Abstract
Purpose
We report a multicenter experience using endovascular embolization as the first line approach for treatment of anterior cranial fossa (ACF) dural arteriovenous fistula (DAVF).
Methods
All patients with DAVFs located in the anterior cranial fossa who were treated with endovascular technique as a first line approach were included. Demographics, clinical presentation, angioarchitecture, strategy, complications, immediate angiographic, and follow-up results were included in the analysis.
Results
Twenty-three patients met the inclusion criteria (18 male and 5 female). Age ranged from 14 to 79 years (mean 53 years). Twelve patients presented with hemorrhage. Twenty-eight endovascular procedures were performed. The overall immediate angiographic cure rate after endovascular treatment was 82.6% (19/23 patients). The angiographic cure rate of the transvenous strategy was significantly superior to the transarterial strategy (p ≤ 0.001). There was 1 complication in 28 total procedures (3.6%). Angiographic follow-up was available in 21 out of the 23 patients with a mean of 25 months (range 2 to 108 months). In these 21 patients, the DAVF was completely cured in 20 (95%). At last follow-up, all patients had a modified Rankin scale (mRS) 0 to 2.
Conclusion
Our experience suggests that endovascular treatment for ACF DAVFs has an acceptable safety profile with high rates of complete occlusion, particularly with transvenous approach. Whenever possible, transvenous approach should be preferred over transarterial approach as first line strategy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dural arteriovenous fistulas (DAVFs) located in the anterior cranial fossa (ACF) are rare lesions [1,2,3,4,5,6]. Because there is no significant venous sinus in the anterior cranial fossa, these lesions almost always demonstrate cortical venous drainage and therefore are aggressive lesions that require prompt attention and treatment since the hemorrhagic risk is significant [1,2,3,4,5,6,7,8,9,10].
The safety and efficacy of endovascular treatment for most types of DAVFs has been extensively documented in the literature [8,9,10,11]. However, endovascular treatment of ACF DAVFs has posed significant technical challenges. From the transarterial approach (TA), these lesions are usually supplied by ethmoidal branches of the ophthalmic arteries, and therefore distal navigation and embolization carries a risk of blindness. The transvenous approach (TV) has been used successfully; however, since it requires the navigation through small tortuous cortical veins to achieve the fistulous point, this technique is not without risks [1,2,3,4,5,6]. Moreover, these lesions can be effectively treated by craniotomy and microsurgical clip ligation [1, 2, 6].
We report our multicenter experience using endovascular embolization as the first line treatment of anterior cranial fossa DAVFs. We also compared the occlusion rates of TA versus TV.
Methods
Eight high volume neurovascular centers (> 500 neuroendovascular procedures per/year) were recruited to submit retrospective angiographic and clinical outcome data on consecutive patients with ACF DAVFs treated by endovascular techniques.
After approval from our Institutional Review Board was obtained (IRBnet 1150442), the databases of patients treated in our institutions were reviewed. All patients with ACF DAVFs who were treated from August 2010 to August 2019 with embolization as the first line strategy were included.
All procedures were performed by trained neuroendovascular surgeons. The use of general anesthesia and intraprocedural heparinization as well as the choice of endovascular access was decided according to the treating surgeon and center’s preference depending on the lesion characteristics. Transarterial approach (TA) was performed in the usual standard fashion. A 5 or 6 Fr guide-catheter was placed in the respective ICA or ECA. A microcatheter was then navigated over the microwire and selective microcatheterization of the arterial supply to the fistula was performed. Transvenous approach (TV) was performed by either transfemoral or transjugular access followed by guide-catheter placement in the superior sagittal sinus and selective microcatheterization of the draining vein to reach the “foot” of the vein. The choice of which devices and embolic agents were used in either TA and TV was decided by the treating surgeon taking into account preference and local experience. The microcatheters used include Headway 17 (Microvention), Headway Duo (Microvention), Scepter balloon microcatheter (Microvention), Marathon (Medtronic), Apollo (Medtronic), and Sonic (Balt). At the end of the embolization procedure, the microcatheter was removed in all cases. The embolic agents used included Onyx (Medtronic), Phil (Microvention), and coils. Demographics, clinical presentation, arterial supply, venous drainage, TA versus TV, complications, immediate angiographic, and follow-up results were included in the analysis. Cure was defined as complete occlusion with no angiographic evidence of residual or recurrent DAVF in the immediate and follow-up imaging respectively. Transarterial or transvenous failure was defined as persistent opacification of the draining vein at the end of the procedure.
Statistical analysis (GraphPad Software, Inc., La Jolla, CA) was performed using Fisher’s exact test for categorical variables association whenever appropriate. Findings were statistically significant at a two-tailed p < 0.05.
Results (Table 1)
Demographics and clinical presentation
During the study period, 28 patients were diagnosed with ACF DAVF. All patients were considered candidates for endovascular treatment. Five patients refused endovascular treatment; of those, 4 were not treated, and one opted for open surgery. There were 23 patients included in the analysis, 18 male and 5 female. Ages ranged from 14 to 79 years (mean 53 years). All patients had a baseline modified Rankin scale (mRS) 0–2 prior to the treatment, except 3 patients that presented with hemorrhage and had mRS of 3 (2 patients) or 4 (one patient). Twelve patients presented with hemorrhage. In the hemorrhagic cohort, the most common symptoms were headaches in 10, with concomitant seizures in 4, and loss of consciousness in 2. The hemorrhage location was subarachnoid with associated intraparenchymal component in 6 patients, subarachnoid only in 4 patients and intraparenchymal only in 2 patients. In the nonhemorrhagic cohort (11 patients), 4 patients presented with headaches, and 7 patients were asymptomatic, and the DAVF was an incidental finding.
Angiographic architecture
In this series, arterial supply from the ophthalmic artery (OA) was present in all cases (bilateral OA in 16 cases and single OA in 7 cases). Associated arterial supply from the internal maxillary and middle meningeal arteries was seen in 7 and 6 patients respectively. Cortical venous drainage was seen in 21 patients (91.3%). In two patients, the DAVF drained through the superior ophthalmic vein.
Endovascular strategy results: transarterial vs transvenous
Twenty-eight endovascular procedures were performed. Eighteen patients were treated in a single session. When comparing hemorrhagic versus nonhemorrhagic presentation, there was no statistical difference in regard to the approach (p = 0.09) or angiographic cure (p = 1).
Transarterial embolization, using nonadhesive liquid embolic agents was the initial strategy in 9 patients (Onyx in 8 patients and Phil in 1 patient), with the ophthalmic artery being the most common pedicle except for 2 patients where the middle meningeal artery was used. Of these 9 patients, 7 required additional treatment to achieve immediate angiographic cure (5 treated by transvenous approach and 2 with open surgery). Therefore, the failure (persistent opacification of the draining vein at the end of the procedure) rate for transarterial embolization alone was 77.8%. There were no angiographic changes in the arterial supply to the choroidal-retinal blush in the patients treated by TA embolization.
Transvenous embolization using nonadhesive liquid embolic agents (Onyx in 11 patients, Phil in 3 patients), coils (4 patients), or combination of coils and Onyx (1 patient), was used in 19 patients (14 as the initial strategy and 5 post failed TA approach). Of the 19 patients treated by TV, immediate angiographic cure was achieved in 17 (89.5%) after a single TV treatment session.
The overall immediate angiographic cure after endovascular treatment (TA and TV) is 82.6% (19/23 patients). Two patients that failed TA embolization were treated with open surgery. In the TV group, two patients were not cured at the end of the procedure. One patient had partial occlusion of the DAVF and has refused further treatment remaining asymptomatic on clinical follow-up at 3 years. The other patient suffered a complication during the TV approach. In this case, a venous perforation was noted during retrograde microcatheterization of a tortuous, irregular frontal cortical vein using a Scepter balloon microcatheter. The procedure was stopped, and a cone beam CT demonstrated subdural hemorrhage with mass effect. The patient was taken for emergent surgery with decompressive hemicraniectomy and surgical treatment of the DAVF. The patient’s clinical condition improved and, at a 2-month, follow-up mRS was 1. This complication has been further described in a recently published technical video [12]. This was the only complication of our series in 28 procedures accounting for 3.6% of total procedures and 5.3% of the TV treatments. There were no microcatheters left in place, with all microcatheters being successfully removed at the end of the procedures.
In our series, the angiographic cure rate of TV was significantly superior to the TA (p ≤ 0.001). There was no statistical difference in the complication rates between TV and TA.
Follow-up
Angiographic follow-up was available in 21 out of the 23 patients at a mean of 25 months (range 2 to 108 months). In these 21 patients, the DAVF was completely cured in 20 (95%). At last follow-up, all patients had mRS 0 to 2.
Discussion
Anterior cranial fossa dural arteriovenous fistulas (ACF DAVFs), also referred to as ethmoidal or cribriform plate DAVFs, are rare lesions [1,2,3,4,5,6]. Because of their anatomical location, many still favor open surgery as the first line treatment [2, 13]. In spite of that, our study demonstrates that endovascular treatment of ACF DAVFs can be performed safely yielding high complete occlusion rates.
Anterior cranial fossa DAVFs are usually supplied by ethmoidal branches of the ophthalmic arteries, although supply from the middle meningeal arteries and internal maxillary arteries has also been described [3,4,5]. In our series, all lesions where supplied by at least one ophthalmic artery; concomitant internal maxillary (IMAX = 7) and middle meningeal (MMA = 6) supply was seen in 56% of cases. Since there is no significant dural venous sinus in the ACF, these DAVFs usually present with cortical venous drainage; less commonly orbital venous drainage has also been demonstrated [5]. Because their usual draining venous pattern is through cortical veins, these lesions have a high risk of hemorrhage and therefore should be considered for treatment. In our series, 21 patients demonstrated cortical venous drainage, and 2 had orbital drainage.
Over the years, ACF DAVFs have been treated with bifrontal craniotomy and surgical ligation/occlusion though a subfrontal or interhemispheric approach [2]. This approach is highly effective [1,2,3,4,5,6]; however, it is not without risks including infection, retraction injury, anosmia, and brain edema [2, 6]. Complication rates for surgical treatment ranging from 5 to 9% have been reported in the literature [4, 6]. Because of those risks, some authors recommend open surgery only if the lesion is not accessible or has failed endovascular treatment [6, 13]. In our series, 3 patients failed embolization (2 TA and 1 TV) and underwent open surgical treatment.
The transarterial treatment of DAVFs using nonadhesive liquid embolic agents as primary embolic agents is well established [8,9,10,11]. However, the vast majority of these cases do not involve ACF lesions. In one of the early publications, Cognard et al. [8] studied 30 cases of DAVFs associated with cortical venous drainage treated with Onyx; in this study, cure was achieved in 24 cases with a good safety profile. Another study with a large number of patients treated with transarterial embolization using Onyx demonstrated that when Onyx was used as a sole embolic agent angiographic cure was seen in 87% of the DAVFs [11].
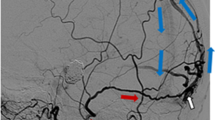
Despite these recent advances, endovascular approaches to treat ACF DAVFs continue to pose significant challenges. From the transarterial perspective, super-selective microcatheterization and embolization through the ophthalmic artery carries the risks of dissecting the ophthalmic artery, or unwanted reflux/embolization of the central retinal artery, which will potentially lead to monocular blindness. Moreover, microcatheter retention, cranial neuropathy (when embolization is performed through the MMA or IMAX), as well as failure to deliver the embolic agent due to difficult tortuous arteries have been described [1,2,3,4,5,6, 8, 11, 13,14,15,16,17,18,19,20]. This may explain why TA embolization alone using nonadhesive liquid embolic agents (Onyx or Phil) had an occlusion rate of only 23% in our series. Similar suboptimal results using TA were also seen in Robert et al. [4] series where 10 patients were treated by endovascular approach in thirteen embolization sessions. In their series, the TA through the ophthalmic artery was the first choice and was successful as a single approach in only 45.5% of cases. In two cases microcatheterization of the ophthalmic artery was not possible despite the use of adjunct devices such as balloons. For cases that failed the initial approach through the ophthalmic artery, a secondary approach through the anterior branch of the MMA yielded occlusion in 50%. The TV approach was used successfully in 2 cases (100% success rate). In two cases, endovascular treatment failed, and the patients required open surgery. There were no complications from the endovascular procedures. However, when the anatomy is favorable, TA may have increased chances of success (Fig. 1).
A 57-year-old presenting with headache, nausea and photophobia. MRI demonstrates a small venous aneurysm with associated edema in the left anterior cranial fossa (Fig. 1a—arrow). Small subarachnoid hemorrhage with intraventricular extension is demonstrated (Fig. 1a—arrowhead). Angiography demonstrates a favorable transarterial anatomy with supply through the ophthalmic artery to the nidus (Fig. 1b and c—arrow). Please note that there is occlusion of the main draining vein at the junction with the superior sagittal sinus (Fig. 1b and c—double arrows). Because of that secondary vein harboring a venous aneurysm (Fig. 1b and c—arrowhead) provides drainage for the fistula. The favorable transarterial anatomy allows for successful distal navigation and positioning of the tip of the Apollo detachable-tip microcatheter into the fistulous nidus (Fig. 1d). The lesion is then successfully embolized. Fig. 1e demonstrates the Onyx cast. After the embolization, the microcatheter is removed in its entirety. Please note the Onyx penetration into the fistulous connections reaching the proximal aspect of the draining vein resulting in immediate complete angiographic occlusion (Fig. 1f). The choroidal-retinal blush is preserved
Li et al. [3] presented a series of 6 consecutive patients with Cognard type IV ACF DAVFs treated by trans-arterial embolization using Onyx. The treatment was effective with complete obliteration of the fistula in 5 out of the 6 cases. The authors reported no mortality or permanent morbidity related to the procedure, although two patients developed transient chemosis and retroorbital discomfort. A case-by-case strategy has also been advocated by some authors. Cannizzaro et al. [13] presented a multicenter series of 16 patients with ACF DAVFs of which 13 were treated, 7 by surgery, and 6 by an endovascular approach. All lesions were completely occluded, and all patients had favorable neurological outcomes. The authors concluded that despite still being traditionally considered surgical lesions, selected cases can be safely and effectively treated endovascularly.
In another series, Agid et al. [6] reported on 24 patients with ACF DAVFs. Eleven patients were treated with surgery and 2 with radiosurgery. Eleven patients were treated by transarterial endovascular embolization using N-butyl cyanoacrylate (NBCA). Surgery was effective in occluding the fistula in all cases. In the endovascular cohort, cure was achieved in 7 (63.6%). The 4 cases that failed embolization underwent successful surgery with disconnection of the fistula. There were no complications in the endovascular group. One patient in the surgical group developed brain edema and confusion after surgery.
Because of the shortcomings related to TA, transvenous approaches have emerged as an important alternative for primary treatment of DAVFs as well as for cases of failed TA. This has been facilitated by recent advances in endovascular technology with development of newer generation of distal access guide-catheters which allow distal navigation through the dural sinuses reaching the anterior aspect of the superior sagittal sinus [2]. From this position a microcatheter can be gently navigated over a microwire retrogradely through the draining cortical vein until the “foot” of the vein is reached [2, 12]. This microcatheter placement allows embolization with coils, which occlude the draining vein at its origin, or liquid embolic agents with retrograde filling of the arterial network feeding the DAVF at the same time that it occludes the draining vein. In fact, transvenous embolization using either nonadhesive liquid embolic agents (Onyx or Phil) or coils was used in 19 patients in our series (Fig. 2). Of these, 14 were treated as the initial strategy and 5 after failed attempts with TA.
A 66-year-old patient presenting with altered mental status and frontal subarachnoid hemorrhage. Angiography revealed an ACF DAVF supplied by the ophthalmic artery draining into a frontal cortical vein (Figs. 2a and b—arrow). Note that the ophthalmic artery is small in size and very tortuous. Decision was then made to perform transvenous approach. A 6-French distal access catheter was advanced through a jugular access and positioned in the most anterior aspect of the superior sagittal sinus (Fig. 2c—arrow). An Apollo detachable-tip microcatheter is then carefully navigated retrogradely through the draining frontal cortical vein until it reaches the “foot” of the draining vein (Fig. 2d). Embolization is then performed using Onyx. Note the embolic agent filling the “foot” of the vein as well as the arterial feeders due to retrograde penetration of the embolic agent (Fig. 2e—arrow). Final images demonstrate immediate complete angiographic occlusion of the DAVF (Fig. 2f)
Transvenous embolization using Onyx has been reported by Albuquerque et al. [20]. They reported success treating 3 high risk DAVF with Onyx transvenously. Similar results were reported by Spiotta et al. [2] in a series of 3 patients with ACF DAVFs that were successfully treated via a transfemoral venous approach with no complications. Our series indicates that TV is superior to TA (p ≤ 0.001) as first line strategy for treatment of ACF DAVFs achieving cure rate of 89.5% (17 of 19 patients) after a single TV treatment session. However, TV also involves important risks. Microcatheter and microwire navigation and manipulation through small, tortuous cortical veins that not infrequently present with some degree of venopathy can lead to vessel rupture and devastating consequences. Our only complication (1 out of 28 procedures) was a venous perforation that resulted in subdural hemorrhage with mass effect requiring decompressive hemicraniectomy. This patient recovered well with a mRS of 1 at 2 months.
We also demonstrate that the occlusion rates are high at midterm follow-up. Of the 21 patients with a mean follow-up of 25 months, the DAVF was completely cured in 20 (95%).
Our study has some limitations. First, as a retrospective review across several institutions, the selection of cases for treatment was based on the operators’ personal experience potentially resulting in selection bias. Second, the heterogeneous presentation of the disease (hemorrhage and nonhemorrhagic) may affect treatments and outcomes; however, when comparing hemorrhagic versus nonhemorrhagic presentation in our series, there was no statistical difference in regard to the approach (TA vs TV) (p = 0.09) or angiographic cure (p = 1). Third, the endovascular techniques and the choice of approach were not standardized. Lastly, the team of treating physicians adjudicated the angiographic and the clinical results, which introduces bias. Nonetheless, the study demonstrates real-world experience in treating these complex uncommon lesions.
In summary, our experience suggests that endovascular treatment for ACF DAVFs is a safe and effective treatment option with high rates of complete occlusion, particularly using the transvenous approach. Whenever possible, the transvenous approach should be preferred over transarterial embolization as first line strategy.
References
Halbach VV, Higashida RT, Hieshima GB, Wilson CB, Barnwell SL, Dowd CF (1990) Dural arteriovenous fistulas supplied by ethmoidal arteries. Neurosurgery 26(5):816–823
Spiotta AM, Hawk H, Kellogg RT, Turner RD, Chaudry MI, Turk AS (2014) Transfemoral venous approach for Onyx embolization of anterior fossa dural arteriovenous fistulae. J Neurointerv Surg 6(3):195–199
Li C, Wu Z, Yang X, Li Y, Jiang C, He H (2014) Transarterial treatment with Onyx of Cognard type IV anterior cranial fossa dural arteriovenous fistulas. J Neurointerv Surg 6(2):115–120
Robert T, Blanc R, Smajda S, Ciccio G, Redjem H, Bartolini B, Fahed R, Piotin M (2016) Endovascular treatment of cribriform plate dural arteriovenous fistulas: technical difficulties and complications avoidance. J Neurointerv Surg 8(9):954–958
Inoue A, Tagawa M, Kumon Y, Watanabe H, Shoda D, Sugiu K, Ohnishi T (2015) Ethmoidal dural arteriovenous fistula with unusual drainage route treated by transarterial embolization. J Neurointerv Surg 7(4):e15
Agid R, Terbrugge K, Rodesch G, Andersson T, Söderman M (2009) Management strategies for anterior cranial fossa (ethmoidal) dural arteriovenous fistulas with an emphasis on endovascular treatment. J Neurosurg 110(1):79–84
van Dijk JM, terBrugge KG, Willinsky RA, Wallace MC (2002) Clinical course of cranial dural arteriovenous fistulas with long-term persistent cortical venous reflux. Stroke 33(5):1233–1236
Cognard C, Januel AC, Silva NA Jr, Tall P (2008) Endovascular treatment of intracranial dural arteriovenous fistulas with cortical venous drainage: new management using Onyx. AJNR Am J Neuroradiol 29(2):235–241
Dabus G, Bernstein RA, Hurley MC, Shaibani A, Bendok BR, Russell EJ (2010) Reversal of diffusion restriction after embolization of dural arteriovenous fistula: case report. Neurosurgery 67(4):E1147–E1151 discussion E1151
Nogueira RG, Dabus G, Rabinov JD, Eskey CJ, Ogilvy CS, Hirsch JA, Pryor JC (2008) Preliminary experience with onyx embolization for the treatment of intracranial dural arteriovenous fistulas. AJNR Am J Neuroradiol 29(1):91–97
Hu YC, Newman CB, Dashti SR, Albuquerque FC, McDougall CG (2011) Cranial dural arteriovenous fistula: transarterial Onyx embolization experience and technical nuances. J Neurointerv Surg 3(1):5–13
Roa JA, Dabus G, Dandapat S, Hasan D, Samaniego EA (2020) Ethmoidal dural arteriovenous fistulas: endovascular transvenous embolization technique. J Neurointerv Surg 12:610
Cannizzaro D, Peschillo S, Cenzato M, Pero G, Resta MC, Guidetti G, Burdi N, Piccirilli M, Santoro A, Lanzino G (2018) Endovascular and surgical approaches of ethmoidal dural fistulas: a multicenter experience and a literature review. Neurosurg Rev 41(2):391–398
Kelly ME, Turner R, Gonugunta V, Rasmussen PA, Woo HH, Fiorella D (2008) Monorail snare technique for the retrieval of an adherent microcatheter from an onyx cast: technical case report. Neurosurgery 63(1 Suppl 1):ONSE89 discussion ONSE89
Alamri A, Hyodo A, Suzuki K, Tanaka Y, Uchida T, Takano I, Kowata K, Iwatate K, Suzuki R (2012) Retrieving microcatheters from Onyx casts in a series of brain arteriovenous malformations: a technical report. Neuroradiology 54(11):1237–1240
Saatci I, Geyik S, Yavuz K, Cekirge HS (2011) Endovascular treatment of brain arteriovenous malformations with prolonged intranidal Onyx injection technique: long-term results in 350 consecutive patients with completed endovascular treatment course. J Neurosurg 115(1):78–88
Weber W, Kis B, Siekmann R, Kuehne D (2007) Endovascular treatment of intracranial arteriovenous malformations with onyx: technical aspects. AJNR Am J Neuroradiol 28(2):371–377
Nyberg EM, Chaudry MI, Turk AS, Turner RD (2013) Transient cranial neuropathies as sequelae of Onyx embolization of arteriovenous shunt lesions near the skull base: possible axonotmetic traction injuries. J Neurointerv Surg 5(4):e21
Newman CB, Park MS, Kerber CW, Levy ML, Barr JD, Pakbaz RS (2012) Over-the-catheter retrieval of a retained microcatheter following Onyx embolization: a technical report. J Neurointerv Surg 4(4):e13
Albuquerque FC, Ducruet AF, Crowley RW, Bristol RE, Ahmed A, McDougall CG (2013) Transvenous to arterial Onyx embolization. J Neurointerv Surg 6(4):281–5
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Guilherme Dabus, MD: Consultant Medtronic, Microvention, Penumbra, Cerenovus.
Peter Kan. MD: Consultant: Stryker, Microvention, Cerenovus.
Carlos Diaz, MD: Consultant: Medtronic.
Boris Pabon, MD: Consultant: Medtronic, Microvention, Stryker, MIVI, Syntheon.
Juan Andres-Mejia, MD: None.
Italo Linfante, MD: Consultant: Medtronic, Stryker, Prolong Pharmaceuticals.
Jonathan A. Grossberg, MD: Georgia Research Alliance Grant; Consultant: Cognition Medical.
Brian M. Howard, MD: None.
Civan Islak, MD: Consultant: Microvention.
Naci Kocer, MD: Consultant: Microvention.
Osman Kizilkilic, MD: None.
Viraj Moholkar, MD: None.
Anna L. Kuhn, MD: None.
Ajit S. Puri, MD: Consultant: Stryker, Medtronic, Cerenovus, Microvention, CereVasc, Merit; Received research grants from Stryker, Medtronic, Cerenovus, Microvention.
Santiago Ortega-Gutierrez, MD: Consultant for Stryker.
Edgar A. Samaniego, MD: Consultant for Microvention, Medtronic.
Michael W. McDermott, MD: None.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study. The data included in the manuscript has been all de-identified.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dabus, G., Kan, P., Diaz, C. et al. Endovascular treatment of anterior cranial fossa dural arteriovenous fistula: a multicenter series. Neuroradiology 63, 259–266 (2021). https://doi.org/10.1007/s00234-020-02536-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02536-3