Abstract
Purpose
Understanding the underlying pathophysiology and the patterns of disease spread is crucial in accurate image interpretation. In this pictorial review, the common and important inflammatory processes of the temporal bone in children will be discussed, and key computed tomography (CT) and magnetic resonance imaging (MRI) features described.
Methods
Inflammatory processes are categorized by anatomical location: the petrous apex and the inner, middle and outer ear. A complete review of the literature is provided.
Results
Cholesteatoma, cholesterol granuloma and mucoceles are inflammatory processes that occur across the anatomical subsites of the temporal bone, whilst site-specific inflammatory processes include labyrinthitis ossificans in the inner ear and keratosis obturans in the external ear. Infection is a key cause of inflammation in the temporal bone, and specific infections include petrous apicitis, otitis media and necrotizing otitis externa. Finally, important mimics and do-not-touch lesions are considered. CT and MRI are complementary in assessing these disorders, as two of the most important diagnostic clues are the presence of bone erosion, best appreciated on CT, and true diffusion restriction as seen on MRI. Flow charts to assist in the diagnosis of paediatric temporal bone inflammatory disease are also provided.
Conclusion
Paediatric temporal bone inflammatory processes are common and can have severe clinical sequelae. Timely intervention, facilitated by correct radiological diagnosis, can often prevent progression of disease, loss of hearing and systemic illness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In this radiological review, inflammatory processes affecting the four major anatomical regions of the temporal bone—the petrous apex, the inner, middle and external ear—in paediatric patients will be examined, and key radiological findings highlighted as well as significant mimics and pitfalls.
Precise knowledge of the radiological appearance of inflammatory pathologies in the petrous bone can guide the differential diagnosis, which is often critical in the management of these children, and CT and MRI are often complementary in reaching the correct diagnosis.
Infection is clearly an important cause of inflammation, but often the distinction between infective and other inflammatory processes is made on clinical and laboratory markers. Therefore, important imaging phenotypes of infection in the temporal bone will be discussed (for example, in the specific cases of petrous apicitis and necrotizing otitis externa), but an extensive discussion of specific infective organisms is beyond the scope of this review.
Petrous apex
The petrous apex is an important region because of the close anatomical relationship with the cranial nerves. The classic Gradenigo’s triad characterizing petrous apicitis (otomastoiditis, abducens palsy and facial pain due to trigeminal involvement) occurs because of the presence at the petrous apex of Dorello’s canal, below the superior sphenopetrosal ligament, carrying the sixth cranial nerve to the cavernous sinus, and the Meckel’s cave, adjacent to the trigeminal impression on the petrous temporal bone, carrying the fifth cranial nerve. Further, anteromedially is the cavernous sinus itself, and posterolaterally are the internal auditory canals and the inner and middle ear contents, all of which are potentially at risk from expanding petrous apex lesions. Therefore, lesions in this location can present with a mixture of cranial nerve-related symptoms as well as localized pain and hearing loss [1].
Several pathological processes and anatomical variants can involve the apex. A simple imaging approach to a petrous apex lesion is outlined in Fig. 1.
Petrous apicitis
The most important cause of non-neoplastic bone destruction in the petrous apex is petrous apicitis, although the bone destruction occurs late in the disease course. In children, it is typically secondary to acute otitis media, but may be primary as well. Classically apicitis presents with permeative bone destruction best demonstrated on CT, unless the infective process is caught at early stages, when the only finding at CT can be a fluid collection in the petrous apex (Fig. 2a). In these cases, MRI is of paramount importance, demonstrating signs of osteomyelitis, with increased short tau inversion recovery (STIR) signal, enhancement and often a fluid collection (Fig. 2b). This may coalesce into an abscess with peripheral enhancement and internal restricted diffusion, and there may be adjacent cranial nerve or dural enhancement [2] (Fig. 2c, d).
Petrous apicitis in the context of left skull base infection in a 6-year-old boy. Axial CT with bone window shows fluid opacification and subtle erosion of the left petrous apex (arrow in a); axial T2 shows extensive oedema involving the left skull base and the apex (arrow in b), note the normal hypointense apex on the right; axial apparent diffusion coefficient (ADC) map (c) and post-contrast T1 fat-sat (d) show an area of restriction and lack of enhancement in keeping with an abscess (arrows); note is made of diffuse thickening and enhancement of the meninges overlying the anterior temporal region (dashed arrow in d), the VII and VIII cranial nerves in the left cerebellopontine angle cistern and the contralateral internal auditory canal
Key review areas following diagnosis are the internal carotid artery, which can be compromised, and the venous sinuses, which may be thrombosed due to involvement of transosseous venous channels, as well as the intracranial and nasopharyngeal compartments for spread of infection and abscess formation [3]. Urgent antibiotic therapy is the treatment of choice with surgery reserved for abscess formation, although it can be difficult to isolate a specific organism [4]. The surgical approach depends on the anatomy, and is most frequently infracochlear. Multidisciplinary involvement is crucial in these children [3].
Other inflammatory conditions
Other inflammatory conditions of the petrous apex typically present with bone expansion due to slow growth, although there may be some trabecular destruction. These include cholesterol granulomas, cholesteatomas and mucoceles. All of these are usually expansile on CT and demonstrate high T2 signal and potentially mild peripheral enhancement on MRI.
A cholesterol granuloma is a well-defined lesion which demonstrates high T1 signal, and can expand in any direction from the petrous apex; although they may be stable, follow-up is required to assess for enlargement, and if they are enlarging or symptomatic, surgery is considered (Fig. 3). The precise cause is uncertain, but they are thought to arise secondary to repeated low level haemorrhage into a pneumatized apex [5].
Cholesteatomas, as in other areas (see further discussion under the Middle Ear section), characteristically demonstrate fluid-like signal (low T1 and high T2 signal) with intense diffusion restriction (a clue for the diagnosis) and require surgery. Mucoceles, which are rare in the petrous apex even in adults, demonstrate variable T1 signal dependent on their protein content but no diffusion restriction; however, these are almost never seen in children [2].
‘Do not touch’ lesions
The key mimics to consider are the so-called ‘do not touch’ lesions. The main culprits are asymmetrical fatty marrow and petrous apex effusions. In both cases, the absence of bone expansion or destruction is reassuring.
Asymmetric fatty marrow is an incidental finding and simply requires demonstration of fat signal on all sequences (increased signal in T1 and T2 turbo spin echo -TSE- and decreased signal in STIR or fat-saturated sequences) (Fig. 4a).
Petrous apex effusion occurs by definition within a pneumatized petrous apex. 4 to 7% of petrous apices are asymmetrically pneumatized. In the absence of bone expansion or destruction and absence of diffusion restriction, it does not require follow-up, although imaging may be considered if there is clinical doubt (Fig. 4b). Doubt can sometimes arise due to proteinaceous content causing increased T1 signal within the effusion, raising the concern for a cholesterol granuloma; again, the absence of erosion of the bony trabeculae suggests the correct diagnosis [1].
A further potential mimic is the so-called cephalocele—a herniation of the contents of Meckel’s cave laterally into the petrous apex, which is actually a meningocele (containing no brain parenchyma) (Fig. 5). The cephalocele is contiguous with a defect in the posterolateral aspect of Meckel’s cave [6] and is best demonstrated on high-resolution T2-weighted sequences. Although in adults, these are usually incidental, they have been associated with cerebrospinal fluid leaks and recurrent meningitis in children. They may require intervention when related to a proven cerebrospinal fluid leak or causing recurrent infection [7].
Petrous apex cephalocele. Axial heavily weighted T2 thin slice (a) and T1 weighted (b) images and apparent diffusion coefficient (ADC) map (c) demonstrate a fluid-signal expansile lesion in the right petrous apex. Coronal heavily weighted T2 thin slice image (d) demonstrates connection between Meckel’s cave (small arrow) and cephalocele (large arrow)
Important mimics
Aneurysms of the petrous segment of the internal carotid artery can demonstrate heterogeneous signal and therefore could be confused with inflammatory pathology on MRI, but angiographic sequences can clarify this.
Tumours, particularly chondrosarcomas, metastases or plasmacytomas, and Langerhans’ cell histiocytosis should also be considered where there is bone destruction in the petrous apex [8].
Inner ear
Labyrinthitis ossificans
The main inflammatory condition of the inner ear is labyrinthitis. It is important to detect this early because of potential progression to labyrinthitis ossificans, a major cause of sensorineural hearing loss in children. An inflammatory reaction leads to the membranous labyrinth being filled initially with fibrous and then ossific tissue. At this point, treatment with cochlear implantation may be challenging and carry much worse outcomes [9].
The list of potential causes of labyrinthitis ossificans is long; infection is by far the most frequent, but it can also occur following trauma [10], autoimmune disease [11], and labyrinthine haemorrhage, for example in patients with sickle cell disease [12]. In infective cases, the source of infection can be from the intracranial compartment (meningogenic spread of infection is the most common), the middle ear (tympanogenic spread) or via the blood stream (haematogenous spread). Meningogenic spread is thought to extend via the cochlear aqueduct or vestibule, and is more commonly bilateral, whereas tympanogenic spread occurs via the round or oval window and is usually unilateral [13].
Importantly, imaging in the initial phase may be negative, although it is also possible to have enhancement within the labyrinth on MRI in bacterial infection. One study in 23 paediatric patients with bacterial meningitis showed that enhancement in the labyrinth had 87% sensitivity and 100% specificity for predicting subsequent sensorineural deafness [14]. However, a high index of suspicion should be maintained in the absence of supportive imaging findings when sensorineural hearing loss follows a middle ear or intracranial infection.
High-resolution T2-weighted imaging has been shown to be sensitive to the identification of the fibrous stage (i.e. before the labyrinth is completely obstructed by bone). It reveals areas of signal loss in the labyrinth (particularly the scala tympani and initially in the basal turn) and can also predict the degree of cochlear obstruction, which is useful for surgical planning [15] (Fig. 6a). A potential, but extremely rare in paediatric patients, differential diagnosis for focal cochlear signal loss on high-resolution T2-weighted imaging is an intracochlear schwannoma [16].
Thin-slice CT of the temporal bone demonstrates areas of ossification within the labyrinth well (Fig. 6b) but is not sensitive for the fibrous stage when the CT is negative; however, if used in conjunction with MR, the discrepancy between the two can delineate the degree of fibrous, as opposed to ossific, disease. Radiologically the major differential diagnosis in cases of severe labyrinthitis ossificans (with complete ossification) is labyrinthine aplasia, which is, however, characterized by the absence of the cochlear promontory and a small otic capsule. The history in this context is also completely different and characterized by congenital deafness [17].
Middle ear
From a clinical point of view, the middle ear in children is generally not imaged in acute otitis media, but imaging may need consideration if there is recurrent/persistent infection despite antibiotics or if there is concern for associated complications. Inflammation in the middle ear can be difficult to diagnose as often there is only opacification on CT, which is nonspecific. A key feature to note in paediatric patients is the degree of mastoid development; this can be delayed or arrested by middle ear inflammation in childhood, and can later have a secondary effect of decreased ability to buffer changes in air pressure, therefore predisposing to further middle ear inflammation [18].
Simple opacification without clinical symptoms or bone erosion, known as secretory otitis, is often related to Eustachian tube dysfunction [19], and the nasopharynx should be carefully examined for an obstructive lesion. Acute otitis media, if imaged, is nonspecific on CT but may demonstrate middle ear and mastoid opacification with or without fluid levels. Complications include coalescent mastoiditis (with loss of mastoid trabeculae), venous sinus thrombosis, subdural empyema and abscess formation in various compartments—subperiosteal, Bezold (i.e. extending to the neck), perisinus or intracranial epidural [20, 21].
Longstanding inflammation of the middle ear (chronic otitis media) can simply be otitis media with effusion, which does not require imaging. However, two sequelae of chronic otitis media are important on imaging as they can present with ossicular erosion: cholesteatoma and postinflammatory ossicular erosion (also known as adhesive chronic otitis media or tympanic retraction).
Cholesteatoma—general concepts
The European Academy of Otology and Neurotology (EAONO)/Japanese Otological Society (JOS) consensus defines cholesteatoma as ‘a mass formed by the keratinizing squamous epithelium in the tympanic cavity and/or mastoid and subepithelial connective tissue and by the progressive accumulation of keratin debris with/without a surrounding inflammatory reaction’, and acknowledges that it is not neoplastic but that the pathogenesis is not well understood [22]. In children, it may be congenital or acquired. The annual incidence of acquired cholesteatomas is estimated at 3–15 cases per 100,000 children, and 70–96% of paediatric cholesteatomas are acquired [23]. The detection rate of congenital compared to acquired cholesteatoma in children is increasing [24].
In children, the occurrence of cholesteatoma with syndromic associations has only suggested a link with genes involved in ear embryogenesis, with cases reported in connection with branchio-oto-renal syndrome, microtia with small tympanic cavity [25], and also in cleft disorders (although this may relate to the predisposition to chronic otitis media). A specific genetic association has not been discovered as yet [26].
The differential diagnosis between different types of cholesteatoma can be made clinically via otoscopy, and this is often more straightforward than radiological differentiation (Fig. 7). However, there are a number of radiological signs that can help with the distinction.
Otoscopy. Normal right ear (a) with lateral process of malleus (white arrow), pars flaccida (dashed black arrow) and pars tensa (solid black arrow) of tympanic membrane. In congenital cholesteatoma (b), a white mass (white arrow) is visible in the anterior superior quadrant. Retraction of the tympanic membrane over the ossicles is demonstrated in c (white arrow). In d, a retraction pocket (white arrow, d) and an attic cholesteatoma (black arrow) are shown in the left ear
A helpful MRI finding in cholesteatoma is diffusion restriction on non-echo planar DWI and/or multi-shot DWI images, performed in the coronal plane to reduce artefact affecting standard DWI (Fig. 8e). The non-echo planar DWI sequence has a high sensitivity and specificity for detecting cholesteatoma. A meta-analysis including four paediatric studies showed a sensitivity of 86% and specificity of 94%, although there was significant heterogeneity between the studies [27]. T2-weighted images demonstrate high signal, and there is only mild peripheral enhancement on delayed contrast-enhanced T1 sequences [28].
Congenital cholesteatoma
Congenital cholesteatoma, thought to develop from stranded epithelial, rests in the middle ear, crucially occurs behind an intact eardrum and is not causatively related to chronic otitis media. It presents on examination as a white retrotympanic mass and is typically (but not exclusively) located in the anterior superior quadrant of the middle ear cavity. Bone erosion is present much less frequently. It occurs later in the disease process and to a lesser extent than in the acquired variety [29].
Acquired cholesteatoma
Cholesteatoma in its acquired form is divided into the more common pars flaccida cholesteatoma and the less common pars tensa cholesteatoma. Both arise in the setting of chronic middle ear inflammation with tympanic membrane retraction or perforation. There is a disruption of the normal external migration of the squamous epithelium at the tympanic membrane. Therefore, there is soft tissue opacification of the middle ear cavity with associated bone erosion (Fig. 8). Acquired cholesteatoma can be primary or secondary. In secondary cases, the cholesteatoma extends through a pre-existing (e.g. traumatic or iatrogenic) tympanic perforation [22].
The pars flaccida cholesteatoma is characteristically located in Prussak’s space (the lateral epitympanic recess) and is associated with erosion of the scutum and the ossicles, predominantly the long process of the incus and the head of the malleus. This has a tendency to erode and displace the ossicles medially, and extends into the mastoid via the aditus ad antrum and the tegmen tympani and lateral semicircular canal are at risk of erosion [30].
Pars tensa cholesteatoma generally occurs in the posterior part of the middle ear cavity, affecting the facial recess and sinus tympani (Fig. 9). There is erosion of the manubrium of the malleus and the stapedius tendon as well as the long process of the incus and a resulting lateral ossicular displacement. The facial nerve canal should be scrutinized for erosion [21].
Postinflammatory changes
The distinction of cholesteatoma from other forms of chronic otitis media, specifically tympanic retraction or postinflammatory ossicular erosion, can be difficult on imaging, particularly on CT. The scutum and the head of the malleus are less often involved in postinflammatory erosion. It is also more typical in postinflammatory erosion to see erosion of other structures and consequent loss of two specific radiological signs, which should always normally be present:
-
1.
The ‘two parallel lines’ sign of the manubrium of the malleus and long process of the incus [21] is no longer visualized due to the erosion of the long process of the incus (i.e. posterior line)
-
2.
The ‘right angle sign’, which is the appearance of the incudostapedial joint in the coronal plane, is also lost (Fig. 10) [31].
Postinflammatory erosion. Axial CT image in (a) demonstrates normal ‘2 parallel line’ sign of the malleus neck anteriorly and the lenticular process of the incus posteriorly, in a normal patient. In postinflammatory erosion (b), there is middle ear opacification with loss of the posterior line. There is preservation of the tympanic membrane (orange line in c) and scutum seen on coronal CT, but there is erosion of the normal ‘right angle’ sign between the long process (blue line in d) and lenticular process (green line) of the incus
However, on MRI, the absence of diffusion restriction in postinflammatory erosion should allow distinction, and although contrast is now rarely given in this context, the presence of contrast enhancement also suggests postinflammatory changes over cholesteatoma. Other postinflammatory complications include ossicular fixation, fibrous tissue formation (classically causing peristapedial tenting at the oval window niche), bone formation, or collagen deposition (tympanosclerosis) along tendons or adjacent to the ossicular chain, or along the tympanic membrane (myringosclerosis) where there can be thin calcific plaques [32].
Cholesterol granuloma
Cholesterol granuloma of the middle ear is very rare and usually represents recurrent haemorrhage in a chronically inflamed middle ear. It demonstrates similar signal characteristics as elsewhere in the temporal bone with high T1 and T2 signal and potentially bone destruction. It may also have a peripheral low T2 rim secondary to repeated haemorrhage. MRI with diffusion is helpful to distinguish it from cholesteatoma, as it may demonstrate bone erosion and thus the two entities appear identical on CT [33]. Clinically, it usually presents as a darker mass on otoscopy [34].
Potential mimics
The differential of middle ear lesions should also include systemic diseases such as Langerhans’ cell histiocytosis, and tumours (for example glomus tumours). There are also rarer causes such as extramedullary haematopoiesis in anaemia; these children can also present with treatment-related deafness, and imaging findings must be considered in the context of the pattern of hearing loss.
A flow chart to guide differential diagnosis in the case of middle ear opacification and bony erosion on CT is presented in Fig. 11.
External ear
Serious Inflammatory conditions of the external ears are rare in children and rarely involve the bone. The two key inflammatory conditions of the external ear are keratosis obturans and external auditory canal cholesteatoma. They warrant consideration together because of the potential similarity in their clinical presentation. Both can present with pain and conductive hearing loss, potentially with associated discharge.
Keratosis obturans
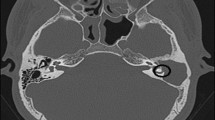
Keratosis obturans is more frequent in younger patients but is generally rare in children [35]. It causes plugging of the external auditory canal with desquamated keratin; the mechanism of this is uncertain but is thought to be related to a localized allergic process [35]. The key finding is again the lack of bone erosion; keratosis obturans presents as a well-defined soft tissue mass with expansion but no erosion of the auditory canal. It is more likely to be bilateral and does not cause any local invasion (Fig. 12) [36].
Cholesteatoma
External auditory canal cholesteatoma causes local bone erosion (most commonly of the inferior and posterior walls) with possible focal bony spicules within the soft tissue mass. It can invade locally, with the facial nerve particularly at risk [37]. The MRI characteristics are as described in the previous sections (Fig. 13). Surgery is invariably required [38].
Necrotizing otitis externa
A rare but very important external ear condition is necrotizing otitis externa. This is more common in elderly—particularly diabetic—patients. Any form of immunosuppression increases the risk, and therefore children with malignancy or on immunosuppressive therapy are vulnerable, as are adolescent diabetics [39]. Patients present with persistent otitis externa, extreme otalgia and systemic symptoms. The infection is thought to spread from the external auditory canal to the infratemporal fossa via the fissures of Santorini (a small canal in the anterior cartilage of the external auditory meatus), whilst leading to osteomyelitis and cranial nerve palsies [40]. CT demonstrates bone erosion with associated abnormal soft tissue around the external auditory canal and within the infratemporal fossa, and MRI demonstrates skull base osteomyelitis with adjacent abscess and phlegmon [41] (Fig. 14). In children, the middle ear is more frequently involved, and there is more rapid involvement of the facial nerve [39]. Malignancy must be excluded in these patients. The middle ear is typically spared in adults.
Necrotizing otitis externa. Coronal T2-weighted image (a) demonstrates opacification of the external auditory canal with isointense signal, which is also seen in the infratemporal fossa. Contrast-enhanced axial T1-weighted image (b) demonstrates extensive enhancing soft tissue in the right infratemporal fossa and parapharyngeal and carotid spaces
The differential considerations of the inflammatory conditions in this context are benign and malignant external ear tumours [42], vascular malformations [43] and temporal bone osteoradionecrosis [44].
Conclusion
Inflammatory disorders of the paediatric petrous temporal bone are rare but important, as early intervention can often prevent progression, loss of hearing and/or systemic illness. CT and MRI are complementary in the assessment of the petrous temporal bone. A careful assessment for bone erosion is the key to differentiating the underlying pathologies. In children, it is particularly important to be aware of patterns of disease spread and potential syndromic associations in order to develop an appropriate strategy for interpreting imaging studies.
References
Chapman PR, Shah R, Cure JK, Bag AK (2011) Petrous apex lesions: pictorial review. AJR Am J Roentgenol 196(3 Suppl):WS26–WS37 Quiz S40-23. https://doi.org/10.2214/ajr.10.7229
Radhakrishnan R, Son HJ, Koch BL (2014) Petrous apex lesions in the pediatric population. Pediatr Radiol 44(3):325–339quiz 323-324. https://doi.org/10.1007/s00247-013-2836-5
Janjua N, Bajalan M, Potter S, Whitney A, Sipaul F (2016) Multidisciplinary care of a paediatric patient with Gradenigo's syndrome. BMJ Case Rep 2016. https://doi.org/10.1136/bcr-2015-214337
Rossor TE, Anderson YC, Steventon NB, Voss LM (2011) Conservative management of Gradenigo’s syndrome in a child. BMJ Case Rep 2011:bcr0320113978. https://doi.org/10.1136/bcr.03.2011.3978
Hoa M, House JW, Linthicum FH, Go JL (2013) Petrous apex cholesterol granuloma: pictorial review of radiological considerations in diagnosis and surgical histopathology. J Laryngol Otol 127(4):339–348. https://doi.org/10.1017/s0022215113000091
Moore K, Fischbein N, Harnsberger H, Shelton C, Glastonbury C, White D, Dillown W (2001) Petrous apex cephaloceles. Am J Neuroradiol 22(10):5
Motojima T, Fujii K, Ishiwada N, Takanashi J, Numata O, Uchino Y, Yamakami I, Kohno Y (2005) Recurrent meningitis associated with a petrous apex cephalocele. J Child Neurol 20(2):168–170. https://doi.org/10.1177/08830738050200021801
Razek AA, Huang BY (2012) Lesions of the petrous apex: classification and findings at CT and MR imaging. Radiographics 32(1):151–173. https://doi.org/10.1148/rg.321105758
Aschendorff A, Klenzner T, Laszig R (2005) Deafness after bacterial meningitis: an emergency for early imaging and cochlear implant surgery. Otolaryngol Head Neck Surg 133(6):995–996
Aralasmak A, Dincer E, Arslan G, Cevikol C, Karaali K (2009) Posttraumatic labyrinthitis ossificans with perilymphatic fistulization. Diagn Interv Radiol 15:239–241
Casselman J, Majoor M, Albers F (1994) MR of the inner ear in patients with Cogan syndrome. Am J Neuroradiol 15(1):131–138
Saito N, Nadgir RN, Flower EN, Sakai O (2010) Clinical and radiologic manifestations of sickle cell disease in the head and neck. Radiographics 30(4):1021–1034. https://doi.org/10.1148/rg.304095171
Huang B, Zdanski C, Castillo M (2012) Pediatric sensorineural hearing loss, part 2: syndromic and acquired causes. Am J Neuroradiol 33(3):399–406
Kopelovich J, Germiller J, Laury A, Shah S, Pollock A (2011) Early prediction of postmeningitic hearing loss in children using magnetic resonance imaging. Arch Otolaryngol Head Neck Surg 137(5):441–447
Booth TN, Roland P, Kutz JW Jr, Lee K, Isaacson B (2013) High-resolution 3-D T2-weighted imaging in the diagnosis of labyrinthitis ossificans: emphasis on subtle cochlear involvement. Pediatr Radiol 43(12):1584–1590. https://doi.org/10.1007/s00247-013-2747-5
Neault M, Zhou G, Kenna M, Poe D (2010) Atypical presentation of intracochlear schwannoma in a child. Int J Pediatr Otorhinolaryngol Extra 5(4):186–189
Joshi V, Navlekar S, Kishore G, Reddy K, Kumar E (2012) CT and MR imaging of the inner ear and brain in children with congenital sensorineural hearing loss. Radiographics 32(3):683–698. https://doi.org/10.1148/rg.323115073
Miura M, Takahashi H, Honjo I, Hasebe S, Tanabe M (1998) Influence of the gas exchange function through the middle ear mucosa on the development of sniff-induced middle ear diseases. Laryngoscope 108(5):683–686. https://doi.org/10.1097/00005537-199805000-00011
Smith M, Scoffings D, Tysome J (2016) Imaging of the Eustachian tube and its function: a systematic review. Neuroradiology 58(6):543–556
Vazquez E, Castellote A, Piqueras J, Mauleon S, Creixell S, Pumarola F, Figueras C, Carreno JC, Lucaya J (2003) Imaging of complications of acute mastoiditis in children. Radiographics 23(2):359–372. https://doi.org/10.1148/rg.232025076
Trojanowska A, Drop A, Trojanowski P, Rosińska-Bogusiewicz K, Klatka J, Bobek-Billewicz B (2012) External and middle ear diseases: radiological diagnosis based on clinical signs and symptoms. Insights Imaging 3(1):33–48. https://doi.org/10.1007/s13244-011-0126-z
Yung M, Tono T, Olszewska E, Yamamoto Y, Sudhoff H, Sakagami M, Mulder J, Incesulu A, Trabalzini F, Ozgirgin N (2017) EAONO/JOS joint consensus statements on the definitions, classification and staging of middle ear cholesteatoma. J Int Adv Otol 13(1):1–8
Kuo C, Shiao A, Yung M, Sakagami M, Sudhoff H, Wang C, Hsu C, Lien C (2015) Updates and knowledge gaps in cholesteatoma research. Biomed Res Int 2015:1–17. https://doi.org/10.1155/2015/854024
Park KH, Park SN, Chang KH, Jung MK, Yeo SW (2009) Congenital middle ear cholesteatoma in children; retrospective review of 35 cases. J Korean Med Sci 24(1):126–131. https://doi.org/10.3346/jkms.2009.24.1.126
Talenti G, Pinelli L, Davies B, Wyatt M, Nash R, D'Arco F (2018) Petrous bone CT findings in patient with 3MC syndrome. - PubMed - NCBI. Otol Neurotol 39(8):e743–e745
Jennings B, Prinsley P, Philpott C, Willis G, Bhutta M (2018) The genetics of cholesteatoma. A systematic review using narrative synthesis. Clin Otolaryngol 43:55–67. https://doi.org/10.1111/coa.12900
Lingam R, Bassett P (2017) A meta-analysis on the diagnostic performance of non-echoplanar diffusion-weighted imaging in detecting middle ear cholesteatoma: 10 years on. Otol Neurotol 38(4):521–528. https://doi.org/10.1097/MAO.0000000000001353
Lingam R, Kumar R, Vaidhyanath R (2019) Inflammation of the temporal bone. Neuroimaging Clin N Am 29(1):1–17
Barath K, Huber A, Stampfl P, Varga Z, Kollias S (2011) Neuroradiology of cholesteatomas. Am J Neuroradiol 32(2):221–229
Lemmerling M, De Foer B, VandeVyver V, Vercruysse J-P, Verstraete K (2008) Imaging of the opacified middle ear. Eur J Radiol 66(3):363–371. https://doi.org/10.1016/j.ejrad.2008.01.020
Koch B, Hamilton B, Hudgins P, Harnsberger H (2016) Temporal bone. In: Diagnostic imaging: head and neck. Elsevier, Salt Lake City, pp 1046–1047
Lemmerling M, De Foer B, Verbist B, VandeVyver V (2009) Imaging of inflammatory and infectious diseases in the temporal bone. Neuroimaging Clin N Am 19(3):321–337
Nevoux J, Lenoir M, Roger G, Denoyelle F, Ducou Le Pointe H, Garabedian EN (2010) Childhood cholesteatoma. Eur Ann Otorhinolaryngol Head Neck Dis 127(4):143–150. https://doi.org/10.1016/j.anorl.2010.07.001
Adamo DA, Fender QA, Hyde BJ, Carlson ML, Koeller K, Lane JI (2017) A pictorial essay of middle ear pathology correlating temporal bone CT and MRI with archived otoscopic photographs. Neurographics 7(2):76–87. https://doi.org/10.3174/ng.2170191
Park S, Jung Y, Oh J (2015) Clinical characteristics of keratosis obturans and external auditory canal cholesteatoma. Otolaryngol Head Neck Surg 152(2):326–330
Juliano A, Ginat D, Moonis G (2013) Imaging review of the temporal bone: part I. anatomy and inflammatory and neoplastic processes. Radiology 269(1):17–33. https://doi.org/10.1148/radiol.13120733
McCoul E, Hanson M (2011) External auditory canal cholesteatoma and keratosis obturans: the role of imaging in preventing facial nerve injury. Ear Nose Throat J 90(12):E1–E7
Jang C, Kim Y, Seong J, Kang S, Jung E, Sung C, Kim S, Cho Y (2016) Clinical characteristics of pediatric external auditory canal cholesteatoma. Int J Pediatr Otorhinolaryngol 87:5–10
Rubin J, Yu V, Stool S (1988) Malignant external otitis in children. J Pediatr 113(6):965–970
Rubin Grandis J, Branstetter B, Yu V (2004) The changing face of malignant (necrotising) external otitis: clinical, radiological, and anatomic correlations. Lancet Infect Dis 4(1):34–39
Grandis J, Curtin H, Yu V (1995) Necrotizing (malignant) external otitis: prospective comparison of CT and MR imaging in diagnosis and follow-up. Radiology 196(2):499–504
White R, Ananthakrishnan G, McKean S, Brunton J, Hussain S, Sudarshan T (2012) Masses and disease entities of the external auditory canal: radiological and clinical correlation. Clin Radiol 67(2):172–181
Saddoud N, Kcahou S, Louati A, Daghfous M (2018) External ear arteriovenous malformation. Otol Neurotol 39(3):e216–e217
Sharon JD, Khwaja SS, Drescher A, Gay H, Chole RA (2014) Osteoradionecrosis of the temporal bone: a case series. Otol Neurotol 35(7):1207–1217. https://doi.org/10.1097/MAO.0000000000000321
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
This was not a research study and therefore informed consent was not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
Inflammation and infection of the temporal bone in children are common and can have a high morbidity and mortality if unrecognised.
Computed tomography and magnetic resonance imaging are complementary in the diagnosis of inflammatory temporal bone disease in children.
Analysing for the presence of and pattern of bone erosion is often a key diagnostic feature in these conditions.
Rights and permissions
About this article
Cite this article
Campion, T., Taranath, A., Pinelli, L. et al. Imaging of temporal bone inflammations in children: a pictorial review. Neuroradiology 61, 959–970 (2019). https://doi.org/10.1007/s00234-019-02258-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-019-02258-1