Abstract
Purpose
The management of residual or persistent intracranial aneurysms after flow-diversion therapy is not well defined in the literature. In this multicentric study, we report clinical and angiographic outcomes of 11 patients that underwent retreatment for 12 aneurysms initially treated with flow-diverter stents.
Methods
The median patient age was 53 years. Aneurysms (median size, 7.3 mm) were located at the internal carotid artery in 9 cases, and at the posterior circulation in 3. Treatment strategies, complications, and angiographic outcome were retrospectively assessed.
Results
Retreatment was feasible in all cases and performed by overlapping flow-diverter implantation. Overall, 12 side vessels were covered during retreatment, whereof 10 (83.3%) remained patent until mid-term follow-up. There were no further technical or symptomatic complications and no treatment-related morbidity. Angiographic follow-up (median, 17 months) showed improved aneurysm occlusion in all patients. Complete or near-complete aneurysm occlusion was achieved in 11 aneurysms (91.7%).
Conclusion
Required retreatment after failed flow-diversion therapy can be performed with adequate safety and efficacy by placement of additional flow-diverter stents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the introduction of the Pipeline Embolization Device (PED, Covidien, Mansfield, MA, USA) in 2008, the concept of flow-diversion has evolved into an established technique for endovascular treatment of intracranial aneurysms [1]. In particular, flow-diverter devices (FDDs) have proven advantageous for the treatment of large, fusiform, and dissecting aneurysms that are otherwise difficult to treat [2,3,4,5,6].
Due to their flow-laminating capabilities, FDDs reduce the blood flow into the aneurysm and thereby lead to progressive aneurysm thrombosis over time [7]. Numerous comparative studies have shown that FDDs can provide more durable aneurysm occlusion when compared with other techniques such as conventional and balloon- or stent-assisted coiling, still offering acceptable morbidity and mortality rates [8,9,10].
However, there is a subset of—mainly large and complex—aneurysms in which complete occlusion does not occur after FDD treatment, even at long-term follow-up [11, 12]. The risk for rupture or re-rupture of those incompletely occluded aneurysms after flow-diversion is uncertain. The technical options for retreatment are limited—other than for aneurysm remnants and recurrences that were previously treated with other techniques: the dense mesh design and high metal coverage of FDDs make catheterization of the aneurysm sac with a microcatheter through the struts in general not possible. Different from aneurysms after sole coiling, microsurgical clipping does not prove feasible either [13]. Therefore, retreatment strategies for this group of aneurysms are basically limited to overlapping implantation of more FDDs or definitive occlusion of the vessel. In an experimental study, Fahed et al. assessed occlusion rates of residual aneurysms after initial flow-diversion that were retreated by either FDD implantation alone or in combination with injection of liquid embolic agent between the layers of the FDDs. The authors reported improved aneurysm occlusion at follow-up; however, residual aneurysms were present in all cases. Moreover, parent artery occlusion occurred in 50% of aneurysms retreated with liquid embolic agent [14]. Although some studies investigated the risk factors for treatment failures related to flow-diversion [15,16,17], clinical reports on retreatment strategies and angiographic outcome are scarce in the available literature.
The objective of the current study was to present our dual-center experience in retreating aneurysms with residual filling after initial FDD implantation. We retrospectively evaluated retreatment strategies and report clinical and angiographic outcome.
Methods
This is a retrospective review of consecutive patients treated with FDDs at two German neurovascular centers between January 2010 and December 2018. All patients that underwent retreatment of the index aneurysm were identified and enrolled in the present study. There were no specific exclusion criteria. In accordance with the institutional guidelines, an ethics committee approval could be waived for this retrospective study. The study was conducted in accordance with the 1964 Helsinki Declaration.
Data collection
The following parameters were collected retrospectively from the medical records: patient age, sex, rupture status, initial FDD treatment (date, FDD type, use of adjunctive devices), and retreatment strategy (date, treatment technique). The neurological status was assessed at baseline, after treatment and at follow-up visits.
All neurological complications (e.g., aphasia, hemiparesis) and self-reported symptoms (e.g., impairment of vision, prolonged headache, dizziness) were recorded. We also report technical complications that did not result in neurological deficits including side vessel occlusion, in-stent stenosis, and procedural asymptomatic thromboembolic and hemorrhagic events. Functional outcome was evaluated by the modified Rankin scale (mRS) at discharge from hospital and at follow-up visits. Treatment-related morbidity was defined as any increase in the mRS score after the procedure. In patients with procedure-related cerebral infarction, the stroke severity was determined by using the National Institutes of Health Stroke Scale (NIHSS), whereby a score ≥ 4 points was considered major stroke. We further report all technical adverse events such as thromboembolic and hemorrhagic events that were not associated with clinical symptoms.
Pre-interventional 4-vessel digital subtraction angiography (DSA) was reviewed to determine aneurysm location, morphology, size, and neck width. Aneurysm morphology was categorized as saccular, fusiform, dissecting, and blister-like. Based on aneurysm size and neck width, the dome-to-neck ratio was calculated for all aneurysms except for fusiform aneurysms.
Immediate and follow-up aneurysm occlusion was evaluated with the O’Kelly-Marotta (OKM) grading scale for flow-diversion: A = total filling (> 95%); B = subtotal filling (5–95%); C = entry remnant (< 5%); and D = complete occlusion [18]. A favorable aneurysm occlusion was defined as OKM C + D. Furthermore, the extent of intra-aneurysmal contrast stasis was categorized as: 1 = no stasis; 2 = moderate stasis; and 3 = significant stasis. Moreover, we evaluated if overstented side vessels remained patent at the end of the intervention or at follow-up.
Procedure
The description of the procedure applies to both the initial and the retreatment. In the current study, the following FD types were used: Pipeline Embolization Device (PED; Medtronic, Dublin, Ireland), Flow Re-direction Endoluminal Device (FRED; MicroVention, Aliso Viejo, CA, USA), Silk (Balt Extrusion, Montmorency, France), and Derivo Embolization Device (DED; Acandis, Pforzheim, Germany). All interventions were performed via a transfemoral approach with the patient under general anesthesia in a biplane angiosuite (Philips, Best, the Netherlands, and Siemens, Erlangen, Germany). The patients received 5000 IE of heparin after groin puncture, followed by aliquots of 1000 IU/h until the end of the procedure.
The FDDs were deployed via a convenient 0.027″ microcatheter (Headway 27, MicroVention, Aliso Viejo, CA, USA, or Neuroslider 27, Acandis, Pforzheim, Germany). The appropriate size of the FDDs was determined based on the proximal parent artery diameter measured on 2D DSA. The implantation of multiple FDDs or adjunctive devices such as stents or the Woven EndoBridge (WEB, MicroVention, Aliso Viejo, CA, USA) was left to the neurointerventionalist’s discretion and usually reserved for aneurysms with large size or complex anatomy. In case immediate aneurysm occlusion was requested (e.g., ruptured aneurysm), adjunctive coiling of the aneurysm sac was performed accordingly.
After initial FDD implantation, angiographic follow-up was scheduled at 6 and 24 months after the procedure using the DSA or magnetic resonance angiography according to our institutional guidelines. In case of incomplete aneurysm occlusion, the indication of retreatment was made individually after discussion within an interdisciplinary neurovascular team among neuroradiologists and vascular neurosurgeons. The decision of retreatment was made considering the potential risk of rupture or re-rupture and the patient characteristics (e.g., patient age, comorbidities, patient preference). In general, aneurysms with residual OKM A and B filling after 6 months were subjected to retreatment. Among neck remnants (OKM C), retreatment was only performed for complex aneurysms that carry per se an increased rupture risk and are prone for further aneurysm growth (e.g., giant and fusiform aneurysms). The endovascular retreatment method was left to the discretion of the operator and consisted mainly of implantation of additional FDDs.
Antiplatelet regimen
Scheduled patients were treated with 100 mg acetylsalicylic acid (ASA) and clopidogrel 75 mg starting 5–7 days (d) prior to the procedure until 4 months postoperatively. ASA was continued life-long. In subarachnoid hemorrhage (SAH) patients, tirofiban (Aggrastat, Merck, West Point, PY, USA) was administered according to the manufacturer’s guidelines starting just before stent placement and continued for 16–24 h after the procedure, followed by oral double antiplatelet therapy as stated above for scheduled cases. Platelet inhibition testing was performed using ASA, P2Y12 (Verify Now, Accumetrics, San Diego, CA, USA) and vasodilator-stimulated phosphoprotein-phosphorylation (VASP) assays [19]. Levels between 350 and 550 ARU (ASA Response Units) for ASA and 30–60% for clopidogrel were defined as sufficient platelet inhibition. Insufficient response to either drug was counteracted by dose escalation (e.g., clopidogrel 150 mg/d) or substitution with prasugrel (60-mg bolus, 10 mg/d).
Results
Patient and aneurysm characteristics
During the study period, a total of 284 patients underwent flow-diversion for 316 aneurysms. Thereof, angiographic follow-up was available for 235 aneurysms. After careful discussion of the cases within an interdisciplinary team, retreatment was performed for 11 patients with 12 aneurysms (5.1%). The median patient age at initial flow-diverter treatment was 53 years (range, 33–72 years) and 7 patients (63.6%) were female. Three patients were treated for a ruptured aneurysm in the acute phase of SAH (27.3%). There were no recurrent aneurysms that had been treated before. Nine aneurysms (75.0%) were located at the internal carotid artery and one at the vertebral artery (8.3%). One SAH patient had an aneurysm at the basilar tip (8.3%) and at the superior cerebellar artery (8.3%), which were initially treated by a single flow-diverter. Aneurysm morphology was saccular in 8 cases (66.7%), fusiform in 2 (16.6%), dissecting in 1 (8.3%), and blister-like in 1 (8.3%). The median aneurysm size was 7.3 mm (range, 1–26 mm) and the median neck width was 4.7 mm (range, 1–18 mm). Patient and aneurysm characteristics at baseline are presented in Table 1.
Initial treatment
Procedural specifics of initial FDD treatment are outlined in Table 2. Three aneurysms were treated by a single FDD only (25.0%), while the remaining aneurysms were treated by multiple FDDs (n = 7, 58.3%) and/or in combination with other devices (coiling, n = 2; stent, n = 1; WEB, n = 1) due to complex aneurysm morphology. Side vessels were covered in 10 cases, in which 10 of 11 side vessels (90.9%) remained patent at the end of the procedure. Immediate complete aneurysm occlusion (OKM D) was achieved in two patients (16.7%), while a varying degree of aneurysm occlusion was obtained in the remaining cases.
There was one procedural complication. In this case, retraction of a FDD due to misplacement resulted in vessel wall injury of the ICA and consecutive carotid-cavernous fistula. The fistula could be occluded by implanting 3 PEDs. The patient did not have any symptoms after the intervention.
Retreatment
Angiographic follow-up at a median of 59 days (range, 2–360) revealed entry remnants (OKM C) in 3 aneurysms (25.0%), subtotal filling (OKM B) in 7 (58.3%) and total filling (OKM A) in 2 (16.7%). The rationale for retreatment in each patient is listed in Table 2. The median interval between initial and retreatment was 118 days (range, 7–427 days). In three SAH patients, retreatment was performed between 7 and 22 days after initial treatment as we assumed an increased risk for re-rupture due to incomplete aneurysm occlusion (cases 1, 7, and 8). In all cases, retreatment consisted of additional FDD implantation (1 FDD in 9 cases, 2 FDDs in 2 cases). Adjunctive coiling was performed in one patient. In this case, the distal end of the initially implanted FRED dislocated partially into the aneurysm sac resulting in aneurysm regrowth. After embolization of the residual aneurysm with coils via the distal end of the FRED, a DED was implanted to ensure accurate vessel wall apposition.
Retreatment with additional FDDs was feasible in all cases, leading to immediately improved aneurysm occlusion in 4 cases (36.4%). During retreatment, 12 side vessels were covered in 9 cases, whereof two (16.6%) were occluded at the end of the procedure. In both cases, occluded side vessel occlusion was uneventful and did not have any neurological symptoms. During the procedures, there were no further thromboembolic or hemorrhagic events and there were no neurological complications. Hence, the technical complication rate was 16.6% and the symptomatic complication rate was 0%. All patients had an unchanged mRS score at discharge and at follow-up (Table 3).
At last available follow-up (median, 514 days; range, 186–1681 days), complete occlusion (OKM D) was obtained in 9 cases, entry remnants (OKM C) in 2 and subtotal filling (OKM B) in 1. Hence, favorable aneurysm occlusion was attained in 11 patients (91.7%). Progressive aneurysm thrombosis was observed in all cases. At follow-up, no further covered side vessels were occluded.
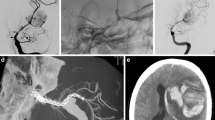
Illustrative cases of retreatment after FDD implantation are presented in Figs. 1, 2, and 3.
A patient presented with a ruptured aneurysm at the basilar artery/P1-junction (a, b). Due to the complex anatomy and the ruptured aneurysm status, the treatment strategy consisted of implantation of a PED 2.5 × 10 mm (c) and adjunctive coiling (3 coils), achieving immediate contrast stasis within the aneurysm sac (OKM B2; d). Short-term follow-up shows subtotal aneurysm filling (OKM B1; e). Due to initial aneurysm rupture, we expected a relevant re-rupture risk from the residual aneurysm and treated it by implantation of an additional PED (3.5 × 14 mm; f). Angiographic follow-ups at 6 months show complete aneurysm occlusion and subclinical intimal hyperplasia (g) that regressed almost completely at further follow-up (h). After PED implantation the right posterior cerebral artery and the superior cerebellar artery are retrogradely perfused via the right posterior communicating artery (h)
Digital subtraction angiography (DSA) shows an unruptured aneurysm (aneurysm size, 10 mm; neck width, 5 mm) at the paraophthalmic segment of the right ICA (a). After deployment of two PEDs (4 × 15 mm and 4 × 14 mm), a reduced inflow and significant contrast stasis (OKM B3) could be immediately achieved (b). Four-month angiographic follow-up shows a constant aneurysm remnant (OKM B3; c). After interdisciplinary discussion, the aneurysm was retreated by implantation of a PED 3.75 × 14 mm (d). Four-month angiographic follow-up after retreatment shows progressive aneurysm occlusion with a residual aneurysm neck (OKM C3; e). The neck remnant reduced slightly with persistent significant contrast filling (OKM C3) at long-term follow-up after 56 months (f)
A large asymptomatic Pcom-aneurysm with tortuous anatomy of the ICA (a: pa, p: lateral view). First treatment was performed by implantation of a FRED flow-diverter and additional coiling in jailing technique (c, d). Twelve months after the treatment, massive enlargement of the previously occluded aneurysm sac was noted (e, f) and dislocation of the distal markers of the flow-diverter into the sac was identified (arrows). After recoiling, catheterization of the stent was possible with a microwire (g) and localization of the wire within the flared ends of the stent was verified by gentle balloon inflation (eclipse). Subsequently, a microcatheter was exchanged (h) and an overlapping Leo stent was implanted (i, k). Three months later, an overlapping Derivo flow-diverter was implanted (l, m, n). The angiogram after 6 months showed complete occlusion of the aneurysm (o: pa, p: lateral view)
Discussion
In the current study, we report our dual-center experience in treatment of residual aneurysms after previous FDD implantation. The predominant treatment strategy consisted of placement of additional FDDs, which was feasible and resulted in progressive aneurysm occlusion in all cases. There was no morbidity or mortality related to retreatment. To the best of our knowledge, the present series is the first to report clinical and anatomic results of retreatment after initial flow-diversion treatment with residual aneurysm perfusion.
Efficacy
In general, FDDs provide progressive aneurysm thrombus resulting in comparably high aneurysm occlusion rates at long-term follow-up. To date, the best data on FDDs are available for the PED. In the cumulative population of the IntrePED (International Retrospective Study of the Pipeline Embolization Device), PUFS (Pipeline for Uncoilable or Failed Aneurysms Study), and ASPIRe (Aneurysm Study of Pipeline in an Observational Registry) trials, complete aneurysm occlusion was achieved in 75.0% at 6 months and 85.5% at 12 months after PED implantation [20]. The overall aneurysm retreatment rate in these three trials was 3.0%, which is considerably lower when compared with that in conventional coiling with reported retreatment rates up to 20% [21,22,23].
Daou et al. analyzed potential risk factors of failed aneurysm occlusion after PED implantation in a large single-center series. The authors reported residual aneurysms in 16.4%, and 6.8% underwent retreatment. Older patient age, prior stent implantation before flow-diversion, location in the distal anterior circulation, and longer follow-up duration were independently associated with treatment failure [16]. Shapiro et al. reported residual aneurysms in 19% and described fusiform shape, decreasing dome-to-neck ratio, and previous stent implantation as independent risk factors for treatment failure. The authors revealed FDD malapposition, inadequate coverage of the aneurysm neck by the FDD, and presence of a branching vessel from the aneurysm sac as potential mechanisms for delayed or insufficient aneurysm thrombosis [17]. Moreover, Kan et al. reported residual aneurysms in fetal posterior communicating artery aneurysms [15].
In our cohort, retreatment was performed in 5.1%, which is in the range of the abovementioned studies. Due to a paucity of studies and the overall small number of cases, the remaining rupture risk of aneurysms with residual or recurrent filling after FDD treatment and the indications for retreatment are not clear. Aneurysm recurrences after coiling are usually the sequel of compaction leading to exposure of some part of the aneurysm wall to the pulsatile blood flow making it susceptible for rupture [24]. However, FDDs divert the blood flow away from the aneurysm itself resulting in reduced inflow and reduced wall shear stress and hemostasis within the aneurysm sac. Hence, one may assume that the risk of rupture of residual aneurysms after flow-diversion is low—even if complete occlusion of the aneurysm itself is not achieved. For these reasons, not all flow-diverter-treated aneurysms with residual filling are generally subjected to retreatment [16]. On the other hand, there are reports on postinterventional aneurysm rupture after flow-diverter treatment [25, 26] and an increase of pressure within the aneurysm due to alterations of the flow in the parent artery and subsequent cerebral autoregulation is discussed in these cases [24]. However, to the best of our knowledge, there is no longitudinal data available, which defines the rupture risk of incompletely occluded aneurysms after flow-diversion.
In the present series, we report 12 aneurysms that were subjected to retreatment due to residual aneurysm filling. The study cohort contains three aneurysms with neck remnants (OKM C) that were retreated. These aneurysms had a complex morphology (i.e., fusiform) or were large-sized and were thus expected to carry a relevant risk for aneurysm rupture or re-rupture.
Owing to the limited options of retreatment after FDD implantation, our treatment strategy consisted mainly of placement of additional FDDs. Complete occlusion of the parent artery, which can be performed in patients with sufficient collaterals, was not performed in any of our cases. This approach proved feasible and resulted in improved aneurysm occlusion in all cases. At mid-term follow-up, the rates of complete and favorable aneurysm occlusion were 75.0% and 91.7%, respectively, which correspond to the angiographic results reported by previous studies on FDDs [20].
Safety
In general, flow-diversion is associated with higher morbidity and mortality rates when compared with conventional coiling, which is mainly related to ischemic complications [27, 28]. For instance, morbidity and mortality occurred in 5.4% in the IntrePED study [8] and 6.8% in the ASPIRe study [29]. These morbidity rates are not negligible and require careful pondering of treatment risks and benefits.
In this context, there is evidence that the complication rate correlates with the number of implanted FDDs [27]. Chalouhi et al. reported that complications occurred more often with implantation of multiple PEDs (15%) than with a single PED (5%), while there was no significant difference regarding the degree of aneurysm occlusion. For this reason, the authors recommend to keep the number of implanted FDDs to a minimum [30]. In the current series, most aneurysms were initially treated by multiple stents due to a complex morphology, large size, and/or insufficient immediate contrast stasis after placement of the first FDD. All patients received additional FDDs during retreatment; however, we did not observe any procedure-related morbidity. There were two occlusions of covered side vessels, which were regarded as technical complications. However, the patients remained asymptomatic in both cases and there were no further technical or symptomatic complications during a cumulative follow-up period of 19.4 patient years, hence indicating that retreatment with FDDs is reasonably safe, when required.
The use of multiple FDDs correlates with an increased risk for side vessel occlusion, which can, depending on the location, result in severe neurological deficits such as visual impairment, or ischemic complications in general [31]. In our series, side vessels arising from the parent artery were covered by the FDD in all but one patient; however, 76.9% of these vessels remained patent until last follow-up. Similar to our results, side vessels were occluded in 20% in the study by Bhogal et al. [32], 21% in the study by Puffer et al. [33], and 27% in the study by Brinjikji et al. [34]. Evidence suggests that angiographic side vessel occlusion after flow-diverter treatment does not necessarily cause neurological deficits [32] which also applies to the patients in the current series.
Limitations
The present series has several limitations. Although we performed a dual-center analysis of consecutive patients during a 9-year period, only a small number of patients could be identified. Moreover, the majority of patients had complex aneurysms; hence, the study sample is not representative. Although all patients were available for angiographic follow-up, the long-term clinical and angiographic results remain unclear. Furthermore, our series is retrospective; hence, minor neurological deficits might be underreported.
Acknowledging these limitations, our study provides novel insight into the treatment options for incomplete aneurysm occlusion after flow-diversion and might help other neurointerventionalists in their treatment decisions. Further studies will be necessary to confirm our results and to develop a treatment algorithm for aneurysm residuals after flow-diversion.
Conclusions
In our dual-center series, retreatment of incompletely occluded aneurysms after initial flow-diverter treatment consisted of additional FDD implantation and resulted in progressive aneurysm occlusion in all cases. There were no complications and morbidity related to retreatment. Larger studies are required to validate our results.
References
Wakhloo AK, Gounis MJ (2014) Revolution in aneurysm treatment: flow diversion to cure aneurysms: a paradigm shift. Neurosurgery 61(CN_suppl_1):111–120
Shankar JJS, Tampieri D, Iancu D, Cortes M, Agid R, Krings T, Wong J, Lavoie P, Ghostine J, Shettar B (2015) SILK flow diverter for complex intracranial aneurysms: a Canadian registry. J Neurointerv Surg. https://doi.org/10.1136/neurintsurg-2015-011708
Chalouhi N, Tjoumakaris S, Starke RM, Gonzalez LF, Randazzo C, Hasan D, McMahon JF, Singhal S, Moukarzel LA, Dumont AS (2013) Comparison of flow diversion and coiling in large unruptured intracranial saccular aneurysms. Stroke 44(8):2150–2154
Awad AJ, Mascitelli JR, Haroun RR, De Leacy RA, Fifi JT, Mocco J (2017) Endovascular management of fusiform aneurysms in the posterior circulation: the era of flow diversion. Neurosurg Focus 42(6):E14
Goertz L, Dorn F, Kraus B, Borggrefe J, Schlamann M, Forbrig R, Turowski B, Kabbasch C (2018) Safety and efficacy of the Derivo Embolization Device for the treatment of ruptured intracranial aneurysms. J Neurointerv Surg. https://doi.org/10.1136/neurintsurg-2018-014166
Kraus B, Goertz L, Turowski B, Borggrefe J, Schlamann M, Dorn F, Kabbasch C (2018) Safety and efficacy of the Derivo Embolization Device for the treatment of unruptured intracranial aneurysms: a multicentric study. J Neurointerv Surg. https://doi.org/10.1136/neurintsurg-2018-013963
Goertz L, Dorn F, Kraus B, Borggrefe J, Forbrig R, Schlamann M, Liebig T, Turowski B, Kabbasch C (2019) Improved occlusion rate of intracranial aneurysms treated with the Derivo Embolization Device: one-year clinical and angiographic follow-up in a multicenter study. World Neurosurg 126:e1503–e1509
Kallmes DF, Hanel R, Lopes D, Boccardi E, Bonafé A, Cekirge S, Fiorella D, Jabbour P, Levy E, McDougall C (2015) International retrospective study of the Pipeline Embolization Device: a multicenter aneurysm treatment study. Am J Neuroradiol 36(1):108–115
Brinjikji W, Murad MH, Lanzino G, Cloft HJ, Kallmes DF (2013) Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke 44(2):442–447
Chiu A, Cheung A, Wenderoth J, De Villiers L, Rice H, Phatouros C, Singh T, Phillips T, McAuliffe W (2015) Long-term follow-up results following elective treatment of unruptured intracranial aneurysms with the Pipeline Embolization Device. Am J Neuroradiol 36(9):1728–1734
Saatci I, Yavuz K, Ozer C, Geyik S, Cekirge H (2012) Treatment of intracranial aneurysms using the Pipeline flow-diverter embolization device: a single-center experience with long-term follow-up results. Am J Neuroradiol 33(8):1436–1446
Lubicz B, Collignon L, Raphaeli G, Pruvo J-P, Bruneau M, De Witte O, Leclerc X (2010) Flow-diverter stent for the endovascular treatment of intracranial aneurysms. Stroke 41(10):2247–2253
Ding D, Starke RM, Liu KC (2014) Microsurgical strategies following failed endovascular treatment with the Pipeline Embolization Device: case of a giant posterior cerebral artery aneurysm. J Cerebrovasc Endovasc Neurosurg 16(1):26–31
Fahed R, Darsaut TE, Kotowski M, Salazkin I, Raymond J (2018) Re-treatment of residual aneurysms after flow diversion: an experimental study. Neuroradiol J 31(3):270–279
Kan P, Duckworth E, Puri A, Velat G, Wakhloo A (2016) Treatment failure of fetal posterior communicating artery aneurysms with the Pipeline Embolization Device. J Neurointerv Surg 8(9):945–948
Daou B, Atallah E, Chalouhi N, Starke RM, Oliver J, Montano M, Jabbour P, Rosenwasser RH, Tjoumakaris SI (2018) Aneurysms with persistent filling after failed treatment with the Pipeline Embolization Device. J Neurosurg 1(aop):1–7
Shapiro M, Becske T, Nelson PK (2017) Learning from failure: persistence of aneurysms following Pipeline embolization. J Neurosurg 126(2):578–585
O’kelly C, Krings T, Fiorella D, Marotta T (2010) A novel grading scale for the angiographic assessment of intracranial aneurysms treated using flow diverting stents. Interv Neuroradiol 16(2):133–137
Schwarz UR, Geiger J, Walter U, Eigenthaler M (1999) Flow cytometry analysis of intracellular VASP phosphorylation for the assessment of activating and inhibitory signal transduction pathways in human platelets. Thromb Haemost 81(03):1145–1152
Kallmes DF, Brinjikji W, Cekirge S, Fiorella D, Hanel RA, Jabbour P, Lopes D, Lylyk P, McDougall CG, Siddiqui A (2017) Safety and efficacy of the Pipeline Embolization Device for treatment of intracranial aneurysms: a pooled analysis of 3 large studies. J Neurosurg 127(4):775–780. https://doi.org/10.3171/2016.8.jns16467
Chalouhi N, Jabbour P, Singhal S, Drueding R, Starke RM, Dalyai RT, Tjoumakaris S, Gonzalez LF, Dumont AS, Rosenwasser R (2013) Stent-assisted coiling of intracranial aneurysms: predictors of complications, recanalization, and outcome in 508 cases. Stroke 44(5):1348–1353
Chalouhi N, Tjoumakaris S, Gonzalez L, Dumont A, Starke R, Hasan D, Wu C, Singhal S, Moukarzel L, Rosenwasser R (2014) Coiling of large and giant aneurysms: complications and long-term results of 334 cases. Am J Neuroradiol 35(3):546–552
Piotin M, Blanc R, Spelle L, Mounayer C, Piantino R, Schmidt PJ, Moret J (2010) Stent-assisted coiling of intracranial aneurysms: clinical and angiographic results in 216 consecutive aneurysms. Stroke 41(1):110–115
Cebral J, Mut F, Raschi M, Scrivano E, Ceratto R, Lylyk P, Putman C (2011) Aneurysm rupture following treatment with flow-diverting stents: computational hemodynamics analysis of treatment. Am J Neuroradiol 32(1):27–33
Turowski B, Macht S, Kulcsár Z, Hänggi D, Stummer W (2011) Early fatal hemorrhage after endovascular cerebral aneurysm treatment with a flow diverter (SILK-Stent). Neuroradiology 53(1):37–41
Kulcsár Z, Houdart E, Bonafé A, Parker G, Millar J, Goddard A, Renowden S, Gal G, Turowski B, Mitchell K (2011) Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. Am J Neuroradiol 32(1):20–25
Brinjikji W, Lanzino G, Cloft HJ, Siddiqui AH, Boccardi E, Cekirge S, Fiorella D, Hanel R, Jabbour P, Levy E, Lopes D, Lylyk P, Szikora I, Kallmes DF (2016) Risk factors for ischemic complications following Pipeline Embolization Device treatment of intracranial aneurysms: results from the IntrePED study. AJNR Am J Neuroradiol 37(9):1673–1678. https://doi.org/10.3174/ajnr.A4807
Algra AM, Lindgren A, Vergouwen MDI, Greving JP, van der Schaaf IC, van Doormaal TPC, Rinkel GJE (2018) Procedural clinical complications, case-fatality risks, and risk factors in endovascular and neurosurgical treatment of unruptured intracranial aneurysms: a systematic review and meta-analysis. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2018.4165
Kallmes DF, Brinjikji W, Boccardi E, Ciceri E, Diaz O, Tawk R, Woo H, Jabbour P, Albuquerque F, Chapot R (2016) Aneurysm study of Pipeline in an observational registry (ASPIRe). Int Neurol 5(1–2):89–99
Chalouhi N, Tjoumakaris S, Phillips J, Starke R, Hasan D, Wu C, Zanaty M, Kung D, Gonzalez L, Rosenwasser R (2014) A single Pipeline Embolization Device is sufficient for treatment of intracranial aneurysms. Am J Neuroradiol 35(8):1562–1566
Rouchaud A, Leclerc O, Benayoun Y, Saleme S, Camilleri Y, D’Argento F, Boncoeur M-P, Robert P-Y, Mounayer C (2015) Visual outcomes with flow-diverter stents covering the ophthalmic artery for treatment of internal carotid artery aneurysms. Am J Neuroradiol 36(2):330–336
Bhogal P, Ganslandt O, Bäzner H, Henkes H, Pérez MA (2017) The fate of side branches covered by flow diverters–results from 140 patients. World Neurosurg 103:789–798
Puffer RC, Kallmes DF, Cloft HJ, Lanzino G (2012) Patency of the ophthalmic artery after flow diversion treatment of paraclinoid aneurysms. J Neurosurg 116(4):892–896
Brinjikji W, Lanzino G, Cloft HJ, Kallmes DF (2014) Patency of the posterior communicating artery after flow diversion treatment of internal carotid artery aneurysms. Clin Neurol Neurosurg 120:84–88
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
LG, NH, TL, WA, NA, and BK acquired the data. LG, CK, and FD developed the project, analyzed the data, and drafted the manuscript. All authors revised the paper critically for important intellectual content and provided final approval of the version published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
FD and CK serve as consultants for Acandis GmbH (Pforzheim, Germany). CK and TL serve as proctors for MicroVention Inc./Sequent Medical (Aliso Viejo, CA, USA). The other authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Goertz, L., Hesse, N., Liebig, T. et al. Retreatment strategies for recurrent and residual aneurysms after treatment with flow-diverter devices. Neuroradiology 62, 1019–1028 (2020). https://doi.org/10.1007/s00234-020-02389-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02389-w