Abstract
Purpose
Endovascular treatment of unruptured intracranial aneurysms may increase cerebral microbleeds (CMBs) in postprocedural T2*-weighted MRIs, which may be a risk for future intracerebral hemorrhage. This study examined the characteristics of postprocedural CMBs and the factors that cause their increase.
Methods
The patients who underwent endovascular treatment for unruptured intracranial aneurysms from April 2016 to February 2018 were retrospectively analyzed. Treatment techniques for endovascular treatment included simple coiling, balloon-assisted coiling, stent-assisted coiling, or flow diverter placement. To evaluate the increase in CMBs, a head MRI including diffusion-weighted imaging and T2*-weighted MRIs was performed on the preprocedural day; the first postprocedural day; and at 1, 3, and 6 months after the procedure.
Results
Among the 101 aneurysms that were analyzed, 38 (37.6%) showed the appearance of new CMBs. In the multivariate analysis examining the causes of the CMB increases, chronic kidney disease, a higher number of preprocedural CMBs, and a higher number of diffusion-weighted imaging–positive lesions on the first postprocedural day were independent risk factors. Furthermore, a greater portion of the increased CMBs was found in cortical and subcortical lesions of the treated vascular perfusion area within 1 month after the procedure.
Conclusion
In endovascular treatment for unruptured intracranial aneurysms, CMBs tended to increase in patients with small vessel disease before the procedure, and it was also implicated in hemorrhagic changes after periprocedural microinfarction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antiplatelet therapy, in combination with multiple drugs to prevent periprocedural thrombotic complications associated with endovascular treatment for unruptured intracranial aneurysms, has become a standard treatment because of the spread of stent-assisted coil embolization and flow diverter placement. However, there is a concern that the administration of multiple antiplatelet drugs may increase the risk of bleeding complications. One of the significant bleeding complications in endovascular treatment of aneurysms is delayed intracerebral hemorrhage (ICH), especially after flow diverter placement [1,2,3]. Head-gradient-echo T2*-weighted MRIs (T2*WI) after endovascular treatment for unruptured intracranial aneurysms often show emergence of new cerebral microbleeds (CMBs). The presence of CMBs is generally considered to be a risk factor for future cerebral hemorrhage and may also be related to delay ICH [4,5,6,7]. Although previous study reported that new CMBs after neuroendovascular surgery developed in 8.0–21.9% of patients, the occurrence rate of CMBs after endovascular treatment for intracranial aneurysms has not been well known [8,9,10]. Additionally, the involvement of antiplatelet therapy and other triggers for the increase of CMBs after endovascular treatment for intracranial aneurysms has not been thoroughly investigated yet.
In this study, we analyzed MRI scans before and after endovascular treatment for unruptured intracranial aneurysms and examined the characteristics of CMBs, such as their triggers, distribution, and timing of appearance.
Methods
Study population
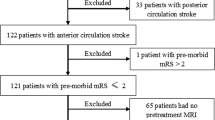
After Institutional Review Board approval from our hospital was obtained for this study, we enrolled consecutive adult patients who were scheduled to undergo endovascular treatments for untreated unruptured intracranial aneurysms, between April 2016 and February 2018. Cases with complex aneurysms treated combined traditional open surgery and endovascular technique, those in which T2*WI or diffusion-weighted images (DWI) in MRI were not imaged at the scheduled period, and those with intraprocedural thrombus formation or postprocedural symptomatic cerebral ischemic lesions who were treated by additional antithrombotic treatment were excluded.
Data on the clinical characteristics of participants were obtained from our single-center prospective database, including age, sex, comorbidities (hypertension, dyslipidemia, diabetes mellitus, and chronic kidney disease [eGFR < 60 ml/min/1,73 m2]), stroke history, smoking status, maximum aneurysm diameter and neck diameter, treatment technique, and platelet reactivity measured using the VerifyNow assay (Accumetrics, San Diego, CA, USA). Aneurysms and the neck diameters of aneurysms were determined using preprocedural 3D reformatted images derived from rotational catheter angiograms. Treatment techniques were classified into one of the following four categories: simple coiling, balloon-assisted coiling, stent-assisted coiling, and flow diverter placement.
Treatment methods
All patients were administered antiplatelet therapy with oral aspirin (100 mg/day) and clopidogrel (75 mg/day) 2 weeks before treatment. Aspirin reaction unit (ARU) and P2Y12 reaction unit (PRU) values using the VerifyNow assay were measured 24 h before surgery. If the platelet inhibition did not achieve the satisfactory levels, ARU > 550 or PRU > 240, the patient received additional cilostazol (200 mg/day) before procedure and continued for 1 week. For simple coiling and balloon-assisted coiling, the combination of two drugs was administered for 1 week after the procedure and then was switched to a single drug, which was continued for 1 month [11]. For stent-assisted coiling, the two drugs were administered for 3 to 6 months and then were switched to a single drug [12, 13]. For flow diverter placement, the two drugs were administered for at least 6 months and then were switched to a single drug [14]. Antihypertensive medication was aggressively prescribed to achieve blood pressure lowering below 140/90 mmHg for all patients with hypertension and continued after discharge.
All treatments were performed under general anesthesia using standard approaches from the common femoral artery. During the procedure, the activated clotting time was checked every hour with a target of 250 to 300 s, and heparin was injected intravenously as needed. The appropriate treatment technique was selected according to the characteristics of the aneurysm. Simple coiling was chosen if the aneurysm morphology was saccular with a narrow neck. Aneurysms with an unfavorable angioarchitecture including dome-to-neck ratio < 2 or a neck diameter ≥ 4 mm required adjunctive techniques, such as balloon-assisted or stent-assisted coiling (Neuroform [Stryker, Kalamazoo, MI, USA], Enterprise [Johnson & Johnson Codman, Miami, FL, USA], or low-profile visualized intraluminal support [MicroVention Terumo, Tustin, CA, USA]). According to the Japanese government approval of flow diverters (pipeline embolization device [Covidien, Irvine, CA, USA]), we chose flow diverter placement for the aneurysms of ≥ 10 mm in maximum diameter with a neck of ≥ 4 mm arising between the petrous and the superior hypophyseal artery segments of the internal carotid artery.
MRI evaluation
Head MRI imaging, including DWI and T2*WI, was performed using a 3.0-T MRI system (Signa Excite HDx 3.0T; GE Healthcare, Chicago, IL, USA). The protocol for DWI imaging included a repetition time if 5500 ms, an echo time of 73 ms, a matrix size 128 × 192 mm, a b-value of 1000 s/mm2, a slice thickness of 5 mm, and an interslice gap of 1 mm. The protocol for T2*WI included a repetition time of 560 ms, an echo time of 16 ms, a flip angle of 18°, a matrix size of 180 × 256 mm, a slice thickness of 5 mm, and an interslice gap of 1 mm. DWI was imaged the day after the procedure, and positive lesions indicating ischemia were counted. T2*WI was imaged on the preprocedural day; the first postprocedural day; and at 1, 3, and 6 months after the procedure, and CMBs were counted. CMBs in T2*WI were defined as low intensity, punctate, or patchy signals that were smaller than 10 mm [15].
The distribution of increased CMBs was divided into two categories: (1) deep CMBs in the basal ganglia, thalamus, caudate nucleus, internal and external capsules, and in the brainstem and cerebellum under the tent, and (2) lobar CMBs in the cortex and subcortex. Furthermore, whether or not CMBs were in the perfusion area of the blood vessels of the treated aneurysm was evaluated. The MRI scans obtained from the head were evaluated by two neurosurgeons (E.H. and S.S.). Disagreements between these neurosurgeons were resolved by discussion with a third reviewer.
Statistical analysis
Patients were classified into two subgroups according to the occurrence of CMBs. Clinical characteristics were compared between these subgroups using the Pearson chi-square test, the unpaired t test, or the Mann-Whitney U test as appropriate. The multivariate logistic regression analysis was performed for CMBs while adjusting for factors significantly associated with CMBs in univariate analyses. Values of p < 0.05 were considered statistically significant. Interobserver agreement for assessing the appearance of CMBs and DWI-positive lesions was assessed using the kappa statistic. Statistical analyses were performed using the JMP version 12.1.0 statistical software (SAS Institute, Cary, NC, USA).
Results
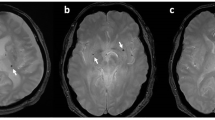
One hundred thirty-one aneurysms in 126 consecutive patients who underwent endovascular treatment for unruptured intracranial aneurysms were enrolled. After excluding 30 aneurysms in accordance with the exclusion criteria, 101 aneurysms were included. Of these cases, 38 (37.6%) showed the appearance of new CMBs during follow-up and there was no difference between treatment techniques (simple or balloon-assisted coiling 38.1%, stent-assisted coiling 39.2%, flow diverter placement 34.5%). All lesions were asymptomatic. The appearance of DWI-positive lesions on the day after the procedure was detected in 73 (72.3%) cases and there was no significant difference between treatment techniques (simple or balloon-assisted coiling 65.5%, stent-assisted coiling 72.5%, flow diverter placement 81.0%). Figure 1 shows one of these cases. The kappa statistics for the appearance of CMBs showed a high level of agreement (κ = 0.81), and the appearance of DWI-positive lesions also showed an excellent agreement (κ = 0.94). The characteristics of patients, aneurysms, and images were divided into two groups according to whether new CMBs appeared or not. Univariate analysis of the factors that increase CMBs revealed that chronic kidney disease (p = 0.038), the presence of preprocedural CMBs (p < 0.001), the maximum diameter of the aneurysm (p = 0.003), and the presence of many DWI-positive lesions on the day after the procedure (p = 0.001) were significantly involved (Table 1). A multivariate analysis showed that chronic kidney disease (odds ratio, 6.47; 95% confidence interval, 1.44–35.50; p = 0.015), the higher number of CMBs before the procedure (odds ratio, 1.69; 95% confidence interval, 1.17–2.69; p = 0.003; per 1 lesion), and the higher number of DWI-positive lesions on the day after procedure (odds ratio, 1.10; 95% confidence interval, 1.02–1.20; p = 0.013; per 1 lesion) were independent risk factors for increased CMBs (Table 2).
Stent-assisted coiling for a 5.7 mm unruptured basilar tip aneurysm. a Left vertebral artery angiogram obtained immediately after the procedure, showing near-complete obliteration of the aneurysm and good patency of the parent artery. b Diffusion-weighted imaging on the first day after procedure, revealing positive lesions. c T2*-weighted MRI obtained 1-month post-procedure, revealing a new cerebral microbleed in the left occipital lobe parenchyma (arrowhead)
The rate of increase in CMBs was between 34 and 38% among all treatment techniques, and the majority of cases increased within 1 month after the procedure. All CMBs found within 1 month after the procedure were still visible on the 3 and 6 months. Regarding the site of increase, most of the new CMBs were located in cortical and subcortical lesions and the perfusion area of the blood vessels of the treated aneurysm following all treatment techniques (Table 3).
Although delayed ICH was not detected after coil embolization, only one case after flow diverter placement at a left internal carotid artery aneurysm had delayed ICH. In this case, CMBs increased in T2*WI 1 month after the procedure and delayed ICH was found in the left frontal lobe 1.5 months after the procedure.
Discussion
Delayed ICH is known to be one of the complications of endovascular treatment for unruptured intracranial aneurysms, and it is reported to occur in 1.5 to 8.5% of flow diverter placements and 0.46 to 2.2% of coiling cases [1,2,3, 16,17,18,19]. We evaluated the factors that increase CMBs after endovascular treatment and their distribution characteristics based on the assumption that the appearance of CMBs, which is a potential risk factor for future cerebral hemorrhage [4,5,6,7], will lead to delayed ICH. In our study, 37.6% of the cases exhibited increased CMBs, and there was no difference between treatment techniques. Previous reports regarding CMBs after endovascular treatment for intracranial aneurysms, which are very few, demonstrated the same occurrence rate after flow diverter placement (36.7%) but a lower rate after stent-assisted coiling (11.1%) [20]. The high occurrence rate of CMBs in our study might be associated with the high prevalence of hypertension and preprocedural CMBs at baseline. The major findings of this study were that chronic kidney disease, the presence of a higher number of postoperative DWI-positive lesions, and a higher number of preprocedural CMBs were independent risk factors. Furthermore, CMBs increased within 1 month after treatment, and the distribution tended to be higher for lobar CMBs in the perfusion area of the blood vessels of the treated aneurysm.
CMBs generally have been reported to result from hemorrhagic changes after microinfarction as well as blood leakage due to an impaired blood-brain barrier and neurovascular unit from hypertensive microangiopathy and amyloid angiopathy [21, 22]. The results from this study of the factors that increased CMBs after endovascular treatment suggest at least two mechanisms. One mechanism is that the administration of antiplatelet drugs induces vascular leakage. Having CMBs before treatment indicates that microangiopathy predisposed to bleeding was already present. Furthermore, several studies have shown that the presence of CMBs is associated with chronic kidney disease [23,24,25]. The two may occur together by a similar mechanism such as hypertensive microvascular damage because the kidney and brain have similar microvascular beds, and inflammation and endothelial dysfunction in chronic kidney disease affects the microvascular system of the brain [24]. Blood leakage may be induced by the administration of an antiplatelet drug in the presence of such microangiopathy.
Another mechanism is hemorrhagic infarction. Several previous reports have suggested the involvement of hemorrhagic infarction as a cause of delayed ICH [1,2,3, 20]. These findings might result from flow diverter placement, which is more likely to lead to delayed ICH than other treatment techniques and is estimated to cause many postprocedural DWI-positive lesions. Similarly, in this study, CMBs increased as the number of postoperative DWI-positive lesions increased, suggesting the involvement of hemorrhagic infarction. Furthermore, in our study, the findings that most of the increased CMBs were located in cortical and subcortical lesions of treated vascular perfusion areas and appeared within 1 month after a procedure may also have been the result of hemorrhagic infarction.
Long-term dual antiplatelet therapy increases bleeding risk in secondary prevention of cerebral infarction [26], but it remains unclear whether antiplatelet therapy used for endovascular treatment of unruptured intracranial aneurysms increases CMBs and symptomatic cerebral hemorrhages. Several studies have shown that PRU values are associated with postoperative bleeding complications [19, 27,28,29]. To our best knowledge, little has been reported on the association between new CMBs appearance and platelet function. In our study, we examined preprocedural platelet function using ARU and PRU, but the values of ARU and PRU were no discrepancy between two groups. This result may suggest that increased CMBs are not so much because of the effects of antiplatelet drugs but because of factors that increase the likelihood of bleeding, such as the aforementioned angiopathy or hemorrhagic infarction due to microinfarction.
This study has several limitations. First, the study followed a single-center design; thus, the results may be specific to our techniques and equipment. Second, our study did not consider the progression of hypertension. In all cases with hypertension, drug therapy was proactively performed to achieve blood pressure lowering below 140/90 mmHg and continued after discharge by family physicians, but the concrete values of blood pressure after discharge was not recorded in our hospital database. In general, there is a correlation between CMBs and hypertension. This relationship may have influenced the fact that no significant differences were observed in hypertension, which was one of the factors that increased CMBs. Third, air bubble embolisms during the procedure, small atheromatous embolisms due to catheter manipulation in the aorta, or other vessels or foreign bodies (hydrophilic coating materials, metal fragments) can be represented as hypointense signals on T2*WI [30]. Fourth, there is a potential risk of missing CMBs because T2*WI protocol has an interslice gap of 1 mm. However, all CMBs recognized once were still visible on the later MRI in this study. Finally, only one patient after flow diverter placement had delayed ICH, and the direct correlation between CMBs and delayed ICH could not be shown. The low number of treatments using flow diverters, which are more likely to cause delayed ICH, may also have influenced these findings. A larger number of cases must be considered in the future.
Conclusions
In endovascular treatment for unruptured intracranial aneurysms, postprocedural CMBs increased in 36.6% of cases. Risk factors for increased CMBs were chronic kidney disease, a higher number of CMBs before treatment, and a higher number of DWI-positive lesions after a procedure. CMBs were likely to increase in patients with small vessel disease before a procedure, and hemorrhagic changes after periprocedural microinfarction were likely involved.
Data availability
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Cruz JP, Chow M, O'Kelly C, Marotta B, Spears J, Montanera W, Fiorella D, Marotta T (2012) Delayed ipsilateral parenchymal hemorrhage following flow diversion for the treatment of anterior circulation aneurysms. AJNR Am J Neuroradiol 33:603–608. https://doi.org/10.3174/ajnr.A3065
Kayan Y, Delgado Almandoz JE, Fease JL, Tran K, Milner AM, Scholz JM (2016) Incidence of delayed ipsilateral intraparenchymal hemorrhage after stent-assisted coiling of intracranial aneurysms in a high-volume single center. Neuroradiology 58:261–266. https://doi.org/10.1007/s00234-015-1624-3
Sim SY, Song J, Oh SY, Kim MJ, Lim YC, Park SK, Shin YS, Chung J (2016) Incidence and characteristics of remote intracerebral hemorrhage after endovascular treatment of unruptured intracranial aneurysms. World Neurosurg 95:335–340. https://doi.org/10.1016/j.wneu.2016.08.057
Fan YH, Zhang L, Lam WW, Mok VC, Wong KS (2003) Cerebral microbleeds as a risk factor for subsequent intracerebral hemorrhages among patients with acute ischemic stroke. Stroke 34:2459–2462. https://doi.org/10.1161/01.STR.0000090841.90286.81
Bokura H, Saika R, Yamaguchi T, Nagai A, Oguro H, Kobayashi S, Yamaguchi S (2011) Microbleeds are associated with subsequent hemorrhagic and ischemic stroke in healthy elderly individuals. Stroke 42:1867–1871. https://doi.org/10.1161/STROKEAHA.110.601922
Charidimou A, Kakar P, Fox Z, Werring DJ (2013) Cerebral microbleeds and recurrent stroke risk: systematic review and meta-analysis of prospective ischemic stroke and transient ischemic attack cohorts. Stroke 44:995–1001. https://doi.org/10.1161/STROKEAHA.111.000038
Wilson D, Charidimou A, Ambler G, Fox ZV, Gregoire S, Rayson P, Imaizumi T, Fluri F, Naka H, Horstmann S, Veltkamp R, Rothwell PM, Kwa VIH, Thijs V, Lee YS, Kim YD, Huang Y, Wong KS, Jäger HR, Werring DJ (2016) Recurrent stroke risk and cerebral microbleed burden in ischemic stroke and TIA: a meta-analysis. Neurology 87:1501–1510. https://doi.org/10.1212/WNL.0000000000003183
Ogawa Ito A, Shindo A, Ii Y, Matsuura K, Tabei KI, Maeda M, Umino M, Suzuki Y, Shiba M, Toma N, Suzuki H, Tomimoto H (2019) Microbleeds after carotid artery stenting: small embolism may induce cerebral microbleeds. Cerebrovasc Dis Extra 9:57–65. https://doi.org/10.1159/000500112
Shi ZS, Duckwiler GR, Jahan R, Tateshima S, Gonzalez NR, Szeder V, Saver JL, Kim D, Ali LK, Starkman S, Vespa PM, Salamon N, Villablanca JP, Viñuela F, Feng L, Loh Y, Liebeskind DS (2015) New cerebral microbleeds after mechanical thrombectomy for large-vessel occlusion strokes. Medicine 94:e2180. https://doi.org/10.1097/MD.0000000000002180
Gao Y, Nie K, Duan Z, Wang S, Ma G, Zhang X, Li C, Zhang Y, Dai C, Wang L (2019) A follow-up study of cerebral microbleeds in patients who received stents for symptomatic cerebral artery stenosis. Ann Vasc Surg 58:338–346. https://doi.org/10.1016/j.avsg.2018.11.031
Nishikawa Y, Satow T, Takagi T, Murao K, Miyamoto S, Iihara K (2013) Efficacy and safety of single versus dual antiplatelet therapy for coiling of unruptured aneurysms. J Stroke Cerebrovasc Dis 22:650–655. https://doi.org/10.1016/j.jstrokecerebrovasdis.2012.02.008
Wakhloo AK, Linfante I, Silva CF, Samaniego EA, Dabus G, Etezadi V, Spilberg G, Gounis MJ (2012) Closed-cell stent for coil embolization of intracranial aneurysms: clinical and angiographic results. AJNR Am J Neuroradiol 33:1651–1656. https://doi.org/10.3174/ajnr.A3034
Rossen JD, Chalouhi N, Wassef SN, Thomas J, Abel TJ, Jabbour PM, Kung DK, Hasan DM (2012) Incidence of cerebral ischemic events after discontinuation of clopidogrel in patients with intracranial aneurysms treated with stent-assisted techniques. J Neurosurg 117:929–933. https://doi.org/10.3171/2012.8.JNS12185
Becske T, Potts MB, Shapiro M, Kallmes DF, Brinjikji W, Saatci I, McDougall CG, Szikora I, Lanzino G, Moran CJ, Woo HH, Lopes DK, Berez AL, Cher DJ, Siddiqui AH, Levy EI, Albuquerque FC, Fiorella DJ, Berentei Z, Marosföi M, Cekirge SH, Nelson PK (2017) Pipeline for uncoilable or failed aneurysms: 3-year follow-up results. J Neurosurg 127:81–88. https://doi.org/10.3171/2015.6.JNS15311
Roob G, Fazekas F (2000) Magnetic resonance imaging of cerebral microbleeds. Curr Opin Neurol 13:69–73. https://doi.org/10.1097/00019052-200002000-00013
Becske T, Kallmes DF, Saatci I, McDougall CG, Szikora I, Lanzino G, Moran CJ, Woo HH, Lopes DK, Berez AL, Cher DJ, Siddiqui AH, Levy EI, Albuquerque FC, Fiorella DJ, Berentei Z, Marosfoi M, Cekirge SH, Nelson PK (2013) Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology 267:858–868. https://doi.org/10.1148/radiol.13120099
Fischer S, Vajda Z, Aguilar Perez M, Schmid E, Hopf N, Bäzner H, Henkes H (2012) Pipeline embolization device (PED) for neurovascular reconstruction: initial experience in the treatment of 101 intracranial aneurysms and dissections. Neuroradiology 54:369–382. https://doi.org/10.1007/s00234-011-0948-x
Brinjikji W, Murad MH, Lanzino G, Cloft HJ, Kallmes DF (2013) Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke 44:442–447. https://doi.org/10.1161/STROKEAHA.112.678151
Sweid A, Starke RM, Herial N, Chalouhi N, Das S, Baldassari MP, Alexander TD, Tjoumakaris S, Gooch MR, Hasan D, Rosenwasser RH, Romo V, Jabbour P (2019) Predictors of complications, functional outcome, and morbidity in a large cohort treated with flow diversion. Neurosurgery. 87:730–743. https://doi.org/10.1093/neuros/nyz508
Nakae R, Nagaishi M, Kawamura Y, Tanaka Y, Hyodo A, Suzuki K (2019) Microhemorrhagic transformation of ischemic lesions on T2*-weighted magnetic resonance imaging after pipeline embolization device treatment. J Neurosurg 130:1997–2004. https://doi.org/10.3171/2017.12.JNS172480
Fazekas F, Kleinert R, Roob G, Kleinert G, Kapeller P, Schmidt R, Hartung HP (1999) Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol 20:637–642
Fisher M (2014) Cerebral microbleeds: where are we now? Neurology 83:1304–1305. https://doi.org/10.1212/WNL.0000000000000871
Kim SH, Shin DW, Yun JM, Lee JE, Lim JS, Cho BL, Kwon HM, Park JH (2017) Kidney dysfunction and cerebral microbleeds in neurologically healthy adults. PLoS One 12:e0172210. https://doi.org/10.1371/journal.pone.0172210
Cho AH, Lee SB, Han SJ, Shon YM, Yang DW, Kim BS (2009) Impaired kidney function and cerebral microbleeds in patients with acute ischemic stroke. Neurology 73:1645–1648. https://doi.org/10.1212/WNL.0b013e3181c1defa
Laible M, Horstmann S, Möhlenbruch M, Wegele C, Rizos T, Schüler S, Zorn M, Veltkamp R (2015) Renal dysfunction is associated with deep cerebral microbleeds but not white matter hyperintensities in patients with acute intracerebral hemorrhage. J Neurol 262:2312–2322. https://doi.org/10.1007/s00415-015-7840-2
Lee M, Saver JL, Hong KS, Rao NM, Wu YL, Ovbiagele B (2013) Risk-benefit profile of long-term dual- versus single-antiplatelet therapy among patients with ischemic stroke: a systematic review and meta-analysis. Ann Intern Med 159:463–470. https://doi.org/10.7326/0003-4819-159-7-201310010-00006
Nishi H, Nakahara I, Matsumoto S, Hashimoto T, Ohta T, Sadamasa N, Ishibashi R, Gomi M, Saka M, Miyata H, Watanabe S, Okata T, Sonoda K, Kouge J, Ishii A, Nagata I, Kira J (2016) Platelet reactivity and hemorrhage risk in neurointerventional procedures under dual antiplatelet therapy. J Neurointerv Surg 8:949–953. https://doi.org/10.1136/neurintsurg-2015-011844
Delgado Almandoz JE, Crandall BM, Scholz JM, Fease JL, Anderson RE, Kadkhodayan Y, Tubman DE (2014) Last-recorded P2Y12 reaction units value is strongly associated with thromboembolic and hemorrhagic complications occurring up to 6 months after treatment in patients with cerebral aneurysms treated with the pipeline embolization device. AJNR Am J Neuroradiol 35:128–135. https://doi.org/10.3174/ajnr.A3621
Ajadi E, Kabir S, Cook A, Grupke S, Alhajeri A, Fraser JF (2019) Predictive value of platelet reactivity unit (PRU) value for thrombotic and hemorrhagic events during flow diversion procedures: a meta-analysis. J Neurointerv Surg 11:1123–1128. https://doi.org/10.1136/neurintsurg-2019-014765
Jeon SB, Kang DW (2007) Neurological Picture. Cerebral air emboli on T2-weighted gradient-echo magnetic resonance imaging. J Neurol Neurosurg Psychiatry 78:871. https://doi.org/10.1136/jnnp.2006.102954
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Higashi, E., Hatano, T., Ando, M. et al. Factors associated with the new appearance of cerebral microbleeds after endovascular treatment for unruptured intracranial aneurysms. Neuroradiology 63, 1079–1085 (2021). https://doi.org/10.1007/s00234-020-02616-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02616-4