The main inclusion criteria were age 18–70 years, first-ever unilateral infarct in the internal carotid artery territory, moderate to severe neurological deficit defined as a NIH stroke scale (NIHSS) [32] score ≥ 7, and the ability to follow a rehabilitation program. In addition to the inclusion–exclusion criteria listed in Table S1, we excluded patients with apraxia, or neglect diagnosed with an extinction NIHSS subscore > 1.
Healthy participants
In addition, we included 31 healthy participants matched for age (± 5 years) and sex to the patients. Exclusion criteria are listed in Table S1.
Demographic and clinical measures
Age, sex, education level, handedness [33], height, weight, and stroke risk factors were collected. Of note, all participants were right-handed. Neurological severity was assessed using NIHSS and sensorimotor deficit using the Fugl-Meyer Score (FMS) [34], with upper limb motor, sensory, and coordination subscores (Table 1). A global cognitive assessment was performed with the Mini-Mental State Exam and the RBANS, exploring five domains (spatial, attention/executive, immediate and delayed memory, language) [35]. Assessments were performed by a stroke neurologist (NIHSS, neuropsychologists (RBANS, behavioral measures), and physiotherapists (FMS).
Behavioral measures
We explored ILH impairment using three behavioral tests. The Purdue Pegboard Test (PPT) (Lafayette Instrument Company, Indiana) [36] was performed as described in http://www.equipement-ergotherapie.com/8-dexterité-manipulation.html, as a standardized quantitative test requiring motor (for grasping) and visuomotor (for reaching) components. The hand dynamometer (Lafayette Instrument Company, Indiana; https://www.prohealthcareproducts.com/100-kg-220lb-hand-grip-dynamometer-lafayette-instruments/) is a validated test to measure handgrip force (Grip). The Motor Screening Task (MST) measures movement time to assess sensorimotor deficits in CANTAB (https://www.cambridgecognition.com/cantab/cognitive-tests/attention/motor-screening-task-mot/). Raw scores were converted to percentiles to adjust for age and sex using published norms [37] and CANTAB norms. Scores below the 5th percentile were considered as impaired. The frequency of ILH impairment was also assessed in patients without cognitive deficit, defined as RBANS > 40.
MRI data acquisition
The MRI protocol included structural and diffusion sequences. All participants were scanned on a 3 T Philips magnet (Achieva 3.0 TTX; Philips, the Netherlands) with a 32-channel head coil. High-resolution (1 mm3) sagittal 3D-T1-weighted (TR 9.9 ms, TE 4.6 ms, flip angle 8°, TI 920 ms, inter shot time 1792 ms) and fluid-attenuated inversion recovery (FLAIR) images (TR 8 s, TE 342 ms) were acquired. Diffusion-weighted images were acquired using single-shot echo-planar imaging (EPI) sequence (TR 11 ms, TE 72 ms, FOV 240 mm, slice thickness 2.0 mm, 70 axial slices, SENSE factor 2, fold-over direction anteroposterior, fat shift direction P, fat suppression, and voxel size 1.67*1.67*2 mm). We acquired 60 noncollinear directions with a b value of 1000 s/mm2 and 10 directions with a b value of 0 s/mm2 that were averaged to give 1 average direction.
MRI data analysis
Structural images were used to manually delineate lesion masks and compute lesion volumes using MRIcron (https://www.nitrc.org/projects/mricron). Diffusion-weighted images were processed with the Diffusionist toolkit derived from FSL software, as previously described [38]. Each DWI image was visually checked and removed if corrupted. Then, after correction of eddy-current distortions, the diffusion tensor was estimated.
We used FA to assess WM disruptions. Voxel-wise FA images were constructed from the resulting tensors. Linear and nonlinear registration transformations were applied to the FSL FA template in the MNI-152 space by incorporating the knowledge of each brain lesion using manually delineated lesion masks [39]. FA was estimated only in the template’s skeleton and outside the lesion mask. We estimated average FA values with atlas-based regions of interest (ROI) approach using the human brain WM JHU atlas [40]. As FA values vary along the CST tract, we selected the JHU atlas that includes 4 ROIs for the CST. FA was estimated in a set of 33 ROIs listed in Table 2 and represented in Fig. S1. Diffusionist toolkit and related documentation can be found at http://mri-diffusionist.com/.
Statistical analysis
ILH impairment was explored using descriptive statistics. First, we explored the relationship between behavioral tasks (PPT, handgrip, and MST percentiles) and clinical scores using Spearman correlations. As both ILH scores and FA measures showed a normal distribution, we tested the linear associations between ROI-derived FA and ILH raw scores using partial Pearson’s correlations controlling for education, age, and sex, with 95% confidence intervals obtained with bootstrapping based on 1000 samples. In addition, FA values were compared between stroke patients and healthy participants using a t-test with bootstrapping based on 1000 samples to provide robust 95% confidences intervals.
We used linear regression models to determine the WM ROIs and thus the tracts associated with ILH impairment. The effects of ROIs, lesion side, volume, BMI, height, and weight were tested and included in the model only if significant. All models were adjusted for the effects of education, age, and sex. The best model was determined with the statistical significance of the factors with the F-test (p < 0.05), model fit estimated with Durbin-Watson test and distribution of residuals, and model accuracy assessed with adjusted R2. Internal validation was performed with bootstrapping based on 1000 samples. Statistical data analyses were performed using SPSS 23.0.
Results
Twenty-nine patients (21 males, 10 right lesions, all right-handed) completed clinical, behavioral, and MRI assessments at 1 month post-stroke (Fig. S2). We also included 29 age-and sex-matched healthy participants (21 males, mean age 51.1 ± 12.2 years, all right-handed) Clinical data are presented in Table 1. Lesion overlap (Fig. 2) highlights that the middle cerebral artery territory was infarcted in all patients.
Overlap of stroke lesions in the 29 patients. Axial slices are displayed with for z MNI coordinates. Left lesions are represented on the left side and right lesions on the right side of each slice (neurologic convention). Note that ipsilesional SCR and SLF were damaged in all patients, and PLIC, ALIC, and ACR in 70% of them
ILH assessment
Behavioral measures are presented in Table 1. ILH was impaired in 12 patients for PPT (41.4%, 95% CI = 24.1–62.1), 17 for grip (58.6%, 95% CI = 41.4–75.9) and 7 for MST (24;1%, 95% CI = 10.3–41.4). In the subgroup of 24 patients without cognitive deficit, rates were not significantly different from the whole group, with PPT impaired in 10 patients (41.7%, 95% CI = 21.7–62.5), grip in16 patients (66.7%, 95% CI = 47.6–86.4), and MST in 7 patients (29.2%, 95% CI = 10.0–47.6).
Factors associated with ILH impairment
PPT, Grip, and MST significantly correlated with clinical but cognitive scores (Table S2). ILH correlated with the paretic hand for PPT, with a trend for grip and MST (Table S3). There was no significant effect of lesion side on ILH performances. FA values were significantly lower in the patients than in the healthy participants for all ROIs but the contralesional pons-CST, PLIC-CST, and PLIC-CRT, and bilateral SCR-CRT (Table S4).
Table 2 reports ILH and FA correlations. PPT correlated with the ipsilesional (i-) CST (CP-, PLIC, and SCR ROIs), contralesional (c-) CP-CST, bilateral SLF, ALIC, ACR, and PCR, CC, and c-SCP. Grip correlated with the same ROIs but i-SCR and i-ALIC, and with i-ICP. MST correlated with bilateral SLF, ACR, PCR, c-ALIC, CC genu and splenium, and lesion volume.
Linear regression models are presented in Table 3. PPT was predicted by ipsilesional PLIC-CST (B = 17.95; p = 0.002) and SLF (B = 20.52; p = 0.008) with no significant effect of education, age, and sex; r2 = 0.696, indicating good model accuracy. Handgrip strength was predicted by ipsilesional CP-CST (B = 109.58; p = 0.016) and contralesional ACR (B = 42.69; p = 0.039), with an effect of male sex, but no education and age; r2 = 0.571, indicating moderate accuracy. Movement time was predicted by CC genu (B = − 1810.03; p = 0.003) ipsilesional-SLF (B = − 917.45; p = 0.015), contralesional pons-CST (B = 1744.31; p = 0.016), and ipsilesional PLIC-CRP (B = − 380.54; p = 0.037), with male sex and high education supporting better performance; r2 = 0.755, indicating good accuracy.
Discussion
Clinical assessment of ILH
We assessed behavioral performances of the less-affected hand (ILH) in 29 patients at 1 month post-stroke. Since the degree of ILH impairment may depend on the type of task that is tested [10], we used three tasks with different motor and visuomotor processes. PPT, grip, and movement time were impaired in 41.4%, 58.6%, and 24.1%, respectively, highlighting that ILH impairment is frequent, in line with previous studies [6, 8, 10, 15, 41]. Among the multiple tasks described in the literature exploring post-stroke ILH impairment, PPT impairment is the most commonly described, while more heterogeneous results are reported for handgrip strength [6, 15] and movement time [42]. Here, the low frequency of MST suggests that this test may be an insensitive measure compared to kinematic measures, [42, 43], and that visuomotor components of ILH impairment may have been underestimated.
We measured FA in the main tracts related to hand motor function [29, 30, 44] and found that all ROIs but pons and PLIC ROIS of the contralesional CST had lower FA values in patients than in healthy participants, indicating microstructural damage to contralesional and interhemispheric white matter tracts following stroke that may compound ILH function.
ILH performances correlated with clinical motor and sensory scores highlighting that ILH impairment scales with sensorimotor stroke severity and Barthel index. The effect of stroke severity was particularly strong for ILH PPT that correlated with the paretic PPT and lesion volume, while trends were observed for grip and MST.
Correlation analyses between ILH and FA in ROIs of the motor network also revealed task dissociations. PPT (including reach movements requiring visuomotor processes and precise grasp requiring motor processes), MST (visuomotor reaching task), and grip strength (pure motor task) were predicted by a combination of different tracts of the motor network. This argues against the idea that a single mechanism may account for ILH impairment that varies in terms of modality and degree.
Mechanisms of ILH impairment
Our findings showed that, depending on the task, several tracts including the CST, c-ACR, CC, i-SLF, and to a lesser extent i-CRP, were associated with ILH impairment.
We found moderate to strong correlations between the three ILH scores and i-CST, while no significant correlation was observed with contralesional CST-CR and CST-PLIC, suggesting that motor processes of ILH impairment are driven by the ipsilesional CST. Furthermore, i-CST predicted ILH impairment, explaining 18.2 and 8.8% of the PPT and grip variance, respectively. Anatomically, the uncrossed fibers of the ipsilesional CST (Fig. 1-S3A) descending in the dorsal funiculus terminate in the ventromedial intermediate zone to propriospinal neurons connected to distal motoneurons through intersegment spinal interneurons. Although the ventromedial intermediate zone is related to the motor function of the trunk and arms [45], propriospinal neurons may connect with distal motoneurons through intersegment spinal interneurons and thus be involved in the motor control of dexterous hand movements [46]. Moreover, other corticospinal pathways projecting to the reticular formation such as the CRP terminate bilaterally to the propriospinal neurons of the ventral and lateral intermediate zone and contribute to motor performance [47]. Our findings, showing that i-PLIC-CRP was a factor of MST performance, strengthen the hypothesis that ipsilesional descending pathways participate in ILH impairment.
ILH scores were also correlated with FA in the bilateral ALIC, ACR, and CC. Linear models showed that c-ACR was a significant factor of handgrip impairment, explaining 23.3% of the variance. ACR has been linked to cognition and particularly to attention in adults with brain injury [48]. Moreover, a part of ACR fibers originate in the SMA, descend through the ALIC [49], and then merge with the CST in the CP [13], which continues in the pons and medulla to decussate at the pyramid caudal end [13, 50]. The involvement of SMA in simple motor tasks is documented by stroke studies, with SMA lesions leading to mild motor deficits [50], and i-SMA fMRI–related activity supporting motor recovery [17, 51]. Therefore, motor control components of ILH impairment may also implicate the contralesional CST through transcallosal and c-ACR fibers from premotor and/or prefrontal areas (Fig. 1-S3.B).
We found that all ILH scores strongly correlated with the CC including the genu, which predicted MST and explained 24.3% of the variance. A role of the CC is motor coordination of bimanual [52] and unilateral hand motor movements through the balance of excitatory and inhibitory interhemispheric interactions [14]. The ipsi- and contralesional motor areas exert a reciprocal influence through transcallosal fibers [53], as evidenced in tracer studies showing reciprocal transcallosal connections for both MI and SMA [54]. In nonhuman primates, SMA lesions impaired the ILH motor program through transcallosal connections to contralesional SMA [55]. Taken together, our findings concur with previous stroke studies [6, 56], suggesting that the information is shared with the contralesional hemisphere through transcallosal fibers, before travelling through the descending motor pathways. This is consistent with the recent literature proposing an active and specific role of the ipsilateral hemisphere in the planning and execution of voluntary movements [19].
ILH impairment also correlated with decreased FA in bilateral SLF and i-SLF predicted PPT and MST explaining 12.0 and 10.3% of the variance, respectively. These findings support the theory that ILH impairment relates to the bilateral hemispheric control of unilateral movement [6, 10, 14, 15]. In this view, the damaged hemisphere would alter movements of both ILH and paretic hands. In the literature, unimanual motor tasks implicating visuomotor components yield bilateral activity in the frontoparietal network [14, 57,58,59]. In our study, PPT and MST that require visuomotor control (in contrast to handgrip) were associated with the SLF, a key structure of the frontoparietal network connecting parietal, premotor, and motor frontal areas in both human [60,61,62] and nonhuman primates [13]. Furthermore, our findings that i-SLF disruptions alter ILH with visuomotor processing are supported by previous works showing an essential role for the SLF in motor planning and kinematic components of movement execution in 30 right-handed healthy participants [44].
Interestingly, ILH impairment did not correlate with cognitive deficits and was not significantly improved in patients without cognitive deficit, suggesting that cognitive impairment did not influence ILH impairment in this study. Nevertheless, as patients with severe apraxia or neglect were excluded from our study, we may have underestimated the effects of cognitive impairment related to apraxia and neglect on ILH impairment [22].
There were significant correlations between lesion volume and MST, with a trend for PPT and grip. Surprisingly, few studies, if any, have explored the relationships between lesion volume and ILH impairment in humans. Our findings are consistent with nonhuman macaque experiments [63] reporting that reaching ILH tasks were compounded by lesion volume.
Limitations
The small sample size is the main limitation of this study. However, this is the first study exploring the microstructural WM disruptions to understand the underlying mechanisms of ILH impairment following stroke. Also, the homogeneity of our population in terms of age, absence of leukoaraïosis, and stroke severity and territory may have compensated, at least for a part, for this limitation. Nevertheless, the small sample may explain why we did not observe any effect of the lesion side, in contrast with others [7]. Another limitation relates to ILH impairment patients’ perception. When patients with impaired ILH were asked if they noticed that their ILH was impaired, most of them answered that their ILH function was worse than before stroke, but much better than the contralateral hand. However, we did not record all patients’ answers.
Conclusion
This study showed that motor-related tract disruptions predict ILH impairment, with a pattern related to the processes engaged in each task: tasks with motor processing were associated with the ipsilateral CST suggesting the involvement of uncrossed CST fibers, while tasks with visuomotor processing were related to i-SLF supporting hand motor control. In addition, our findings revealed a role for the contralesional hemisphere that may modulate the planning and execution of hand movements through prefrontal/premotor areas and transcallosal interactions. Taken together, ILH impairment may result from the summation of several WM disruptions, supporting the concept of degeneracy of the motor network. Our results provide a theoretical basis for integrating ILH impairment in rehabilitation programs to improve functional recovery and for research interventions, such as neuromodulation.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request criteria.
Abbreviations
- FA:
-
fraction of Anisotropy (diffusion MRI measure of white matter integrity)
- FMS:
-
Fugl-Meyer Score (Total score ranging 0 to 226, upper limb motor score subscore 0-66, sensory score 0-24 and coordination score 0-6)
- JHU atlas:
-
Johns Hopkins University atlas based on the MNI-ICBM labels 2-mm template
- ILH:
-
Ipsilateral Hand
- MST:
-
Movement Screening Test = movement time
- NIHSS:
-
National Institute of Health Stroke Scale (neurological severity)
- PPT:
-
Purdue Pegboard Test
- RBANS:
-
Repeatable Battery for the Assessment of Neuropsychological Status (global assessment of cognitive functions with a mean of 100 in healthy participants)
- SD:
-
Standard deviation
- ACR:
-
Anterior Corona Radiata
- ALIC:
-
Anterior Limb of Internal Capsule
- body-CC:
-
body or middle segment of the Corpus Callosum (CC3 and CC4)
- CST:
-
Corticospinal tract
- SCR:
-
Superior Corona Radiata
- PLIC:
-
Posterior Limb of Internal Capsule
- CP:
-
Cerebral Peduncle
- CRP:
-
Cortico Reticulospinal Pathway
- genu-CC:
-
genu of the Corpus Callosum
- ICP:
-
inferior cerebellar peduncle
- MCP:
-
middle cerebellar peduncle
- SCP:
-
superior cerebellar peduncle
- SLF:
-
superior longitudinal fasciculus
- i:
-
ipsilesional
- c:
-
contralesional
References
Pennati GV et al (2020) Recovery and prediction of dynamic precision grip force control after stroke. Stroke 51(3):944–951
Stinear C (2010) Prediction of recovery of motor function after stroke. Lancet Neurol 9(12):1228–1232
Carey LM, Matyas TA (2011) Frequency of discriminative sensory loss in the hand after stroke in a rehabilitation setting. J Rehabil Med 43(3):257–263
Colebatch JG, Gandevia SC (1989) The distribution of muscular weakness in upper motor neuron lesions affecting the arm. Brain 112(Pt 3):749–763
Gowers, W.R., A manual of diseases of the nervous system. Motor Symptoms. Vol. II. 1886, London UK: Churchill. 988.
Jones RD, Donaldson IM, Parkin PJ (1989) Impairment and recovery of ipsilateral sensory-motor function following unilateral cerebral infarction. Brain 112(Pt 1):113–132
Varghese R, Winstein CJ (2019) Relationship between motor capacity of the contralesional and ipsilesional hand depends on the side of stroke in chronic stroke survivors with mild-to-moderate impairment. Front Neurol 10:1340
Semrau JA et al (2017) Robotic Characterization of ipsilesional motor function in subacute stroke. Neurorehabil Neural Repair 31(6):571–582
Plantin J et al (2021) Recovery and prediction of bimanual hand use after stroke. Neurology 97(7):e706–e719
Kitsos GH et al (2013) The ipsilesional upper limb can be affected following stroke. ScientificWorldJournal 2013:684860
Ziemann U et al (1999) Dissociation of the pathways mediating ipsilateral and contralateral motor-evoked potentials in human hand and arm muscles. J Physiol 518(Pt 3):895–906
Nyberg-Hansen R (1968) “The pyramidal tract syndrome” in man in the light of experimental investigations. Tidsskr Nor Laegeforen 88(1):8–14
Schmahmann JD, Pandya DN (2006) Fiber pathways of the brain. Oxford University Press, Oxford, p 654
Chettouf S et al (2020) Are unimanual movements bilateral? Neurosci Biobehav Rev 113:39–50
Noskin O et al (2008) Ipsilateral motor dysfunction from unilateral stroke: implications for the functional neuroanatomy of hemiparesis. J Neurol Neurosurg Psychiatry 79(4):401–406
Dechaumont-Palacin S et al (2008) Neural correlates of proprioceptive integration in the contralesional hemisphere of very impaired patients shortly after a subcortical stroke: an FMRI study. Neurorehabil Neural Repair 22(2):154–165
Favre I et al (2014) Upper limb recovery after stroke is associated with ipsilesional primary motor cortical activity: a meta-analysis. Stroke 45(4):1077–1083
Rehme AK et al (2011) The role of the contralesional motor cortex for motor recovery in the early days after stroke assessed with longitudinal FMRI. Cereb Cortex 21(4):756–768
Bundy DT, Leuthardt EC (2019) The cortical physiology of ipsilateral limb movements. Trends Neurosci 42(11):825–839
Jung HY, Yoon JS, Park BS (2002) Recovery of proximal and distal arm weakness in the ipsilateral upper limb after stroke. NeuroRehabilitation 17(2):153–159
Chestnut C, Haaland KY (2008) Functional significance of ipsilesional motor deficits after unilateral stroke. Arch Phys Med Rehabil 89(1):62–68
Sunderland A et al (1999) Impaired dexterity of the ipsilateral hand after stroke and the relationship to cognitive deficit. Stroke 30(5):949–955
Wetter S, Poole JL, Haaland KY (2005) Functional implications of ipsilesional motor deficits after unilateral stroke. Arch Phys Med Rehabil 86(4):776–781
Edelman GM, Gally JA (2001) Degeneracy and complexity in biological systems. Proc Natl Acad Sci U S A 98(24):13763–13768
Price CJ, Friston KJ (2002) Degeneracy and cognitive anatomy. Trends Cogn Sci 6(10):416–421
Auriat AM et al (2015) A review of transcranial magnetic stimulation and multimodal neuroimaging to characterize post-stroke neuroplasticity. Front Neurol 6:226–226
Kumar P et al (2016) Prediction of upper limb motor recovery after subacute ischemic stroke using diffusion tensor imaging: a systematic review and meta-analysis. J Stroke 18(1):50–59
Lindenberg R et al (2012) Predicting functional motor potential in chronic stroke patients using diffusion tensor imaging. Hum Brain Mapp 33(5):1040–1051
Lindow J et al (2016) Connectivity-based predictions of hand motor outcome for patients at the subacute stage after stroke. Front Hum Neurosci 10:101
Puig J et al (2017) Diffusion tensor imaging as a prognostic biomarker for motor recovery and rehabilitation after stroke. Neuroradiology 59(4):343–351
Jaillard A, et al. (2020) Autologous mesenchymal stem cells improve motor recovery in subacute ischemic stroke: a randomized clinical trial. Transl Stroke Res
Brott T et al (1989) Measurements of acute cerebral infarction: a clinical examination scale. Stroke 20(7):864–870
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9(1):97–113
Fugl-Meyer AR et al (1975) The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med 7(1):13–31
Randolph C et al (1998) The repeatable battery for the assessment of neuropsychological status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol 20(3):310–319
Rapin I, Tourk LM, Costa LD (1966) Evaluation of the Purdue Pegboard as a screening test for brain damage. Dev Med Child Neurol 8(1):45–54
Spreen O, Strauss E, A compendium of neuropsychological test. Second, (eds) (1998) New York. Oxford University Press, USA
Soulard J et al (2020) Motor tract integrity predicts walking recovery: a diffusion MRI study in subacute stroke. Neurology 94(6):e583–e593
Renard F, Urvoy M, Jaillard A (2015) Bayesian stroke lesion estimation for automatic registration of DTI images. 91–103
Oishi K et al (2008) Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. Neuroimage 43(3):447–457
Marque P et al (1997) Impairment and recovery of left motor function in patients with right hemiplegia. J Neurol Neurosurg Psychiatry 62(1):77–81
Metrot J et al (2013) Motor recovery of the ipsilesional upper limb in subacute stroke. Arch Phys Med Rehabil 94(11):2283–2290
Bustren EL, Sunnerhagen KS, Alt Murphy M (2017) Movement kinematics of the ipsilesional upper extremity in persons with moderate or mild stroke. Neurorehabil Neural Repair 31(4):376–386
Budisavljevic S et al (2017) Asymmetry and structure of the fronto-parietal networks underlie visuomotor processing in humans. Cereb Cortex 27(2):1532–1544
Kuypers H (2011) Anatomy of the descending pathways
Tohyama T et al (2017) Contribution of propriospinal neurons to recovery of hand dexterity after corticospinal tract lesions in monkeys. Proc Natl Acad Sci U S A 114(3):604–609
Bradnam LV, Stinear CM, Byblow WD (2013) Ipsilateral motor pathways after stroke: implications for non-invasive brain stimulation. Front Hum Neurosci 7:184
Niogi SN et al (2008) Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain 131(Pt 12):3209–3221
Morecraft RJ et al (2002) Localization of arm representation in the corona radiata and internal capsule in the non-human primate. Brain 125(Pt 1):176–198
Fries W et al (1993) Motor recovery following capsular stroke. Role of descending pathways from multiple motor areas. Brain 116(Pt 2):369–382
Grefkes C et al (2008) Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol 63(2):236–246
Andres FG et al (1999) Functional coupling of human cortical sensorimotor areas during bimanual skill acquisition. Brain 122(Pt 5):855–870
Leichnetz GR (1986) Afferent and efferent connections of the dorsolateral precentral gyrus (area 4, hand/arm region) in the macaque monkey, with comparisons to area 8. J Comp Neurol 254(4):460–492
Gould HJ 3rd et al (1986) The relationship of corpus callosum connections to electrical stimulation maps of motor, supplementary motor, and the frontal eye fields in owl monkeys. J Comp Neurol 247(3):297–325
Brinkman C (1984) Supplementary motor area of the monkey’s cerebral cortex: short- and long-term deficits after unilateral ablation and the effects of subsequent callosal section. J Neurosci 4(4):918–929
Nowak DA et al (2007) Dexterity is impaired at both hands following unilateral subcortical middle cerebral artery stroke. Eur J Neurosci 25(10):3173–3184
Cavina-Pratesi C et al (2018) Human neuroimaging reveals the subcomponents of grasping, reaching and pointing actions. Cortex 98:128–148
Cavina-Pratesi C et al (2010) Functional magnetic resonance imaging reveals the neural substrates of arm transport and grip formation in reach-to-grasp actions in humans. J Neurosci 30(31):10306–10323
Begliomini C et al (2014) An investigation of the neural circuits underlying reaching and reach-to-grasp movements: from planning to execution. Front Hum Neurosci 8:676
Makris N et al (2005) Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex 15(6):854–869
Thiebaut de Schotten M et al (2011) A lateralized brain network for visuospatial attention. Nat Neurosci 14(10):1245–1246
Wang X et al (2016) Subcomponents and connectivity of the superior longitudinal fasciculus in the human brain. Brain Struct Funct 221(4):2075–2092
Darling WG et al (2011) Volumetric effects of motor cortex injury on recovery of ipsilesional dexterous movements. Exp Neurol 231(1):56–71
Acknowledgements
We thank the platform of France Life Imaging network for partly supporting MRI data acquisition through the grant “ANR-11-INBS-0006, the Clinical Investigation Center (CIC) INSERM UMS 002 CHU Grenoble Alpes for data monitoring, and Felix Renard and Bérengère Aubert-Broche for the Diffusionist software (Diffusion MRI processing).
Funding
This work was supported by: Ministère des Solidarités et de la Santé. Programme hospitalier de recherche clinique – PHRC: ISIS-2007PHR04 (NCT00875654) and HERMES-2007-A00853-50 for patient inclusion and clinical and multimodal MRI data acquisition; IRMaGE platform partly funded by the French program “investissement d’avenir “ run by the agence nationale pour la recherche, grant « infrastructure d’avenir en Biologie Santé»—ANR – 11 – INBS-0006 m; and the Indonesia Endowment Fund for Education (LPDP) for F. F. Hannanu PhD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Institutional review board approval by the Institutional Review Board (CPP: 07-CHUG-25).
Consent to participate
A written informed consent from all patients or relatives was obtained before their participation in the study.
Consent to publish
All the authors have read and approved the submission.
Competing interest
The authors have no conflict of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hannanu, F.F., Naegele, B., Hommel, M. et al. White matter tract disruption is associated with ipsilateral hand impairment in subacute stroke: a diffusion MRI study. Neuroradiology 64, 1605–1615 (2022). https://doi.org/10.1007/s00234-022-02927-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-022-02927-8