Abstract
Purpose
To retrospectively evaluate the diagnostic performance of a 1-min contrast-enhanced 3D-FLASH pulse sequence for detecting intracranial enhancing lesions compared to standard contrast-enhanced 3D-MPRAGE pulse sequence.
Methods
Contrast-enhanced 3D-FLASH (acquisition time 49 s) and contrast-enhanced 3D-MPRAGE (4 min 35 s) pulse sequences were performed consecutively in 110 inpatient/emergency department 3T MRI brain examinations and analyzed by two independent neuroradiologist readers. For each sequence, the readers recorded (1) number of enhancing intracranial lesions; (2) intracranial susceptibility artifact (presence or absence; mm depth of intracranial signal loss); and (3) motion artifact (none, mild, moderate, severe). Inter and intra-reader agreement and reader accuracy relative to a reference standard were determined, and sequence comparison with respect to susceptibility and motion artifacts was performed.
Results
There was substantial intra-reader, inter-sequence agreement [reader 1, κ = 0.70 (95% CI: [0.60, 0.81]); reader 2, κ = 0.70 (95% CI: [0.59, 0.82])] and substantial intra-sequence, inter-reader agreement [3D-MPRAGE assessment, κ = 0.76 (95% CI: [0.66, 0.86]); 3D-FLASH assessment, κ = 0.86 (95% CI: [0.77, 0.94]) for detection of intracranial enhancing lesions. For both readers, the diagnostic accuracy of 3D-FLASH and 3D-MPRAGE was similar (3D-MPRAGE: 86.4 and 88.1%; 3D-FLASH: 88.2 and 84.5%), with no inter-sequence diagnostic accuracy discordancy between the sequences for either reader. 3D-FLASH was associated with less susceptibility artifact (p < 0.001 both readers) and less motion artifact (p < 0.001 both readers).
Conclusion
On 3T brain MRI in the inpatient and emergency department setting, 1-min 3D-FLASH pulse sequence achieved comparable diagnostic performance to 4.5 min 3D-MPRAGE pulse sequence for detecting enhancing intracranial lesions, with reduced susceptibility and motion artifacts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Three-dimensional fast low-angle shot (3D-FLASH) and magnetization-prepared rapid gradient echo (3D-MPRAGE) MRI pulse sequences have been used in clinical neuroimaging for over three decades [1, 2]. Both are gradient-echo pulse sequences that have been optimized to acquire 3D data with high (on the order of 1 mm) isotropic spatial resolution in acquisition times suitable for clinical application. Early studies comparing MPRAGE and FLASH techniques for brain and skull base imaging showed that MPRAGE achieved shorter imaging times and reduced motion artifacts. Furthermore, the T1-weighted contrast was reported comparable or better on MPRAGE compared to FLASH [3, 4]. However, more recent studies have indicated that optimized versions of the 3D-FLASH technique, namely, volumetric interpolated brain examination (VIBE), show improved contrast conspicuity compared to 3D-MPRAGE, with comparable or shorter acquisition times (2–6 min) [5].
Our motivation to re-evaluate these pulse sequences arose from observations of 3D-FLASH localizer sequences performed on 3T MRI scans at our institution. At our institution, an “auto-align” sequence (Siemens Healthineers, Erlangen, Germany) is used as a localizer on 3T MRI scanners [6]. The sequence generates T1-weighted volumetric data of the entire head in the sagittal plane with relatively low spatial resolution and an acquisition time of approximately 14 s (see Table 1). The software then calculates the rigid body registration matrix and relates the anatomy to an auto-align atlas available with the package. This registration allows auto-prescription of imaging planes for performing the diagnostic pulse sequences.
We observed that the 3D data from this “auto-align” sequence approached diagnostic quality, with reasonable T1-weighting and excellent conspicuity of gadolinium-based contrast material. This observation motivated our development and clinical implementation of a modified version of the auto-align 3D-FLASH pulse sequence that provides diagnostic quality, volumetric T1-weighted whole brain imaging (Fig. 1) in about 1 min.
Normal contrast-enhanced 3D-FLASH images (a–c) and 3D-MPRAGE images (d–f) in a 22-year-old female with migraines. Images are reformated in the (a, d) sagittal, (b, e) axial, and (c, f) coronal planes. Time of acquisition for the whole brain 3D-FLASH sequence was 49 s, and time of acquisition for the whole brain 3D-MPRAGE sequence was 4 min and 35 s
The purpose of this study was to retrospectively test the diagnostic performance and image quality of our 1-min 3D-FLASH sequence compared to the 4.5-min 3D-MPRAGE sequence routinely used at our institution for the detection of enhancing intracranial lesions on 3T MRI.
Methods
This retrospective study was performed in line with the principles of the Declaration of Helsinki and was approved by our institutional review board and is Health Insurance Portability and Accountability Act (HIPPA) compliant. The requirement for informed consent was waived.
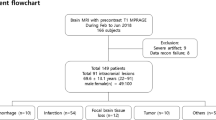
One hundred fifty-three consecutive inpatient and emergency department patients who underwent contrast-enhanced 3T MRI brain examinations at our institution between February 2018 and June 2018 were eligible for study inclusion. Inpatients included any patient admitted for hospitalization at our medical center. Emergency department patients included any patient who presented to our emergency department. All eligible 3T brain MRIs were performed during the emergency department visit or inpatient hospitalization. During this time period, all 3T contrast-enhanced MRI brain protocols in the inpatient/emergency setting included a contrast-enhanced 3D-FLASH and 3D-MPRAGE pulse sequences. We excluded brain MRI examinations if (1) pre-contrast T1-weighted imaging demonstrated abnormal intracranial intrinsic T1 shortening (hyperintense signal) (n = 26); (2) contrast-enhanced 3D-MPRAGE or 3D-FLASH was not performed (n = 15); and (3) the same patient had an earlier contrast-enhanced brain 3T MRI examination already included in our study (n = 2). A total of 110 subjects were ultimately included in our study.
The contrast-enhanced 3D-FLASH and 3D-MPRAGE pulse sequences from each MRI exam were anonymized and randomly assigned to one of two separate research worklists on our PACS. For some subjects, the 3D-FLASH was assigned to the first worklist, and the 3D-MPRAGE was assigned to the second worklist. For the remaining subjects, the opposite order was used. Both of the research worklists had either the contrast-enhanced 3D-FLASH or 3D-MPRAGE sequence for all 110 subjects. All MRI exams were performed on 3T scanners (Magnetom Prisma or Skyra; Siemens Healthineers, Erlangen, Germany) using either a 64-channel or 20-channel head coil (Head/Neck 64 or Head/Neck 20, Siemens Healthineers, Erlangen, Germany). The parameters used for the 3D-FLASH and 3D-MPRAGE techniques, as well as those for the auto-align FLASH pulse sequence from which the 3D-FLASH implementation used for this study was derived, are summarized in Table 1. A slice resolution of 69% was used in the 3D-FLASH sequence resulting in slices spaced at 0.9-mm separation, but with 1.3 mm thickness of each slice. For both techniques, an acceleration factor of 2 was applied using Siemens integrated parallel imaging techniques. Total exam times of contrast-enhanced brain MRIs at our institution are ~ 25–30 min, depending on the specified protocol. 3D-MPRAGE and 3D-FLASH images were always obtained consecutively in each patient. In 63/110 subjects (57%), the 3D-MPRAGE sequence was obtained prior to the 3D-FLASH sequence.
Two board-certified neuroradiologist readers (reader 1, 7 years of experience; reader 2, 1 year of experience) independently reviewed the contrast-enhanced 3D-MPRAGE and 3D-FLASH images on each worklist in PACS. Each worklist was reviewed at two different time points separated by a minimum 1-month interval to avoid recall bias. The readers were tasked with documenting the following for each case based on their analysis of either the contrast-enhanced 3D-MPRAGE or 3D-FLASH: (1) number of enhancing intracranial lesions (n); (2) presence of susceptibility artifact obscuring intracranial anatomy (yes or no); (3) depth of intracranial encroachment of susceptibility artifact (mm); and (4) motion artifact (none, mild, moderate, severe). The readers provided a qualitative description of all enhancing lesions and annotated each lesion with an arrow on PACS which was saved for subsequent review. Readers were instructed not to count regions of non-pathologic intracranial enhancement (e.g., mild post-operative pachymeningeal enhancement, developmental venous anomaly) as “enhancing lesions.” Of note, the readers were supplied separate worklists per reading session. As such, readers could not view prior annotations, neither their own from the first reading session nor those made by the other reader.
After independent reader analysis, a third board-certified neuroradiologist reader (14 years of experience) reviewed the contrast-enhanced 3D-MPRAGE and 3D-FLASH images for all subjects. All independent reader data, reader annotations, and qualitative lesion descriptions were available to reader 3, who had two tasks. The first was to create a criterion standard for number of enhancing lesions per subject. The second was to document and eliminate insignificant discrepancies between readers 1 and 2 based on reader annotations and qualitative descriptions. This involved situations related to the variable counting of a single region of irregular/ill-defined enhancement (e.g., necrotic glioblastoma confined to a single lobe, which one reader counted as a single large enhancing “lesion,” and the other reader counted as multiple juxtaposed enhancing “lesions”) (n = 3), or incorrect counting by one of the readers of benign developmental venous anomalies (n = 4), despite instructions otherwise.
Statistical analyses
Categorical-scaled patient demographics information and categorical patient characteristic information were summarized by frequencies and percentages. Continuous-scaled patient demographic information and patient characteristic information were summarized by the median and interquartile range (IQR) of the empirical distribution.
Concordance between the number of enhancing intracranial lesions detected per case on 3D-MPRAGE and 3D-FLASH images was summarized on a reader by reader basis via the unweighted version of the Kappa statistic (κ), via inter-sequence percent agreement. Inter-reader agreement was evaluated for systematic discordancy via a binomial chi-square test. Percent agreement was estimated by the cross-tabulation of the number of enhancing intracranial lesions detected per case on MPRAGE and FLASH. Percent agreement was defined as the number of cases in which there was exact agreement between the 3D-MPRAGE and the 3D-FLASH enhancing intracranial lesions assessments (i.e., sum of the diagonal elements of the cross-tabulation table) divided by the total number of cases and multiplied by 100%. This estimate was then used to determine an exact 95% confidence interval for percent agreement [7].
Diagnostic accuracy for 3D-MPRAGE and 3D-FLASH was estimated by calculating the percent agreement between the number of enhancing intracranial lesions detected per case by readers 1 and 2 and the criterion measure of number of enhancing intracranial lesions determined by reader 3, via the aforementioned cross-tabulation procedure. An inter-sequence comparison of detection accuracy was conducted via the McNemar’s chi-square test. Fisher’s exact tests determined the association between cases with false negative findings and the order the post-contrast sequence was performed (first or second), and two-tailed independent t tests were used to determine whether false negative errors on 3D-MPRAGE versus 3D-FLASH occurred with lesions of differing size.
A comparison of the frequency of susceptibility artifacts for 3D-MPRAGE and 3D-FLASH pulse sequences was conducted per reader via the McNemar’s chi-square test. Depth of intracranial signal loss from susceptibility artifact was compared via the paired Student’s t test. Motion artifact (none, mild, moderate, severe) on 3D-MPRAGE versus 3D-FLASH images was compared via a binomial chi-square test.
Results
Patient characteristics
Table 2 lists patient characteristics. Among the 110 included patients, there were 59 women and 51 men. Median age was 57 years (IQR: [29, 69 years]). The most common clinical indication for the included contrast-enhanced brain MRI exams was suspected or known intracranial neoplasm (55.5% of exams).
MRI findings
According to the criterion standard, a total of 54 cases contained at least one contrast-enhancing intracranial lesion, and a total of 56 cases contained no contrast-enhancing intracranial lesion. Among the 54 cases that contained an enhancing intracranial lesion, there were 32 cases with intracranial neoplasms, 15 with intracranial infectious/inflammation lesions, 4 with vascular lesions, 3 with demyelinating lesions, 2 with subacute infarctions, and 2 cases of tuberous sclerosis containing enhancing subependymal nodules/giant cell astrocytomas. Four cases contained contrast-enhancing lesions with differing pathologies.
Detection of enhancing intracranial lesions
Both reader 1 and reader 2 showed substantial intra-reader inter-sequence agreement between the number of enhancing intracranial lesions detected by 3D-MPRAGE and detected by 3D-FLASH (reader 1, κ = 0.70 [95%CI: 0.60, 0.81]; reader 2, κ = 0.70 [0.59, 0.82]). Reader 1’s assessment of enhancing lesions on 3D-MPRAGE versus 3D-FLASH showed 88.2% [80.6, 93.6%] agreement. Reader 2’s assessment of enhancing lesions on 3D-MPRAGE versus 3D-FLASH showed 85.5% [77.5, 91.5%] agreement. There was no evidence of systematic discordance between the number of enhancing intracranial lesions detected by 3D-MPRAGE and 3D-FLASH for either reader (p = 0.78 for reader 1, p = 0.45 for reader 2). Supplemental Tables 1 and 2 provide intra-reader comparison data.
There was substantial inter-reader agreement for detecting enhancing intracranial lesions with 3D-MPRAGE (κ = 0.76 [0.66, 0.86]) and with 3D-FLASH (κ = 0.86 [0.77, 0.94]). Inter-reader percent agreement was 85.4% [77.5, 91.5%] with 3D-MPRAGE and 90.0% [82.8, 94.9%] with 3D-FLASH. Supplemental Tables 3 and 4 provide inter-reader comparison data.
The diagnostic accuracy of 3D-MPRAGE was 86.4% [78.5, 92.2%] and 88.1% [80.7, 93.6%] for readers 1 and 2, respectively. The diagnostic accuracy of 3D-FLASH was 88.2% [80.6, 93.6%] and 84.5% [76.4, 90.7%] for readers 1 and 2, respectively. No systematic discordance was found when comparing the diagnostic performance of 3D-MPRAGE and 3D-FLASH to the criterion standard for either reader (p = 0.56 for reader 1, p = 0.21 for reader 2). Supplemental Tables 5–8 provide reader versus criterion standard comparison data per reader per MRI sequence.
Discrepancy features
Cases with false negative errors per reader per MRI sequence were tabulated and compared against the order the MRI sequence was performed. For reader 2, cases with false negative errors with 3D-FLASH associated with 3D-FLASH were being performed as the first post-contrast sequence (p = 0.05). For no other reader-MRI sequence pair was there, a significant association between false negative error and the order an MRI sequence was performed.
For reader 1, there was no significant difference in the mean lesion size of lesions that resulted in false negative errors on 3D-MPRAGE versus 3D-FLASH (4.8 mm [2.9–6.6] versus 3.9 mm [3.1–4.8], p = 0.30). For reader 2, there was no significant difference in the mean lesion size of lesions that resulted in false negative errors on 3D-MPRAGE versus 3D-FLASH (4.6 mm [3.4–5.9] versus 4.3 mm [3.5–5.2], p = 0.71).
Motion and susceptibility artifact
For both readers, there was significantly greater degree of motion artifact on 3D-MPRAGE compared to 3D-FLASH images (p < 0.001 for both readers).
For both readers, there was significantly greater incidence of susceptibility artifact obscuring intracranial anatomy with 3D-MPRAGE versus 3D-FLASH images (p < 0.001 for both readers). For both readers, there was greater depth of intracranial signal loss on 3D-MPRAGE compared to 3D-FLASH images. The mean difference in depth of intracranial signal loss from susceptibility artifact on 3D-MPRAGE versus 3D-FLASH was 13 mm (95% CI: [8, 17 mm] p < 0.001) for reader 1 and 25 mm (95% CI: [21, 29 mm] p < 0.001) for reader 2.
Figures 2, 3, and 4 display comparisons between contrast-enhanced 3D-FLASH and 3D-MPRAGE.
Comparison of contrast-enhanced 3D-MPRAGE and 3D-FLASH images in a 67-year-old male with colon cancer metastases. a 3D-MPRAGE and b 3D-FLASH images display brain metastases in the anterior right frontal lobe, left frontal lobe (two), and left thalamus. 3D-FLASH shows comparable diagnostic performance despite ~ 5-fold reduction in acquisition time relative to 3D-MPRAGE
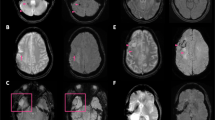
Motion artifact and susceptibility artifact on 3D-MPRAGE versus 3D-FLASH. a, b 54-year-old male with melanoma metastases. a Contrast-enhanced 3D-MPRAGE sequence is degraded by motion artifact, obscuring a small left frontal lobe metastasis that was missed by both neuroradiology readers. b Contrast-enhanced 3D-FLASH sequence is less affected by motion artifact due to its faster acquisition time and more clearly displays the small left frontal lobe metastasis, which both readers detected on this sequence. c, d 14-year-old male with medulloblastoma and a ventricular shunt. The right occipital shunt valve causes local susceptibility artifact which is worse on c 3D-MPRAGE compared to d 3D-FLASH, owing to the longer time to echo and lower receiver bandwidth used for 3D-MPRAGE
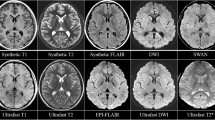
Signal-to-noise ratio in 3D-MPRAGE versus 3D-FLASH. a, b 27-year-old female with multiple sclerosis. An enhancing demyelinating plaque is present in the posterior left frontal lobe white matter (arrows). Both neuroradiologist readers detected the enhancing plaque on the a 3D-MPRAGE sequence where it was more conspicuous. Neither reader detected the lesion on the b 3D-FLASH sequence. The 1-min 3D-FLASH images have lower signal-to-noise ratio than the 4.5-min 3D-MPRAGE images, which might reduce the detectability of very small or mildly enhancing lesions
Discussion
Shortening the acquisition time of MRI pulse sequences while simultaneously maintaining diagnostic quality is a primary goal in pulse sequence development [8]. Shorter acquisition times reduce the potential for motion artifacts, which can severely degrade the diagnostic quality of MR images [9]. Motion artifacts are particularly problematic in acutely ill patients who, for a variety reasons, might be unable to remain still for prolonged image acquisitions, but in whom neurological imaging with MRI might be particularly beneficial and informative. Moreover, shorter MRI acquisition times permit faster patient turnaround times, which potentially increases the utilization of and patient access to MRI [8, 10].
For contrast-enhanced brain MRI, most routine clinical protocols utilize a 3D volumetric T1-weighted acquisition of the brain. The most widely employed of these pulse sequences is an inversion-recovery-prepared gradient-echo pulse sequence, often referred to as MPRAGE [11]. Common implementations of MPRAGE require acquisition times that range from approximately 3.5 to 9 min on 3 T MRI [12,13,14,15,16,17] and are therefore susceptible to degradation by patient motion. At our institution, we recently employed a rapid (49-s acquisition time) diagnostic quality 3D-FLASH sequence for contrast-enhanced brain imaging at 3T MRI. Our study results indicate that, for 3T MRI brain in the inpatient/emergency setting, contrast-enhanced 3D-FLASH has comparable diagnostic performance to 3D-MPRAGE with ~ 5-fold shorter image acquisition time. As expected, the 1-min 3D-FLASH sequence was associated with significantly lower motion artifact compared to the 4.5-min 3D-MPRAGE. Compared to older studies [3, 4], a much shorter acquisition time for diagnostic-quality FLASH has been enabled by advancements in both MR hardware and methodology, including, in particular, higher field strengths, improved receiver systems, vastly improved radio-frequency coils, and parallel imaging methods. As far as diagnostic performance, there is generally a trade-off between signal to noise ratio and motion artifact, both of which affect the detectability of intracranial enhancing lesions. Absent significant motion artifact, the higher signal to noise ratio afforded by 3D-MPRAGE is advantageous, especially for the detection of subtle lesions (Fig. 4). For patients prone to motion, the shorter acquisition time afforded by 3D-FLASH can prove extremely useful (Fig. 3a, b). While motion-navigator radio-frequency pulses can be used in conjunction with MPRAGE to substantially reduce artifacts from head motion [9], this implementation is not available as a standard option on most MRI scanners. Based on our results, we endorse the 1-min 3D-FLASH as a useful adjunct to the longer 3D-MPRAGE for post-contrast imaging of inpatient/emergency patients, especially those prone to motion artifact.
Furthermore, the 3D-FLASH sequence was less degraded by susceptibility artifact compared with 3D-MPRAGE. Susceptibility artifact increases with higher magnetic field strength and longer time to echo and is inversely related to receiver bandwidth [18]. Compared to our 3D-FLASH sequence, 3D-MPRAGE had longer time to echo and lower receiver bandwidth, accounting for the increased degree of susceptibility artifact we observed with 3D-MPRAGE. The 3D-MPRAGE implementation could be modified to have higher receiver bandwidth and shorter time to echo, but at the expense of signal-to-noise ratio.
Aside from MPRAGE, currently there are other newer but well-established 3D T1-weighted pulses sequence available for post contrast imaging. These include “sampling perfection with application-optimized contrasts by using different flip angle evolutions” (SPACE), which is reported to demonstrate superior conspicuity for contrast enhancement than MPRAGE [11], though with acquisition times in a similar range to MPRAGE [5, 11,12,13,14]. Compared with MPRAGE, both VIBE and T1-SPACE sequences tend to show less contrast between gray matter and white matter, producing a more “bland” appearance of the background brain tissue. Furthermore, T1-SPACE shows less physiological enhancement of the intracranial vasculature. Such features that reduce the intrinsic contrast of the background anatomy can be advantageous towards the goal of detecting pathological intracranial enhancement. Future investigation comparing 3D-FLASH with other 3D pulse sequences such as T1-SPACE would be of clinical interest.
Our study is limited by its retrospective design, and our results would be strengthened by efforts at prospective validation. Although the 3D-MPRAGE and 3D-FLASH sequences were anonymized and randomized between reading lists, it is possible that the readers could recognize which of the pulse sequences they were evaluating at a given time, potentially introducing bias in the data collection. We restricted our study to the evaluation of inpatient and emergency room patients, and we have not tested our 3D-FLASH technique in the outpatient setting. Our cohort included relatively few pediatric patients, and further study in this population would be of potential interest since application of rapid imaging, such as a 1-min 3D-FLASH, could help reduce the need for sedation for MRI [19]. We did not evaluate the diagnostic performance of 3D-FLASH per pathologic entity given our overall cohort size, and further investigation may reveal clinical indications or disease processes where the 1-min 3D-FLASH is more or less effective as a clinical diagnostic tool. Our results cannot be extrapolated for 1.5T MRI, and in our institutional experience, the 1-min 3D-FLASH sequence generally suffers from excessive image noise at 1.5T. Pre-contrast T1WI was not included in our analysis, and as such we could not study the effect of T1 shortening on diagnostic performance. Finally, our results only apply to the detection of contrast-enhancing brain lesions, and we have not formally evaluated the utility of 3D-FLASH as a non-contrast MRI sequence. Based on our experience, MPRAGE affords superior gray-white matter differentiation, which is an important attribute for non-contrast T1 weighted brain imaging.
In conclusion, at 3T MRI in the inpatient and emergency department setting, a 1-min contrast-enhanced 3D-FLASH pulse sequence achieved comparable diagnostic performance to a 4.5-min contrast-enhanced 3D-MPRAGE pulse sequence for detecting enhancing intracranial lesions, with shorter acquisition time and reduced susceptibility and motion artifacts. In patients prone to motion artifact, contrast-enhanced 3D-FLASH can serve as a clinically useful adjunct or alternative to 3D-MPRAGE for brain imaging at 3T MRI.
References
Haase A, Frahm J, Matthaei D, Hänicke W, Merboldt KD (1986) FLASH imaging: rapid NMR imaging using low flip-angle pulses. J Magn Reson 213:533–541
Mugler JP, Brookeman JR (1990) Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE). Magn Reson Med 15:152–157
Runge VM, Kirsch JE, Thomas GS, Mugler JP (1991) Clinical comparison of three-dimensional MP-RAGE and FLASH techniques for MR imaging of the head. J Magn Reson Imaging 1:493–500
Held P, Fellner C, Fellner F, Geissler A, Gmeinwieser J (1995) Three-dimensional MP-RAGE--an alternative to conventional three-dimensional FLASH sequences for the diagnosis of viscerocranial tumours? Br J Radiol 68:1316–1324
Wetzel SG, Johnson G, Tan AGS, Cha S, Knopp EA, Lee VS, Thomasson D, Rofsky NM (2002) Three-dimensional, T1-weighted gradient-echo imaging of the brain with a volumetric interpolated examination. Am J Neuroradiol 23:995–1002
van der Kouwe AJW, Benner T, Fischl B, Schmitt F, Salat DH, Harder M, Sorensen AG, Dale AM (2005) On-line automatic slice positioning for brain MR imaging. Neuroimage 27:222–230
Agresti A, Coull BA (1998) Approximate is better than “exact” for interval estimation of binomial proportions. Am Stat 52:119–126
Tsao J (2010) Ultrafast imaging: principles, pitfalls, solutions, and applications. J Magn Reson Imaging 32:252–266
Zaitsev M, Maclaren J, Herbst M (2015) Motion artifacts in MRI: a complex problem with many partial solutions. J Magn Reson Imaging 42:887–901
O’Brien JJ, Stormann J, Roche K et al (2017) Optimizing MRI logistics: focused process improvements can increase throughput in an academic radiology department. AJR Am J Roentgenol 208:W38–W44
Danieli L, Riccitelli GC, Distefano D, Prodi E, Ventura E, Cianfoni A, Kaelin-Lang A, Reinert M, Pravatà E (2019) Brain tumor-enhancement visualization and morphometric assessment: a comparison of MPRAGE, SPACE, and VIBE MRI techniques. AJNR Am J Neuroradiol 40:1140–1148
Kakeda S, Korogi Y, Hiai Y, Ohnari N, Moriya J, Kamada K, Hanamiya M, Sato T, Kitajima M (2007) Detection of brain metastasis at 3T: comparison among SE, IR-FSE and 3D-GRE sequences. Eur Radiol 17:2345–2351
Fukuoka H, Hirai T, Okuda T, Shigematsu Y, Sasao A, Kimura E, Hirano T, Yano S, Murakami R, Yamashita Y (2010) Comparison of the added value of contrast-enhanced 3D fluid-attenuated inversion recovery and magnetization-prepared rapid acquisition of gradient echo sequences in relation to conventional postcontrast T1-weighted images for the evaluation of leptomening. AJNR Am J Neuroradiol 31:868–873
Park J, Kim J, Yoo E, Lee H, Chang J-H, Kim EY (2012) Detection of small metastatic brain tumors: comparison of 3D contrast-enhanced whole-brain black-blood imaging and MP-RAGE imaging. Investig Radiol 47:136–141
Mugler JP, Brookeman JR (1991) Rapid three-dimensional T1-weighted MR imaging with the MP-RAGE sequence. J Magn Reson Imaging 1:561–567
Jeevanandham B, Kalyanpur T, Gupta P, Cherian M (2017) Comparison of post-contrast 3D-T1-MPRAGE, 3D-T1-SPACE and 3D-T2-FLAIR MR images in evaluation of meningeal abnormalities at 3-T MRI. Br J Radiol 90:20160834
Kim D, Heo YJ, Jeong HW et al (2019) Usefulness of the delay alternating with nutation for tailored excitation pulse with t1-weighted sampling perfection with application-optimized contrasts using different flip angle evolution in the detection of cerebral metastases: comparison with MPRAGE imaging. Am J Neuroradiol 40:1469–1475
Huang SY, Seethamraju RT, Patel P, Hahn PF, Kirsch JE, Guimaraes AR (2015) Body MR imaging: artifacts, k-Space, and solutions. Radiographics 35:1439–1460
Kozak BM, Jaimes C, Kirsch J, Gee MS (2020) MRI techniques to decrease imaging times in children. Radiographics 40:485–502
Acknowledgments
We acknowledge the contributions of Aayush Amin (A.R.R.T), (RT), (MR) for assisting in the clinical implementation of 3D-FLASH.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
The requirement for informed consent for this retrospective study was waived by our institutional review board.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Patel, S.H., Batchala, P.P., Schallert, K. et al. 3D fast low-angle shot (FLASH) technique for 3T contrast-enhanced brain MRI in the inpatient and emergency setting: comparison with 3D magnetization-prepared rapid gradient echo (MPRAGE) technique. Neuroradiology 63, 897–904 (2021). https://doi.org/10.1007/s00234-020-02590-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02590-x