Abstract
Purpose
Our aim was to determine the long-term safety and efficacy of the Flow Re-Direction Endoluminal Device (FRED) in this multicenter study with prospective design.
Materials-method
This study included 136 consecutive patients with 155 aneurysms treated between March 2013 and June 2016 in 10 centers. Twenty-two (16.2%) patients presented with rupture of the index aneurysm. Large/giant aneurysms comprised 1/3 of the cohort. Adjuvant coil use during the treatment was 15.5%. The effectiveness measure in the study was the percentage of aneurysms with stable occlusion at follow-up.
Results
Vascular imaging follow-up was performed at least once in 131/136 (96.3%) patients with 148/155 (95.5%) aneurysms up to 75 months (mean: 37.3 months; median: 36 months according to latest follow-up), and 102/155(65.8%) aneurysms in 90/136 (66.2%) patients had ≥ 24-month control. According to the latest controls, the overall stable occlusion rate was 91.9% (95% CI, 87.5 to 96.3%). Three out of 148 aneurysms with follow-up were retreated (2%, 95% CI 0.0 to 4.3%). Adverse events were noted in 19/136 (14%, 95% CI, 9 to 21%) patients with a morbidity of 1.5% (95% CI, 0.0 to 3.5%). Mortality was 1/136 (0.7%, 95% CI, 0.02 to 2.2%) and was unrelated to aneurysm treatment. In-stent stenosis (ISS) was detected in 10/131 of the patients with follow-up (7.6%, 95% CI; 3.1 to 12.2%), only one being symptomatic. No adverse events have occurred in any of the patients with follow-up after 24 months, except the one resulting from ISS.
Conclusion
In the treatment of cerebral aneurysms which were candidates for flow diversion technique, this study showed long-term efficacy of FRED with good safety and occlusion rates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The flow diverter stent (FDS) was initially introduced as a novel endovascular approach to treat cerebral aneurysms with complex morphology such as wide-necked, giant, fusiform, and blister-like aneurysms [1, 2]. Since then, its indications have expanded gradually [3,4,5]. It changes the flow dynamics at the interface between the parent artery and the aneurysm, resulting in stasis of intra-aneurysmal flow, thrombosis in aneurysm sac, and endoluminal reconstruction of the parent artery by endothelialization along the device [6, 7].
There are many FDSs currently available for clinical use [1, 2, 8,9,10,11,12,13]. The Flow Re-Direction Endoluminal Device or FRED (MicroVention, Inc.) has a unique structure of dual-layer stents: an outer layer of 16 wires with a higher radial force and inner layer of 48 wires, as described previously [10, 14,15,16,17,18,19,20,21]. Lower profile version FRED Jr. with modified features has also become available [22]. The publications on FRED are more limited compared to the counterparts that emerged earlier; long-term (≥ 24 months) efficacy and safety have been included to a degree in a few studies [18, 21], and only one single-center study on a relatively small cohort reported long-term results, specifically, so far [23]. Our study is based on multicenter, prospective data collection designed to provide long-term safety and efficacy results of aneurysm treatment with FRED.
Methods
Study design
This study included 136 consecutive patients with 155 aneurysms from 10 centers treated using FRED between March 2013 and June 2016 among a cohort of all aneurysms (n = 656) treated with any kind of flow diverter (Pipeline, FRED/FRED Jr., Silk, P64, Derivo, Surpass). All patients had informed consent for the treatment. An ethics committee (Karadeniz Technical University Faculty of Medicine Ethical Committee: (24237859-85) approved the study.
The parameters to be evaluated were determined prospectively; the coordinating center collected the data upon completion; the entire data were evaluated anonymously by the same jury. The study was planned to remain within the operators’ standard practice to investigate the “real-” world results. In this respect, the choice of flow diverter brand was at operator’s own discretion. The operators did not use FRED exclusively in their practice in this period, i.e., case selection was not totally unbiased.
The aneurysms treated in this study were included in one or more of the following groups: (1) complex saccular aneurysms with a wide neck (neck diameter ≥ 4 mm) or unfavorable dome-neck ratio (≤ 2), (2) large and giant aneurysms with mass effect or that might have mass effect after standard coiling, (3) small aneurysms ≤ 2 mm or blister-like aneurysms which were not favorable for the standard technique, (4) dissecting or fusiform aneurysms, (5) recurrent aneurysms after endovascular treatment or surgery, or (6) aneurysms with a branch directly originating from the sac.
The efficacy of the treatment was determined with the stable occlusion rate according to Cekirge and Saatci Aneurysm Occlusion Classification (CSC) [24] in the latest follow-up and in the follow-up ≥ 24 months. The safety of the treatment was evaluated with the overall incidence of adverse events including angiographic observations or clinical events from the time of treatment until the last follow-up.
Treatment and medication
All procedures were conducted with systemic heparinization after placement of a long introducer sheath via femoral artery. Endovascular treatment was performed with triaxial system in all cases. In some cases, additional coiling was performed using a jailed microcatheter in the aneurysm sac before FRED deployment. These were aneurysms with diameters ≥ 10–15 mm (the cut-off differs according to the operators’ discretion based on presence of nipples, baby aneurysms, increased aspect ratio, partial thrombosis, etc.) and present with subarachnoid hemorrhage (SAH). Coil packing density depended upon the operator’s initiative. In patients with acute SAH (i.e., within 2 weeks after bleeding), flow diversion was used only when the other options (endovascular/surgical) were deemed impossible or presented a higher risk by the judgment of the treating physician.
Antiplatelet therapy was administered in each patient prior to procedure including the patients with acute SAH. Though the antiaggregation protocol varied across centers (Supplementary Table 1), platelet function inhibition was tested in all patients but one. Dual antiplatelet therapy of acetylsalicylic acid (ASA)-clopidogrel was given in 89 patients (65.4%) and ASA-ticlopidine in one because of clopidogrel resistance detected preoperatively. Forty-six patients (33.8%) received prasugrel as mono-drug regimen, but, in one of these patients, prasugrel was switched to ticagrelor (described in the “Results” section).
Follow-up and evaluation
Follow-up imaging was performed using any of digital subtraction angiography (DSA), computerized tomographic angiography (CTA), or contrast enhanced magnetic resonance angiography (CEMRA), based on the routine practice of the centers. DSA was preferred, especially within the first year, unless the patient refused or had any unfavorable medical condition. The vascular imaging results were based on the consensus of the two investigators’ (HD, SC) opinion. In case of discordance, an experienced interventional neuroradiologist (HSC) made the final judgment. Follow-up results were evaluated in these groups: (i) the first control within postoperative 3 to 9 months, (ii) mid-term results (≥ 12 months up to 24 months), (iii) long-term results ≥ 24 months, and (iv) overall results according to the latest available follow-up. In-stent stenosis (ISS) was graded according to the ratio of filling lumen reduction as mild: < 50%, moderate: 50–75% and severe: > 75%. The occlusion status of aneurysms was evaluated according to the scale of CSC [24]:
-
Class 1: Complete occlusion of the aneurysm sac. If there is a branch originating from the aneurysm sac, further evaluation is executed with subgroups:
-
Integrated branch is as follows: IA, fully patent; 1B, reduced in caliber; 1C, not filling antegradely.
-
Class 2: Neck filling.
-
Class 3: Incomplete occlusion with aneurysm filling.
-
Class 4: Because this class is reserved for the immediate postoperative angiography results and ultimate result was the subject of interest, this class was out of the scope in this study.
-
Class 5: “Stable remodeling” refers to filling of the sac at the origin of the incorporated branch or the enlarged tortuous continuation of incorporated branch within the sac which stays same or unchanged for at least 1 year or 2 consecutive angiographies (Fig. 1).
a 3D image of a left MCA trifurcation aneurysm with a bleb, b 3D image showing remodeling of the aneurysm and healing reaction at the aneurysm neck at 1-year control, c–d subtracted (c) and non-subtracted (d) images of 5-year control DSA confirming stable flow remodeling of the aneurysm (class 5) with apparent healing reaction at the neck (arrow)
For the sake of comparison with previous publications, class 5 was called as neck remnant (class 2) according to Raymond-Roy Aneurysm Occlusion Classification (RROC) [25]. O’Kelly-Marotta scale (OKM) [26] equivalence of CSC class 1 would be OKM D; OKM C would include CSC class 2 and 5; OKM B&A fall into group of class 3.
Regarding statistical description, continuous variables were presented as means and ranges and categorical variables as rates.
Results
Patient and aneurysm characteristics
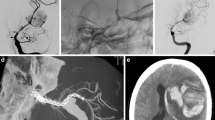
Characteristics of the 136 patients with 155 aneurysms were summarized in Table 1. One hundred eighteen patients (86.8%) were symptomatic. Among 22 patients (16.2%) presenting with SAH, 8 were treated within the first 2 weeks. Anterior circulation aneurysms comprised 145/155 (93.6%) of the treated aneurysms with the majority (99/155, 63.9%) located in the intradural ICA. In the posterior circulation, 7 aneurysms were in the vertebral artery V4 segment; 2 in the basilar trunk (Fig. 2), and one was a dissecting anterior inferior cerebellar artery (AICA) aneurysm (Fig. 3). Fifty-one (32.9%) aneurysms were large or giant. Eleven were recurrent aneurysms after surgery (2) or coiling (9) with 1 patient having a stent in addition to coils.
a 3D angiography 25 days after subarachnoid hemorrhage showing the aneurysm located at the fenestrated basilar trunk, b FRED placed across the aneurysm within the left arm of the fenestrated basilar artery, c 6-month control 3D angiography demonstrating excellent flow remodeling of the basilar artery with complete occlusion of the aneurysm
a DSA 3 weeks after subarachnoid hemorrhage shows a dissecting AICA aneurysm originating distal to the AICA origin from the basilar artery, b 2 FRED placed overlapping across the AICA origin in the basilar artery, c 1-year control angiography revealing aneurysm occlusion and the patency of the AICA (black triangle) (class 1A) due to its flow demand as an example of aneurysm treatment with modifying the flow from a distance. Note mild intimal hyperplasia within the proximal part of FRED (arrow), d 5-year control angiography confirming the stable aneurysm occlusion, patency of the AICA (class 1A) and stable intimal hyperplasia (arrow)
Treatment and procedural results
Technical results
In total, 148 FREDs were used in 136 patients with 155 aneurysms. In 123 patients with single aneurysm, 128 FREDs were used, i.e., in 5 patients two devices were placed for each aneurysm for several reasons: in one patient, the first stent did not cover the aneurysm neck entirely (technical failure); two aneurysms were ruptured recently; one was a giant partially thrombosed ICA aneurysm with higher postoperative rupture risk; in one patient, the posterior inferior cerebellar artery was originating from the remaining aneurysm, and the operator thought one device might not be sufficient for the occlusion due to continuous flow. In the remaining 13 patients harboring multiple aneurysms, 20 FREDs were used to treat 32 aneurysms, and none was covered with more than one device.
Concomitant coiling was performed in 24 aneurysms (15.5%, 95% CI, 9.8 to 21.2%) including 2 giant, 17 large, and 5 small ones.
Safety results
The adverse events were summarized in Table 2. Among the 19/136 patients (14%, 95% CI, 8.1 to 20%) who had adverse events, 2 patients (1.5%, 95% CI, 0.0 to 3.5%) remained with minor neurologic deficit (mRS ≤ 2). Among the 7/136 patients (5.2%) having ischemic complications (which included angiographic observations only or clinical events) and in the perioperative and early postoperative period (≤ 6 months), 5 out of 89 were on dual antiaggregation with clopidogrel and ASA (5.6%, 95% CI, 0.8 to 10.4%), whereas only 1 out of 45 patients was on prasugrel (2.2%, 95% CI, 0.0 to 6.5%), excluding the patient who was not tested preoperatively and then discovered to be resistant. 2/9 thromboembolic complications occurred when the patients were on ASA only: one being symptomatic at 30 months due to ISS and the other having ICA occlusion discovered in the 2nd year control.
The only patient, who was not tested preoperatively, had total stent thrombosis occurring 15 min after device placement while the patient was kept on the table for caution after reversal of the heparin. When the control DSA showed the stent occlusion, after the blood withdrawal for platelet inhibition testing, intraarterial tirofiban was administered first. Because the device remained occluded, a Solitaire AB stent (Medtronic Neurovascular, Irvine, CA) was placed within the FRED, and full patency was achieved. This patient had no clinical event. Because prasugrel resistance was revealed, ticagrelor was commenced instead.
Overall, parent artery occlusion occurred in 5/136 patients (3.7%, 95% CI, 0.5 to 6.8%), none with more than one device placed telescopically; patency was achieved in three of them with additional medication and interventions. The remaining two were detected incidentally when the patients were asymptomatic at the time of discovery and were left untreated.
No patient had intracranial hemorrhage at any time in this series. One patient died because of myocardial infarction 4 months after treatment, mortality by all causes, thus being 0.7% (95% CI, 0.02 to 2.2%).
In-stent stenosis
ISS of any degree was detected in 10 of 131 patients with control imaging (7.6%, 95% CI, 3.1 to 12.2%), excluding two patients who had total ICA occlusion detected during routine control imaging (the reason for occlusion was indeterminate). In none of these patients, more than one device was placed. All but one patient were clinically asymptomatic. All but one patient (described below) had class 1 aneurysm occlusion. In all three patients with severe stenosis (2.3%, 95% CI, 0.0 to 4.9%) including the only symptomatic patient (Fig. 4), PTA was performed.
a 6-month control non-subtracted DSA after FRED treatment with adjunctive coiling of a paraophthalmic ICA aneurysm showing complete aneurysm occlusion (class 1) and patency of the FRED, b 30-month control angiography revealing severe in-stent stenosis due to intimal hyperplasia, c angioplasty performed to treat the severe stenosis, d immediate post-angioplasty non-subtracted angiography showing patency of the FRED restored and normal filling of distal ICA, e post-angioplasty 1-year control angiography demonstrating still patent FRED except for a focal non-significant luminal narrowing
Five (3.8%) patients had mild and two (1.5%) had moderate stenosis in their first control DSA. Their dual antiplatelet therapy/prasugrel was continued until the control DSA showed ISS unchanged or better; ASA substituted then.
Follow-up results
Follow-up results are shown in Table 3. Follow-up vascular imaging was available in 131/136 patients (96.3%) with 148/155 aneurysms (95.5%) at 3 to 75 months post-treatment (mean 37.3 months; median 36 months). At least one control was performed with DSA in 117/136 patients (86%). At least one control between 3 and 9 months was available in 143/155 aneurysms (92.3%), and 148/155 aneurysms (95.5%) had at least one control imaging until 24 months. The patency of the branch incorporated to the sac was expressed as subgroups of CSC, when applicable.
Results of control within the first 3 to 9 months showed complete occlusion (class 1) in 103/143 aneurysms (72%, 95% CI, 64.7 to 79.4%), neck filling (class 2) in 28/143 aneurysms (19.6%, 95% CI, 13.1 to 26.1%), and aneurysmal filling (class 3) in 12/143 aneurysms (8.4%, 95% CI, 3.9 to 12.9%). Complete occlusion increased from 72 to 84.5% when the latest available results are taken into consideration.
According to the latest control, 11/148 aneurysms (7.4%, 95% CI, 3.2 to 11.7%) had stable remodeling so-called class 5 occlusion, all of which had at least 24-month control (range, 24–60 months; mean, 42 months). No case of class 5 occlusion evolved into class 2 or 3 in the follow-up. Therefore, the overall stable occlusion (i.e., class 1 and class 5) rate was 91.9% (95% CI, 87.5 to 96.3%) in this series.
102/155 (65.8%) aneurysms had follow-up of at least 24 months, and in that group, only 6.9% (95% CI, 2 to 11.8%) did not show complete/stable occlusion.
Retreatment
Retreatment was pursued in 2 patients with 3 aneurysms (2%, 95% CI, 0.0 to 4.3%) when the aneurysm filling persisted with no change despite the antiaggregating drug modification in one patient after 24 months (class 3), and in another, the aneurysms were filling (class 2 and 3) with an accompanying ISS increase from mild at 6 months to severe despite continuation of prasugrel. Angioplasty was followed by pipeline device placement in the latter patient, and follow-up DSA confirmed the ICA patency with the aneurysms totally occluded.
Discussion
In this study, 155 aneurysms in 136 patients treated with FRED were analyzed for the angiographic outcome and long-term safety. This series had very high availability (95.5%) of long-term follow-up with a mean of 37.3 months, median of 36 months, up to 75 months.
The reports on FRED are relatively scarce including the ones in systemic reviews. We compared our results with published FRED series having 25 aneurysms or more (Supplementary Table 4) [10, 14, 16,17,18,19,20,21, 23]. Some studies included both FRED and FRED Jr cases [19, 21] which may have affected the results. Our series included a high number of aneurysms only passed by EuFRED [18] and is comparable to FRED Italian Registry [20] with the remaining publications having fewer aneurysms. The ratio of large/giant aneurysms of the cohort and use of adjunctive coiling (none to 41%) differed in the series which may have affected the results.
In the literature, there have been many studies reporting on the safety and efficacy of the flow diverter treatment [1, 2, 13, 27,28,29,30]. In his review, Briganti et al. [28] reported ischemic complications between 1 and 14.2% (mean 4.0%), and hemorrhagic complications ranged from 2.2 to 7.5% (mean 2.9%), resulting in permanent morbidity ranging from 1 to 15% (mean 3.5%) and mortality within a range of 0.5 to 8% (mean 3.4%). Kallmes et al. reported major ischemic stroke rate of 3.7%, major intracranial hemorrhage rate of 2% with the major neurologic morbidity of 5.7%, and mortality of 3.3% [29].
Adverse events occurred between 6.1 and 22% in the FRED series [10, 14, 16,17,18,19,20,21, 23]; given the fact that the reporting criteria varied among series. Our series had adverse event rate of 14% in total including asymptomatic ones. The morbidity and related mortality rates were reported between 0 to 6.2% and 0 to 2.4%, respectively, in these FRED series. Our morbi-mortality rates, 1.5% (mRS ≤ 2) and 0, respectively, are within these ranges. The complication and the morbi-mortality rates in our series and other major FRED studies appear less than those have been reported previously [10, 14, 16,17,18,19,20,21, 23] which may be attributed to the fact that FRED emerged later than the preceding FDSs and the operators had already gone through the learning curve of flow diverter practice. Adjuvant coiling has been performed more liberally; antiplatelet medication has been carried out more diligently, etc.
ISS is a concern in flow-diverter treatment and was reported up to 13.3%, mostly asymptomatic, in the previous FRED series (Supplementary Table 4). In our series, ISS was detected in 7.6%: half of which were ≥ 50%. PTA was performed for severe stenosis (> 75%), not necessarily symptomatic in all cases. PAO was reported up to 16% among the FRED studies including 3.7% of ours, which may have underlying ISS, in some of them.
Regarding the other FDSs, Aguilar Perez M et al. reported ISS of any degree as high as 29.1%, being severe (> 75%) in 2.7% causing no focal deficit in their patients treated with p64 [31]. They suggested that ISS is likely to improve under continued dual antiaggregation, but close monitoring is advised to detect when the stenosis reaches critical level, and then balloon dilatation can be performed with good safety margins. John et al. reported ISS in 9.8% of their patients treated with Pipeline device, and none were symptomatic or required treatment [32].
Our study revealed 2 patients with adverse events after 1 year; at least one was resulting from ISS occurring at 30 months. Luecking et al. also reported on late results of FRED treatment (mean 36.9 months) recently in their single center series, having no adverse event after 3 months [23].
The evaluation of occlusion in the flow diverter treatment is controversial [24,25,26, 33]. In our study, we preferred to use CSC which takes “flow remodeling” into account [24]. When the remaining filling of the aneurysm at a site where a branch is originating (in order to maintain flow into that branch) is unchanged or better in the follow-up, then it is described distinctly as class 5, so-called stable flow remodeling. In other classifications, any filling at the neck region is considered equivalent [25, 26, 33]. In some studies, “adequate occlusion” has been defined to cover neck filling and complete occlusion together, i.e., RROC 1 and 2 or OKM C and D are included in the term “adequate occlusion.” [19,20,21] However, all neck fillings may not behave similarly. Moreover, this latter terminology does not imply the interval change, e.g., the filling at the neck region may increase in the follow-up but still fall into the category of class 2 or OKM C. On the other hand, class 5 in CSC implies that filling is located at a certain location (i.e., at the origin of a vessel), but not in any location (e.g., not eccentric) and remained same at least for 1-year period, which can be considered differently than any other RROC class 2/OKM C filling, and retreatment might be less of a concern. Moreover, the patency of the jailed branch originating from the aneurysm sac is described in CSC, whereas in no other classification, this information takes place [24].
In this series, 92.3% of the aneurysms had a control within 3 to 9 months: 78.7% in 12–24 months and 65.8% in ≥ 24 months. Complete occlusion (class 1) was 72% at 3 to 9 months and increased by time to 84.5%. Class 5, i.e., stable remodeling was called in 7.4% eventually, and all of these patients had ≥ 24-month control; therefore, they are likely to remain stable in the further follow-up. Overall, follow-up of any time period was available in 95.5% of the aneurysms which showed so-called stable occlusion including class 1 and 5 in 91.9% of the aneurysms. In the previous FRED series (Supplementary Table 4), only three studies had any follow-up ≥ 24 months [18, 21, 23]. Although having control up to 43 months, EuFRED had a follow-up median of only 6.6 months [18]. In the study of Guimaraens et al., mean follow-up time was reported as 19 months [21]. The only study reporting the FRED results with a follow-up of ≥ 24 months is that of Luecking et al. [23] on 78 patients with a follow-up availability of 93.6% and overall complete occlusion of 90.6%. Within FRED studies, overall complete occlusion ranged between 57.6 and 90.6%. The complete occlusion rate increased over time in these studies similar to ours [19,20,21]. Some studies reported “adequate occlusion” in addition to complete occlusion, e.g., 81.1% and 73.3% at 1 year in SAFE study [19], 96% and 77% at 12 to 24 months in FRED Italian Registry [20], and 95.9% and 90.6% at a mean follow-up of 36.9 months in the study of Luecking et al. [23], respectively. Guimaraens et al. reported 84.6% adequate occlusion at 1 year [21].
Regarding the efficacy of flow diverters, Briganti et al. found an 81.5% mean rate of complete aneurysm occlusion, which showed progressive increase, in their systematic review [28]. Kallmes et al. reported the complete occlusion rates of 75% at 180 days and 85.5% at 1 year in the pooled analysis of three studies [29]. The 5-year results of PUFS revealed 86.8%, 93.4%, and 95.2% complete occlusion at 1, 3, and 5 years, respectively, confirming the increase in the occlusion rate of flow diverter treatment by time [30]. In summary, the occlusion rate after FRED treatment was not any worse than those in the previously published series of other flow diverters and has the similar tendency to increase over time.
Limitations and strengths of the study
This study has limitations: (i) despite its prospective design, the sites followed their standard of practice; therefore, the technique, selection of flow diverter and auxiliary devices, and the medication protocol varied among the centers; (ii) there was no routine post-treatment sectional imaging ((1) to check any unrecognized or asymptomatic adverse event and (2) to control whether the completely occluded aneurysms disappeared); (iii) the angiographic follow-up protocol varied though the patients were diligently followed clinically and with vascular imaging; and (iv) results were not evaluated by a core lab which may cause bias.
On the other hand, the strengths of the study are as follows: (i) because of the prospective design, the physicians included all consecutive cases which would prevent reporting bias; (ii) all cases were collected in one center; the results were evaluated anonymously by the same jury of physicians; (iii) all patients except one had preoperative platelet function testing; therefore, effective inhibition of thrombocyte aggregation provided a homogenous cohort in that respect; and (iv) because durability is frequently a concern raised against the endovascular treatment, the prolonged follow-up time in a high number of patients in this series is important to support the endovascular treatment.
Conclusion
FRED may offer cerebral aneurysm treatment with good long-term safety and efficacy when flow diversion is the method of choice; however, randomized comparisons are needed for confirmation. This series revealed that in the long term (i.e., after 24 months), it is an exception to encounter an adverse event, and the aneurysms do not get recanalized once they are occluded.
Data availability
The datasets analyzed during the current study are not publicly available due to data protection but are available from the corresponding author upon reasonable request.
References
Nelson PK, Lylyk P, Szikora I, Wetzel SG, Wanke I, Fiorella D (2011) The pipeline embolization device for the intracranial treatment of aneurysms trial. AJNR Am J Neuroradiol 32:34–40
Becske T, Kallmes DF, Saatci I, McDougall CG, Szikora I, Lanzino G, Moran CJ, Woo HH, Lopes DK, Berez AL, Cher DJ, Siddiqui AH, Levy EI, Albuquerque FC, Fiorella DJ, Berentei Z, Marősfoi M, Cekirge SH, Nelson PK (2013) Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology 267(3):858–868. https://doi.org/10.1148/radiol.13120099
Yavuz K, Geyik S, Saatci I, Cekirge HS (2014) Endovascular treatment of middle cerebral artery aneurysms with flow modification with the use of the pipeline embolization device. AJNR Am J Neuroradiol 35(3):529–535. https://doi.org/10.3174/ajnr.A3692
Patel PD, Chalouhi N, Atallah E, Tjoumakaris S, Hasan D, Zarzour H, Rosenwasser R, Jabbour P (2017) Off-label uses of the pipeline embolization device: a review of the literature. Neurosurg Focus 42(6):E4. https://doi.org/10.3171/2017.3.FOCUS1742
Fiorella D, Gache L, Frame D, Arthur AS (2020) How safe and effective are flow diverters for the treatment of unruptured small/medium intracranial aneurysms of the internal carotid artery? Meta-analysis for evidence-based performance goals. J Neurointerv Surg. https://doi.org/10.1136/neurintsurg-2019-015535
Kadirvel R, Ding YH, Dai D, Rezek I, Lewis DA, Kallmes DF (2014) Cellular mechanisms of aneurysm occlusion after treatment with a flow diverter. Radiology 270:394–399. https://doi.org/10.1148/radiol.13130796
Ravindran K, Salem MM, Alturki AY, Thomas AJ, Ogilvy CS, Moore JM (2019) Endothelialization following flow diversion for intracranial aneurysms: a systematic review. AJNR Am J Neuroradiol 40(2):295–301. https://doi.org/10.3174/ajnr.A5955
Byrne JV, Beltechi R, Yarnold J, Birks J, Kamran M (2010) Early experience in the treatment of intra-cranial aneurysms with the Silk flow-diverter: procedural and short term outcomes. PLoSOne 5:e12492
Saatci I, Yavuz K, Ozer C, Geyik S, Cekirge HS (2012) Treatment of intracranial aneurysms using the pipeline flow-diverter embolization device: a single-center experience with long-term follow-up results. AJNR Am J Neuroradiol 33(8):1436–1446. https://doi.org/10.3174/ajnr.A3246
Kocer N, Islak C, Kizilkilic O, Kocak B, Saglam M, Tureci E (2014) Flow Re-direction Endoluminal Device in treatment of cerebral aneurysms: initial experience with short-term follow-up results. J Neurosurg 20:1158–1171
Fischer S, Aguilar-Pérez M, Henkes E, Kurre W, Ganslandt O, Bäzner H, Henkes H (2015) Initial experience with p64: a novel mechanically detachable flow diverter for the treatment of intracranial saccular sidewall aneurysms. AJNR Am J Neuroradiol 36(11):2082–2089. https://doi.org/10.3174/ajnr.A4420
Wakhloo AK, Lylyk P, de Vries J, Taschner C, Lundquist J, Biondi A, Hartmann M, Szikora I, Pierot L, Sakai N, Imamura H, Sourour N, Rennie I, Skalej M, Beuing O, Bonafe A, Mery F, Turjman F, Brouwer P, Boccardi E, Valvassori L, Derakhshani S, Litzenberg MW, Gounis MJ, for the Surpass Study Group (2015) Surpass flow diverter in the treatment of intracranial aneurysms: a prospective multicenter study. AJNR Am J Neuroradiol 36:98–107
Chancellor B, Raz E, Shapiro M et al (2020) Flow diversion for intracranial aneurysm treatment: trials involving flow diverters and long-term outcomes. Neurosurgery 86(Supplement_1):S36–S45. https://doi.org/10.1093/neuros/nyz345
Möhlenbruch MA, Herweh C, Jestaedt L, Stampfl S, Schönenberger S, Ringleb PA, Bendszus M, Pham M (2015) The FRED flow-diverter stent for intracranial aneurysms: clinical study to assess safety and efficacy. AJNR Am J Neuroradiol 36(6):1155–1161. https://doi.org/10.3174/ajnr.A4251
Briganti F, Leone G, Ugga L, Marseglia M, Solari D, Caranci F, Mariniello G, Maiuri F, Cappabianca P (2016) Safety and efficacy of Flow Re-Direction Endoluminal Device (FRED) in the treatment of cerebral aneurysms: a single center experience. Acta Neurochir 158(9):1745–1755. https://doi.org/10.1007/s00701-016-2875-4
Drescher F, Weber W, Berlis A, Rohde S, Carolus A, Fischer S (2017) Treatment of intra- and extracranial aneurysms using the flow-redirection endoluminal device: multicenter experience and follow-up results. AJNR Am J Neuroradiol 38(1):105–112. https://doi.org/10.3174/ajnr.A4964
Luecking H, Engelhorn T, Lang S, Goelitz P, Kloska S, Roessler K, Doerfler A (2017) FRED flow diverter: a study on safety and efficacy in a consecutive group of 50 patients. AJNR Am J Neuroradiol 38(3):596–602. https://doi.org/10.3174/ajnr.A5052
Killer-Oberpfalzer M, Kocer N, Griessenauer CJ, Janssen H, Engelhorn T, Holtmannspötter M, Buhk JH, Finkenzeller T, Fesl G, Trenkler J, Reith W, Berlis A, Hausegger K, Augustin M, Islak C, Minnich B, Möhlenbruch M (2018) European multicenter study for the evaluation of a dual-layer flow-diverting stent for treatment of wide-neck intracranial aneurysms: the European flow-redirection intraluminal device study. AJNR Am J Neuroradiol 39(5):841–847. https://doi.org/10.3174/ajnr.A5592
Pierot L, Spelle L, Berge J, Januel AC, Herbreteau D, Aggour M, Piotin M, Biondi A, Barreau X, Mounayer C, Papagiannaki C, Lejeune JP, Gauvrit JY, Derelle AL, Chabert E, Costalat V (2019) SAFE study (safety and efficacy analysis of FRED embolic device in aneurysm treatment): 1-year clinical and anatomical results. J Neurointerv Surg 11(2):184–189. https://doi.org/10.1136/neurintsurg-2018-014261
Piano M, Valvassori L, Lozupone E, Pero G, Quilici L, Boccardi E (2019) FRED Italian Registry: a multicenter experience with the Flow Re-Direction Endoluminal Device for intracranial aneurysms. J Neurosurg 10:1–8. https://doi.org/10.3171/2019.1.JNS183005
Guimaraens L, Vivas E, Saldaña J et al (2019) Efficacy and safety of the dual-layer flow-diverting stent (FRED) for the treatment of intracranial aneurysms. J Neurointerv Surg. https://doi.org/10.1136/neurintsurg-2019-015371
Möhlenbruch MA, Kizilkilic O, Killer-Oberpfalzer M, Baltacioglu F, Islak C, Bendszus M, Cekirge S, Saatci I, Kocer N (2017) Multicenter experience with FRED Jr Flow Re-Direction Endoluminal Device for intracranial aneurysms in small arteries. AJNR Am J Neuroradiol 38(10):1959–1965. https://doi.org/10.3174/ajnr.A5332
Luecking H, Doerfler A, Goelitz P, Hoelter P, Engelhorn T, Lang S (2020) Two- to five- year follow-up of 78 patients after treatment with the flow redirection endoluminal device. Interv Neuroradiol 26(1):38–44. https://doi.org/10.1177/1591019919878551
Cekirge HS, Saatci I (2016) A new aneurysm occlusion classification after the impact of flow modification. AJNR Am J Neuroradiol 37(1):19–24. https://doi.org/10.3174/ajnr.A4489
Roy D, Milot G, Raymond J (2001) Endovascular treatment of unruptured aneurysms. Stroke 32(9):1998–2004
O'kelly CJ, Krings T, Fiorella D, Marotta TR (2010) A novel grading scale for the angiographic assessment of intracranial aneurysms treated using flow diverting stents. Interv Neuroradiol 16(2):133–137
Brinjikji W, Murad MH, Lanzino G, Cloft HJ, Kallmes DF (2013) Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke 44(2):442–447. https://doi.org/10.1161/STROKEAHA.112.678151
Briganti F, Leone G, Marseglia M, Mariniello G, Caranci F, Brunetti A, Maiuri F (2015) Endovascular treatment of cerebral aneurysms using flow-diverter devices: a systematic review. Neuroradiol J 28(4):365–375. https://doi.org/10.1177/1971400915602803
Kallmes DF, Brinjikji W, Cekirge S, Fiorella D, Hanel RA, Jabbour P, Lopes D, Lylyk P, McDougall CG, Siddiqui A (2017) Safety and efficacy of the pipeline embolization device for treatment of intracranial aneurysms: a pooled analysis of 3 large studies. J Neurosurg 127:775–780. https://doi.org/10.3171/2016.8.JNS16467
Becske T, Brinjikji W, Potts MB, Kallmes DF, Shapiro M, Moran CJ, Levy EI, McDougall CG, Szikora I, Lanzino G, Woo HH, Lopes DK, Siddiqui AH, Albuquerque FC, Fiorella DJ, Saatci I, Cekirge SH, Berez AL, Cher DJ, Berentei Z, Marosfői M, Nelson PK (2017) Long-term clinical and angiographic outcomes following pipeline embolization device treatment of complex internal carotid artery aneurysms: five-year results of the pipeline for uncoilable or failed aneurysms trial. Neurosurgery 80:40–48. https://doi.org/10.1093/neuros/nyw014
Aguilar Pérez M, Bhogal P, Henkes E, Ganslandt O, Bäzner H, Henkes H (2018) In-stent stenosis after p64 flow diverter treatment. Clin Neuroradiol 28(4):563–568. https://doi.org/10.1007/s00062-017-0591y
John S, Bain MD, Hui FK, Hussain MS, Masaryk TJ, Rasmussen PA, Toth G (2016) Long-term follow-up of in-stent stenosis after pipeline flow diversion treatment of intracranial aneurysms. Neurosurgery 78(6):862–867. https://doi.org/10.1227/NEU.0000000000001146
Kamran M, Yarnold J, Grunwald IQ, Byrne JV (2011) Assessment of angiographic outcomes after flow diversion treatment of intracranial aneurysms: a new grading schema. Neuroradiology 53(7):501–508. https://doi.org/10.1007/s00234-010-0767-5
Funding
None
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Isil Saatci (Cekirge): Consultancy and Proctorship agreements with Medtronic Inc. (Minneapolis, USA) and MicroVention Inc. (Aliso Viejo, CA, USA).
Feyyaz Baltacioglu: Financial support for attending symposia and educational programs MicroVention Inc. (Aliso Viejo, CA, USA), Medtronic Inc. (Minneapolis, USA), and Stryker (Neurovascular, Freemont, CA, USA).
H.Saruhan Cekirge: Consultancy and Proctorship agreements with Medtronic Inc. (Minneapolis, USA) and MicroVention Inc. (Aliso Viejo, CA, USA) and shareholder of NDI Technologies, eLUM Technologies Inc., and Vesalio LLC.
The remaining authors declare they have no competing interest.
Ethics approval
• All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
• The study protocol was approved by the ethics committee of the organizing institution (Karadeniz Technical University Faculty of Medicine Ethical Committee: (24237859-85).
• Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dinc, H., Saatci, I., Oguz, S. et al. Long-term clinical and angiographic follow-up results of the dual-layer flow diverter device (FRED) for the treatment of intracranial aneurysms in a multicenter study. Neuroradiology 63, 943–952 (2021). https://doi.org/10.1007/s00234-020-02627-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02627-1